Nomogram Predicting the Risk of Postoperative Major Wound Complication in Soft Tissue Sarcoma of the Trunk and Extremities after Preoperative Radiotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
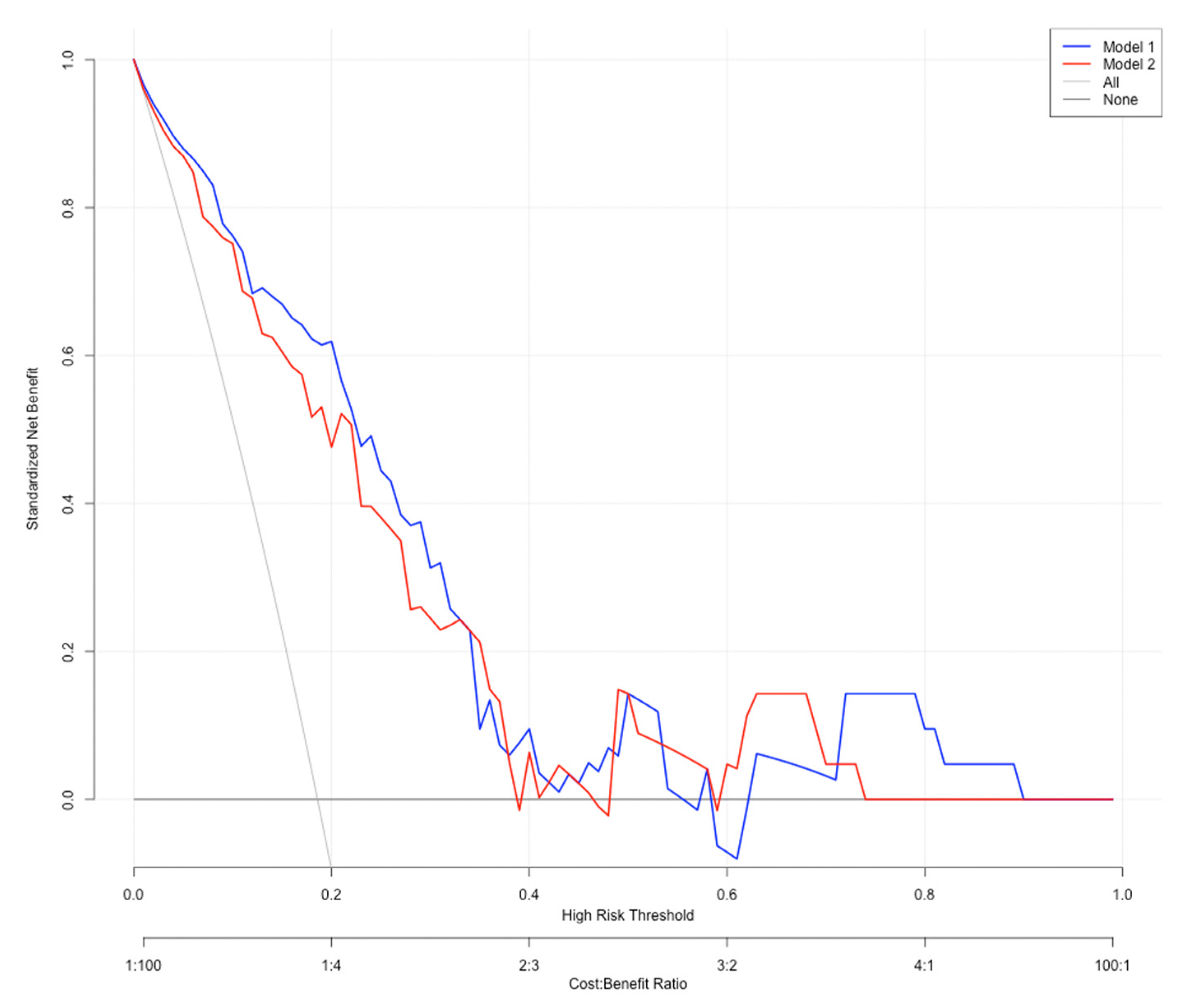
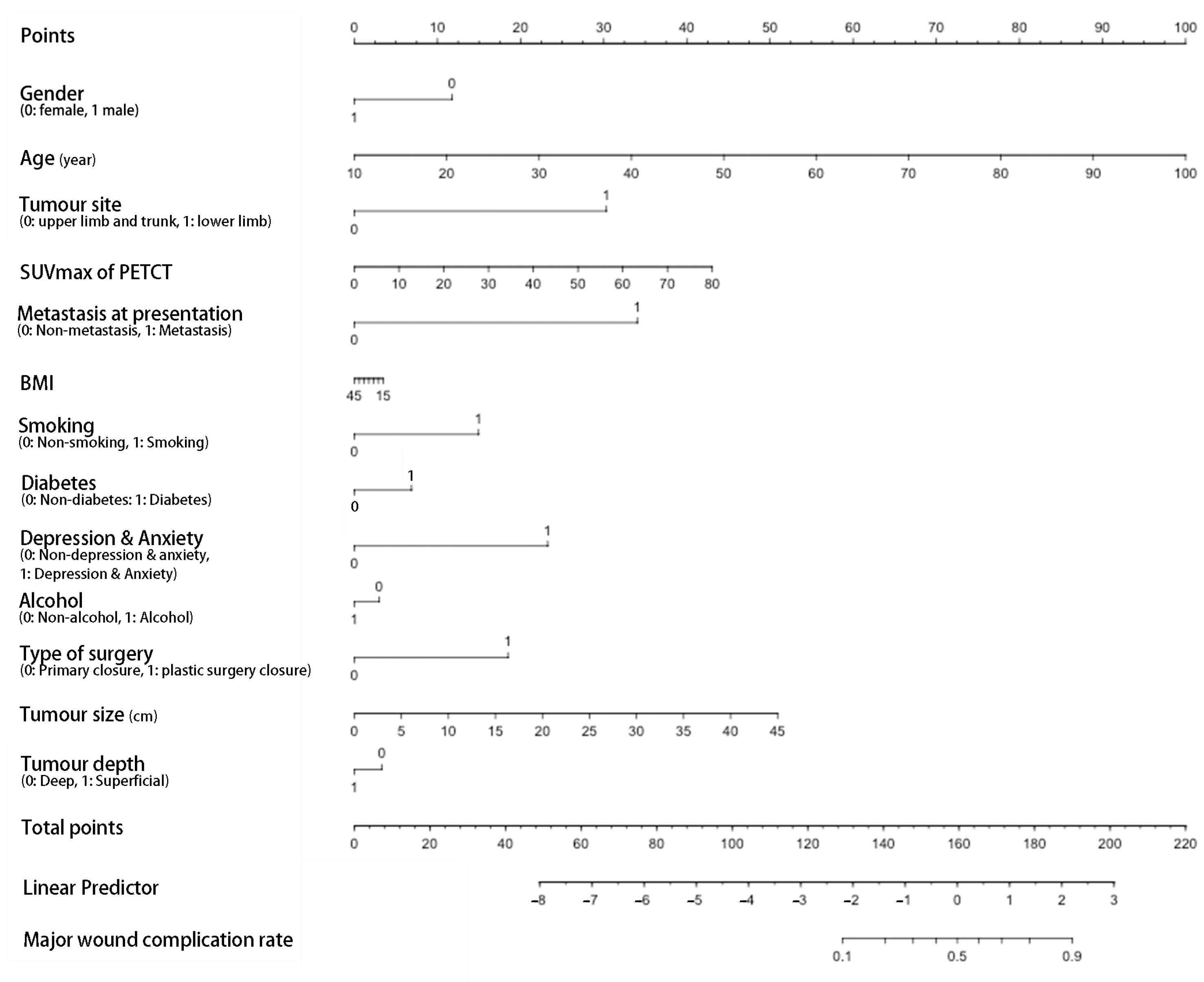
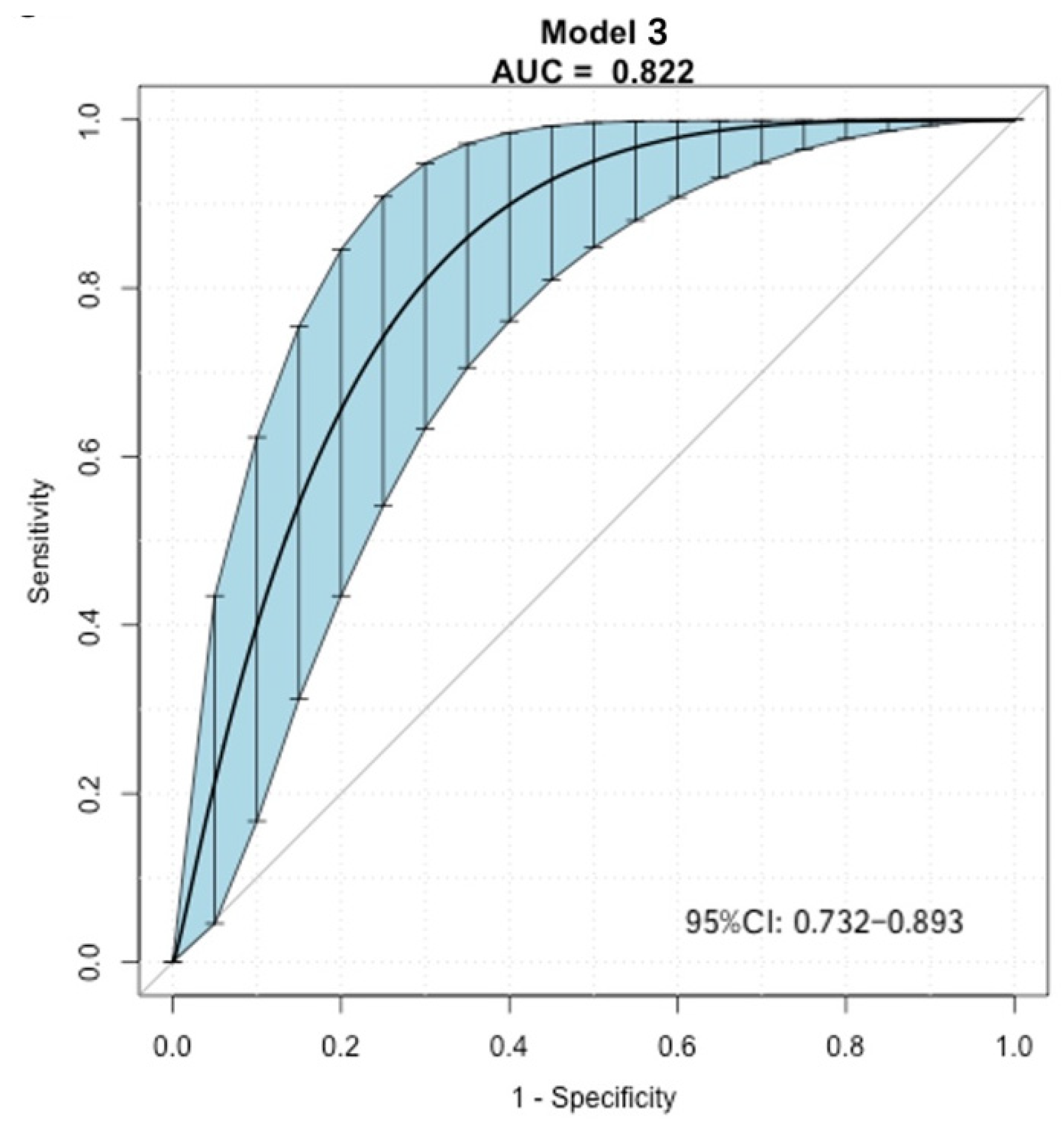
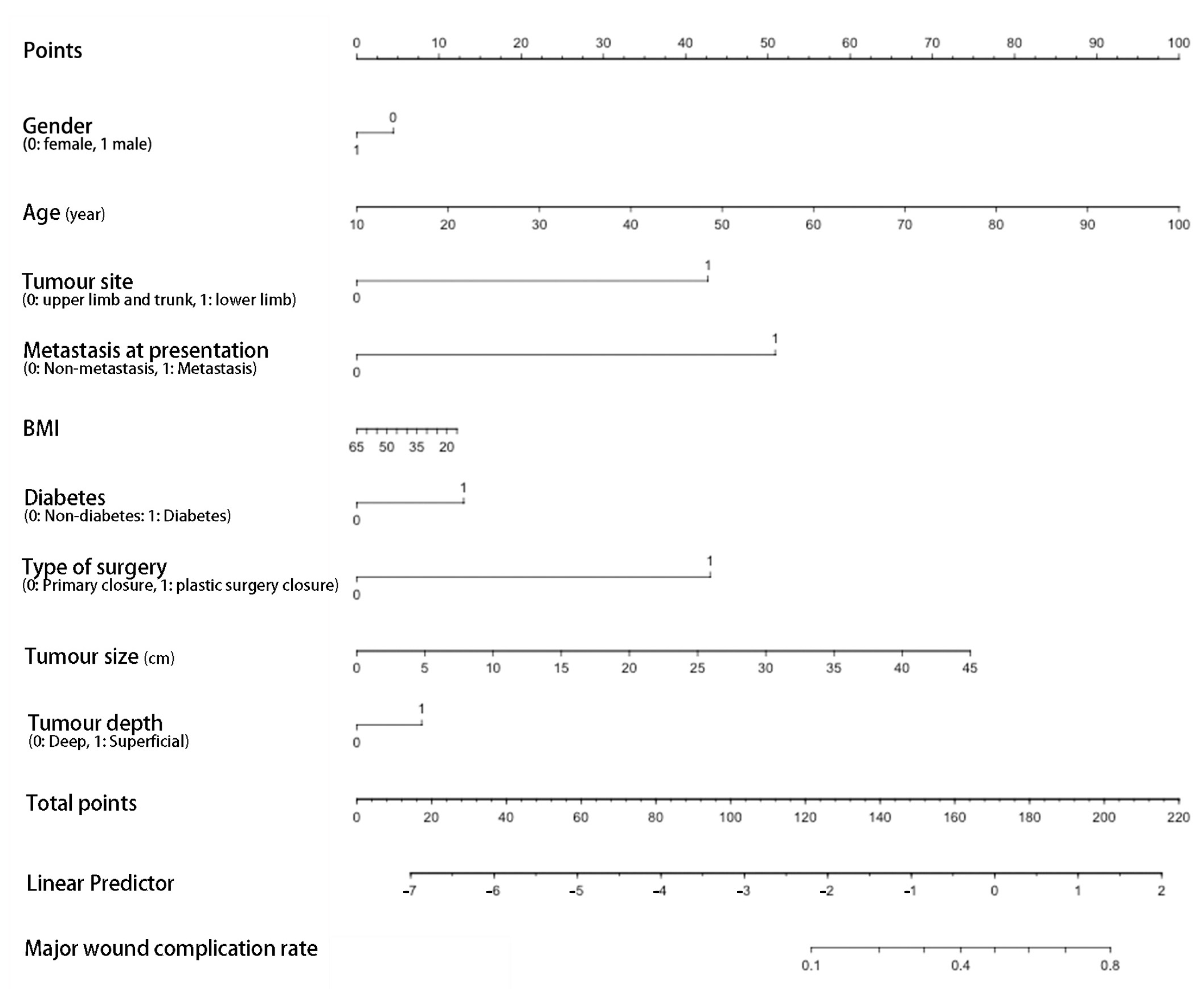
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gronchi, A.; Miah, A.; Dei Tos, A.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.; Bolle, S. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Kane, J.M.; Bui, M.M.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J. NCCN guidelines insights: Soft tissue sarcoma, version 1.2021: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2020, 18, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Rene, N.J.; Castiglioni, A.; Coccaro, N.; Scheitlin, B.; Papa, L. Soft Tissue Sarcomas: Is Pre-operative Radiotherapy Associated With More Acute Wound Complications? Cureus 2021, 13, e15654. [Google Scholar] [CrossRef] [PubMed]
- Kungwengwe, G.; Clancy, R.; Vass, J.; Slade, R.; Sandhar, S.; Dobbs, T.D.; Bragg, T.W.H. Preoperative versus Post-operative Radiotherapy for Extremity Soft tissue Sarcoma: A Systematic Review and Meta-analysis of Long-term Survival. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2443–2457. