Cost-Effectiveness of an Organized Lung Cancer Screening Program for Asbestos-Exposed Subjects
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Population Characteristics and Parameters
3.2. Main Analysis
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. Available online: https://gco.iarc.fr/today (accessed on 5 February 2021).
- Loomis, D.; Guha, N.; Hall, A.L.; Straif, K. Identifying occupational carcinogens: An update from the IARC Monographs. Occup. Environ. Med. 2018, 75, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Douguet, D.; Carteron, H.; Janiaud, P.; Pinhas, N. Health Effects of the Main Types of Exposure to Asbestos; Research Report; National Institute of Health and Medical Research (INSERM): Paris, France, 1997. [Google Scholar]
- IARC. IARC Monographs on the Evaluation on the Carcinogenic Risks to Humans. Asbestos; IARC: Lyon, France, 1977; pp. 14–106.
- Consensus development conference for the elaboration of a clinical medical surveillance strategy for people exposed to asbestos. Paris, France, 15 January 1999. Rev. Mal. Respir. 1999, 16 Pt 2, 1187–1362.
- Haute Autorité de Santé. Post-professional follow-up after asbestos exposure. Rev. Mal. Respir. 2010, 27, e17–e33. [Google Scholar]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Raymakers, A.J.N.; Mayo, J.; Lam, S.; FitzGerald, J.M.; Whitehurst, D.G.T.; Lynd, L.D. Cost-Effectiveness Analyses of Lung Cancer Screening Strategies Using Low-Dose Computed Tomography: A Systematic Review. Appl. Health Econ. Health Policy. 2016, 14, 409–418. [Google Scholar] [CrossRef]
- Zhang, J.; Gold, K.A.; Lin, H.Y.; Swisher, S.G.; Xing, Y.; Lee, J.J.; Kim, E.S.; William, W.N. Relationship between tumor size and survival in non-small-cell lung cancer (NSCLC): An analysis of the surveillance, epidemiology, and end results (SEER) registry. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 682–690. [Google Scholar] [CrossRef]
- Flores, R.; Patel, P.; Alpert, N.; Pyenson, B.; Taioli, E. Association of Stage Shift and Population Mortality among Patients with Non-Small Cell Lung Cancer. JAMA Netw. Open 2021, 4, e2137508. [Google Scholar] [CrossRef]
- Potter, A.L.; Rosenstein, A.L.; Kiang, M.V.; Shah, S.A.; Gaissert, H.A.; Chang, D.C.; Fintelmann, F.J.; Yang, C.-F.J. Association of computed tomography screening with lung cancer stage shift and survival in the United States: Quasi-experimental study. BMJ 2022, 376, e069008. [Google Scholar] [CrossRef]
- Markowitz, S.B.; Dickens, B. Screening for Occupational Lung Cancer: An Unprecedented Opportunity. Clin. Chest Med. 2020, 41, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Ollier, M.; Chamoux, A.; Naughton, G.; Pereira, B.; Dutheil, F. Chest CT scan screening for lung cancer in asbestos occupational exposure: A systematic review and meta-analysis. Chest 2014, 145, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Rampinelli, C.; Bertolotti, R.; Misotti, A.; Lococo, F.; Casiraghi, M.; Spaggiari, L.; Bellomi, M.; Novellis, P.; Solinas, M.; et al. Low-dose computed tomography screening for lung cancer in people with workplace exposure to asbestos. Lung Cancer Amst. Neth. 2019, 131, 23–30. [Google Scholar] [CrossRef]
- Pairon, J.-C.; Andujar, P.; Rinaldo, M.; Ameille, J.; Brochard, P.; Chamming’s, S.; Clin, B.; Ferretti, G.; Gislard, A.; Laurent, F.; et al. Asbestos exposure, pleural plaques, and the risk of death from lung cancer. Am. J. Respir. Crit. Care Med. 2014, 190, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W. Asbestosis: A marker for the increased risk of lung cancer among workers exposed to asbestos. Chest 1999, 115, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Pairon, J.-C.; Laurent, F.; Rinaldo, M.; Clin, B.; Andujar, P.; Ameille, J.; Brochard, P.; Chammings, S.; Ferretti, G.; Galateau-Sallé, F.; et al. Pleural plaques and the risk of pleural mesothelioma. J. Natl. Cancer Inst. 2013, 105, 293–301. [Google Scholar] [CrossRef]
- Paris, C.; Thierry, S.; Brochard, P.; Letourneux, M.; Schorle, E.; Stoufflet, A.; Ameille, J.; Conso, F.; Pairon, J.C.; National APEXS Members. Pleural plaques and asbestosis: Dose- and time-response relationships based on HRCT data. Eur. Respir. J. 2009, 34, 72–79. [Google Scholar] [CrossRef]
- Paris, C.; Thaon, I.; Hérin, F.; Clin, B.; Lacourt, A.; Luc, A.; Coureau, G.; Brochard, P.; Chamming’s, S.; Gislard, A.; et al. Occupational Asbestos Exposure and Incidence of Colon and Rectal Cancers in French Men: The Asbestos-Related Diseases Cohort (ARDCo-Nut). Environ. Health Perspect. 2017, 125, 409–415. [Google Scholar] [CrossRef]
- Institut National d’Etudes Démographiques. Lexique, Mortalité—Tables de Mortalité Françaises. Insee, Vital Sta-tistics and Population Estimates. 2019. Available online: https://www.ined.fr/fr/lexique/mortalite/ (accessed on 5 April 2021).