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Slump, J.; Bastiaannet, E.; Halka, A.; Hoekstra, H.J.; Ferguson, P.C.; Wunder, J.S.; Hofer, S.O.P.; O’Neill, A.C. Risk factors for postoperative wound complications after extremity soft tissue sarcoma resection: A systematic review and meta-analyses. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, C.M.; Hasibuzzaman, M.M.; Rodman, S.N.; Goetz, J.E.; Mapuskar, K.A.; Petronek, M.S.; Steinbach, E.J.; Miller, B.J.; Pulliam, C.F.; Coleman, M.C.; et al. Neoadjuvant Radiotherapy-Related Wound Morbidity in Soft Tissue Sarcoma: Perspectives for Radioprotective Agents. Cancers 2020, 12, 2258. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.; Rebecca, A.; Smith, A.; Casey, W.; Ashman, J.; Gunderson, L.; Curtis, K.; Chang, Y.H.; Beauchamp, C. Risk factors for significant wound complications following wide resection of extremity soft tissue sarcomas. Clin. Orthop. Relat. Res. 2013, 471, 3612–3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liang, C.; Wang, X.; Sun, M.; Kang, L. A computed tomography-based nomogram to predict pneumothorax caused by preoperative localization of ground glass nodules using hook wire. Br. J. Radiol. 2021, 94, 20200633. [Google Scholar] [CrossRef] [PubMed]
- Casabianca, L.; Kreps, S.; Helfre, S.; Housset, M.; Anract, P.; Biau, D.J. Optimal post-operative radiation after soft-tissue sarcoma resection is achieved in less than two thirds of cases. Int. Orthop. 