- Debieuvre, D.; Locher, C.; Asselain, B.; Dayen, C.; Molinier, O.; Falchero, L.; Dujon, C.; Delclaux, B.; Grivaux, M. Evidence of slight improvement in five-year survival in non-small-cell lung cancer over the last 10 years: Results of the French KBP-CPHG real-world studies. Bull. Cancer 2019, 106, 283–292. [Google Scholar] [CrossRef]
- Williams, C.; Lewsey, J.D.; Mackay, D.F.; Briggs, A.H. Estimation of Survival Probabilities for Use in Cost-effectiveness Analyses: A Comparison of a Multi-state Modeling Survival Analysis Approach with Partitioned Survival and Markov Decision-Analytic Modeling. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2017, 37, 427–439. [Google Scholar] [CrossRef]
- Leleu, O.; Basille, D.; Auquier, M.; Clarot, C.; Hoguet, E.; Pétigny, V.; Addi, A.A.; Milleron, B.; Chauffert, B.; Berna, P.; et al. Lung Cancer Screening by Low-Dose CT Scan: Baseline Results of a French Prospective Study. Clin. Lung Cancer 2020, 21, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sturza, J. A review and meta-analysis of utility values for lung cancer. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2010, 30, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Gareen, I.F.; Duan, F.; Greco, E.M.; Snyder, B.S.; Boiselle, P.M.; Park, E.R.; Fryback, D.; Gatsonis, C. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer 2014, 120, 3401–3409. [Google Scholar] [CrossRef]
- Chouaid, C.; Vergnenègre, A. Les coûts du cancer du poumon. Rev. Mal. Respir. Actual. 2018, 10, 192–197. [Google Scholar] [CrossRef]
- Ramos, R.; Masuet, C.; Gossot, D. Lobectomy for early-stage lung carcinoma: A cost analysis of full thoracoscopy versus posterolateral thoracotomy. Surg. Endosc. 2012, 26, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Andreas, S.; Chouaid, C.; Danson, S.; Siakpere, O.; Benjamin, L.; Ehness, R.; Dramard-Goasdoue, M.-H.; Barth, J.; Hoffmann, H.; Potter, V.; et al. Economic burden of resected (stage IB-IIIA) non-small cell lung cancer in France, Germany and the United Kingdom: A retrospective observational study (LuCaBIS). Lung Cancer Amst. Neth. 2018, 124, 298–309. [Google Scholar] [CrossRef]
- McGuire, A.; Martin, M.; Lenz, C.; Sollano, J.A. Treatment cost of non-small cell lung cancer in three European countries: Comparisons across France, Germany, and England using administrative databases. J. Med. Econ. 2015, 18, 525–532. [Google Scholar] [CrossRef]
- Haute Autorité de Santé. Choices in Methods for Economic Evaluation; HAS: Saint-Denis La Plaine, France, 2020; Available online: https://www.has-sante.fr/jcms/r_1499251/en/choices-in-methods-for-economic-evaluation (accessed on 3 June 2020).
- Jain, R.; Grabner, M.; Onukwugha, E. Sensitivity analysis in cost-effectiveness studies: From guidelines to practice. PharmacoEconomics 2011, 29, 297–314. [Google Scholar] [CrossRef]
- Pastorino, U.; Rossi, M.; Rosato, V.; Marchianò, A.; Sverzellati, N.; Morosi, C.; Fabbri, A.; Galeone, C.; Negri, E.; Sozzi, G.; et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2012, 21, 308–315. [Google Scholar] [CrossRef]
- Pastorino, U.; Silva, M.; Sestini, S.; Sabia, F.; Boeri, M.; Cantarutti, A.; Sverzellati, N.; Sozzi, G.; Corrao, G.; Marchianò, A. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: New confirmation of lung cancer screening efficacy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1162–1169. [Google Scholar] [CrossRef]
- Villanti, A.C.; Jiang, Y.; Abrams, D.B.; Pyenson, B.S. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PloS ONE 2013, 8, e71379. [Google Scholar] [CrossRef] [PubMed]
- Black, W.C.; Gareen, I.F.; Soneji, S.S.; Sicks, J.D.; Keeler, E.B.; Aberle, D.R.; Naeim, A.; Church, T.R.; Silvestri, G.A.; Gorelick, J.; et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N. Engl. J. Med. 2014, 371, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- McMahon, P.M.; Kong, C.Y.; Bouzan, C.; Weinstein, M.C.; Cipriano, L.E.; Tramontano, A.C.; Johnson, B.E.; Weeks, J.C.; Gazelle, G.S. Cost-Effectiveness of CT Screening for Lung Cancer in the U.S. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 1841–1848. [Google Scholar] [CrossRef]
- Jaine, R.; Kvizhinadze, G.; Nair, N.; Blakely, T. Cost-effectiveness of a low-dose computed tomography screening programme for lung cancer in New Zealand. Lung Cancer 2018, 124, 233–240. [Google Scholar] [CrossRef]
- Tomonaga, Y.; Ten Haaf, K.; Frauenfelder, T.; Kohler, M.; Kouyos, R.D.; Shilaih, M.; Lorez, M.; de Koning, H.J.; Schwenkglenks, M.; Puhan, M.A. Cost-effectiveness of low-dose CT screening for lung cancer in a European country with high prevalence of smoking-A modelling study. Lung Cancer 2018, 121, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, N.R.; Flanagan, W.M.; Evans, W.K.; Miller, A.B.; Canadian Partnership against Cancer (CPAC); Cancer Risk Management (CRM) Lung Cancer Working. Eligibility for low-dose computerized tomography screening among asbestos-exposed individuals. Scand. J. Work. Environ. Health 2015, 41, 407–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lebègue, D. The Price of Time in Public Decision-Making. Commissariat Général du Plan, Paris. La Documentation Française. 2005. Available online: http://temis.documentation.developpement-durable.gouv.fr/docs/Temis/0050/Temis-0050505/15417_rapport.pdf (accessed on 6 August 2020).