2017, 41, 2401–2405. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.D.; Mo, X.; Andonian, N.T.; Haglund, K.E.; Martin, D.D.; Liebner, D.A.; Chen, J.L.; Iwenofu, O.H.; Chakravarti, A.; Scharschmidt, T.J.; et al. Patterns of major wound complications following multidisciplinary therapy for lower extremity soft tissue sarcoma. J. Surg. Oncol. 2016, 114, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Fisher, C.; Judson, I.; Thomas, J.M. Soft-tissue sarcomas in adults. N. Engl. J. Med. 2005, 353, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elswick, S.M.; Curiel, D.A.; Wu, P.; Akhavan, A.; Molinar, V.E.; Mohan, A.T.; Sim, F.H.; Martinez-Jorge, J.; Saint-Cyr, M. Complications after thigh sarcoma resection. J. Surg. Oncol. 2020, 121, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.C.; Ngan, S.Y.; Wong, K.; Powell, G.; Choong, P.F. Preoperative radiotherapy for soft tissue sarcoma: The Peter MacCallum Cancer Centre experience. Eur. J. Surg. Oncol. 2006, 32, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, L.A.; Esther, R.J.; Erfanian, K.; Green, R.; Kim, H.J.; Sweeting, R.; Tepper, J.E. Wound complications in preoperatively irradiated soft-tissue sarcomas of the extremities. Int J. Radiat. Oncol. Biol. Phys. 2013, 85, 432–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.; Isler, M.; Barry, J.; Mottard, S. Major wound complication risk factors following soft tissue sarcoma resection. Eur. J. Surg. Oncol. 2014, 40, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Slump, J.; Hofer, S.O.P.; Ferguson, P.C.; Wunder, J.S.; Griffin, A.M.; Hoekstra, H.J.; Bastiaannet, E.; O’Neill, A.C. Flap reconstruction does not increase complication rates following surgical resection of extremity soft tissue sarcoma. Eur. J. Surg. Oncol. 2018, 44, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.H.; Chang, D.W.; Shuaib, S.W.; Hanasono, M.M. The effect of neoadjuvant versus adjuvant irradiation on microvascular free flap reconstruction in sarcoma patients. Plast. Reconstr. Surg. 2012, 129, 675–682. [Google Scholar] [CrossRef]
- Cannon, C.P.; Ballo, M.T.; Zagars, G.K.; Mirza, A.N.; Lin, P.P.; Lewis, V.O.; Yasko, A.W.; Benjamin, R.S.; Pisters, P.W. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006, 107, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Temple, C.L.; Ross, D.C.; Magi, E.; DiFrancesco, L.M.; Kurien, E.; Temple, W.J. Preoperative chemoradiation and flap reconstruction provide high local control and low wound complication rates for patients undergoing limb salvage surgery for upper extremity tumors. J. Surg. Oncol. 2007, 95, 135–141. [Google Scholar] [CrossRef] [PubMed]

| Category | Total, n | Non-MaWC n, % | MaWC, n, % | p-Value |
|---|---|---|---|---|
| Number of patients | 126 | 102(81.0%) | 24 (19.0%) | |
| Gender | 0.741 | |||
| Female | 51 (40.5%) | 42 (41.2%) | 9 (37.5%) | |
| Male | 75 (59.5%) | 60 (58.8%) | 15 (62.5%) | |
| Mean age (year) | 62.0 | 57.9 ± 17.1 | 68.5 ± 15.4 | 0.009 |
| Mean BMI | 28.7 | 28.9 ± 7.2 | 27.4 ± 5.9 | 0.361 |
| Diabetes | 0.048 | |||
| Non-diabetes | 110 (87.3%) | 92 (90.2%) | 18 (75.0%) | |
| Diabetes | 16 (12.7%) | 10 (9.8%) | 6 (25.0%) | |
| Smoking | 0.127 | |||
| Non-smoking | 80 (63.5%) | 68(66.7%) | 12 (50.0%) | |
| Smoking | 46 (36.5%) | 34 (33.3%) | 12 (50.0%) | |
| Alcohol | 0.179 | |||
| Non-alcohol | 43 (34.1%) | 32 (31.4%) | 11 (45.8%) | |
| Alcohol | 83 (65.9%) | 70 (68.6%) | 13 (54.2%) | |
| Depression or anxiety | 0.467 | |||
| Non-depression or anxiety | 115 (91.3%) | 94 (92.2%) | 21 (87.5%) | |
| Depression or anxiety | 11 (8.7%) | 8 (7.8%) | 3 (12.5%) | |
| Tumour site | 0.181 | |||
| Upper limb | 11 (8.7%) | 11 (10.8%) | 0 (0.0%) | |
| Proximal lower limb | 75 (59.5%) | 60 (58.8%) | 15 (62.5%) | |
| Distal lower limb | 19 (15.1%) | 13 (12.8%) | 6 (25.0%) | |
| Trunk | 21 (16.7%) | 18(17.6%) | 3 (12.5%) | |
| Tumour depth | 0.892 | |||
| Deep | 101 (80.2%) | 82 (80.4%) | 19 (79.2%) | |
| Superficial | 25 (19.8%) | 20 (19.6%) | 5 (20.8%) | |
| Mean tumour volume (mean ± SD, cm3) | 434.6 | 376.1 ± 173.7 | 710.1 ± 240.2 | 0.065 |
| Mean tumour size (mean ± SD, cm) | 10.2 ± 6.0 | 9.5 ± 5.3 | 13.0 ± 7.9 | 0.018 |
| PETCT SUVmax (Mean ± SD) | 15.0 ±13.8 | 13.9 ± 9.7 | 19.6 ± 16.7 | 0.090 |
| Histology type | 0.686 | |||
| Myxoid liposarcoma | 23 (18.3%) | 19 (18.6%) | 4 (16.7%) | |
| Other liposarcoma | 8 (6.4%) | 5 (4.9%) | 3 (12.5%) | |
| Myxoidfibrosarcoma | 19 (15.1%) | 16 (15.7%) | 3 (12.5%) | |
| Synovial sarcoma | 11 (8.7%) | 10 (9.8%) | 1 (4.2%) | |
| Undifferentiated pleomorphic sarcoma | 13 (10.3%) | 11 (10.8%) | 2 (8.3%) | |
| Leiomyosarcoma | 12 (9.5%) | 11 (10.8%) | 1 (4.2%) | |
| Unclassified pleomorphic sarcoma | 25 (19.8%) | 20 (19.6%) | 5 (20.8%) | |
| Unclassified spindle-cell sarcoma | 6 (4.8%) | 4 (3.9%) | 2 (8.3%) | |
| Others | 9 (7.1%) | 6 (5.9%) | 3 (12.5%) | |
| Metastasis at presentation | 0.028 | |||
| Non-metastasis | 115 (91.3%) | 96 (94.1%) | 19 (79.2%) | |
| Metastasis | 11 (8.7%) | 6 (5.9%) | 5 (20.8%) | |
| Type of surgery | 0.066 | |||
| Primary closure | 117 (92.9%) | 97 (95.1%) | 20 (83.3%) | |
| Plastic surgery closure | 9 (7.1%) | 5 (4.9%) | 4 (16.7%) | |
| Surgery margin | 0.758 | |||
| R0 | 122 (96.8%) | 99 (97.1%) | 23 (95.8%) | |
| R1 | 4 (3.2%) | 3 (2.9%) | 1 (4.2%) |
| Risk Factor | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Age | 1.04 | 1.01–1.08 | 0.009 | 1.08 | 1.02–1.13 | 0.004 |
| Diabetes | 3.07 | 1.01–9.71 | 0.048 | 2.46 | 0.57–10.42 | 0.226 |
| Metastasis at presentation | 4.21 | 1.17–15.22 | 0.028 | 9.12 | 1.21–68.67 | 0.032 |
| Tumour size (cm) | 1.09 | 1.01–1.17 | 0.018 | 1.12 | 1.01–1.24 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Z.; Trent, S.; McCarthy, C.; Cosker, T.; Whitwell, D.; Branford-White, H.; Gibbons, C.L.M.H. Nomogram Predicting the Risk of Postoperative Major Wound Complication in Soft Tissue Sarcoma of the Trunk and Extremities after Preoperative Radiotherapy. Cancers 2022, 14, 4096. https://doi.org/10.3390/cancers14174096
Ouyang Z, Trent S, McCarthy C, Cosker T, Whitwell D, Branford-White H, Gibbons CLMH. Nomogram Predicting the Risk of Postoperative Major Wound Complication in Soft Tissue Sarcoma of the Trunk and Extremities after Preoperative Radiotherapy. Cancers. 2022; 14(17):4096. https://doi.org/10.3390/cancers14174096
Chicago/Turabian StyleOuyang, Zhengxiao, Sally Trent, Catherine McCarthy, Thomas Cosker, Duncan Whitwell, Harriet Branford-White, and Christopher Leonard Maxime Hardwicke Gibbons. 2022. "Nomogram Predicting the Risk of Postoperative Major Wound Complication in Soft Tissue Sarcoma of the Trunk and Extremities after Preoperative Radiotherapy" Cancers 14, no. 17: 4096. https://doi.org/10.3390/cancers14174096
APA StyleOuyang, Z., Trent, S., McCarthy, C., Cosker, T., Whitwell, D., Branford-White, H., & Gibbons, C. L. M. H. (2022). Nomogram Predicting the Risk of Postoperative Major Wound Complication in Soft Tissue Sarcoma of the Trunk and Extremities after Preoperative Radiotherapy. Cancers, 14(17), 4096. https://doi.org/10.3390/cancers14174096