- Mastrangelo, G.; Ballarin, M.N.; Bellini, E.; Bizzotto, R.; Zannol, F.; Gioffrè, F.; Gobbi, M.; Tessadri, G.; Marchiori, L.; Marangi, G.; et al. Feasibility of a screening programme for lung cancer in former asbestos workers. Occup. Med. Oxf. Engl. 2008, 58, 175–180. [Google Scholar] [CrossRef][Green Version]
- Markowitz, S.B. Lung Cancer Screening in Asbestos-Exposed Populations. Int. J. Environ. Res. Public. Health 2022, 19, 2688. [Google Scholar] [CrossRef]
- Welch, L.S.; Dement, J.M.; Cranford, K.; Shorter, J.; Quinn, P.S.; Madtes, D.K.; Ringen, K. Early detection of lung cancer in a population at high risk due to occupation and smoking. Occup. Environ. Med. 2019, 76, 137–142. [Google Scholar] [CrossRef]
- Delva, F.; Laurent, F.; Paris, C.; Belacel, M.; Brochard, P.; Bylicki, O.; Chouaïd, C.; Clin, B.; Dewitte, J.-D.; Le Denmat, V.; et al. LUCSO-1-French pilot study of LUng Cancer Screening with low-dose computed tomography in a smokers population exposed to Occupational lung carcinogens: Study protocol. BMJ Open 2019, 9, e025026. [Google Scholar] [CrossRef]

| Characteristic | ARDCO | NLST | |
|---|---|---|---|
| Cohort | CT Group | Controls | |
| Number of subjects | 14,218 | 26,722 | 26,732 |
| Male sex, n (%) | 13,481 (94.8%) | 15,770 (59.0%) | 15,762 (59.0%) |
| Age at inclusion, years | |||
| <60 | 3332 (23.4%) | 11,442 (42.8%) | 11,424 (42.7%) |
| ≥60 and <75 | 10,490 (73.8%) | 15,279 (57.2%) | 15,305 (57.3%) |
| ≥75 | 396 (2.8%) | 1 (<0.1%) | 3 (<0.1%) |
| Smoker status at inclusion 1 | |||
| Never-smoker | 2943 (20.7%) | 0 (0%) | 0 (0%) |
| Ex-smoker | 6042 (42.5%) | 13,860 (51.9%) | 13,832 (51.7%) |
| Smoker | 835 (5.9%) | 12,862 (48.1%) | 12,900 (48.3%) |
| Asbestos exposure | |||
| Low | 1070 (7.5%) | NA | NA |
| Intermediate | 9660 (67.9%) | NA | NA |
| High | 3488 (24.5%) | NA | NA |
| Parameter | Lung Cancer Incidence (Per 1000 Person-Years) | |||
|---|---|---|---|---|
| All Ages | <60 Years | 60–75 Years | >75 Years | |
| Total population | 2.30‰ | 2.61‰ | 2.21‰ | 2.12‰ |
| Smokers | 6.04‰ | 6.41‰ | 5.57‰ | NA |
| Smokers & former smokers | 2.78‰ | 3.22‰ | 2.30‰ | 1.85‰ |
| Asbestos exposure | ||||
| High | 2.90‰ | 3.03‰ | 2.83‰ | 2.64‰ |
| Intermediate | 2.20‰ | 2.49‰ | 2.15‰ | 1.91‰ |
| High and smoker | 7.07‰ | 6.36‰ | 7.82‰ | NA |
| Pleural plaques | 2.31‰ | 2.42‰ | 2.25‰ | 2.80‰ |
| Subjects with asbestosis | 5.00‰ | NA | 6.06‰ | NA |
| Parameter | Model Values | Probability | Ref | ||
|---|---|---|---|---|---|
| Determinist | Low: Parametric | High: Parametric | Distribution | ||
| Probability of transition between health states without screening | |||||
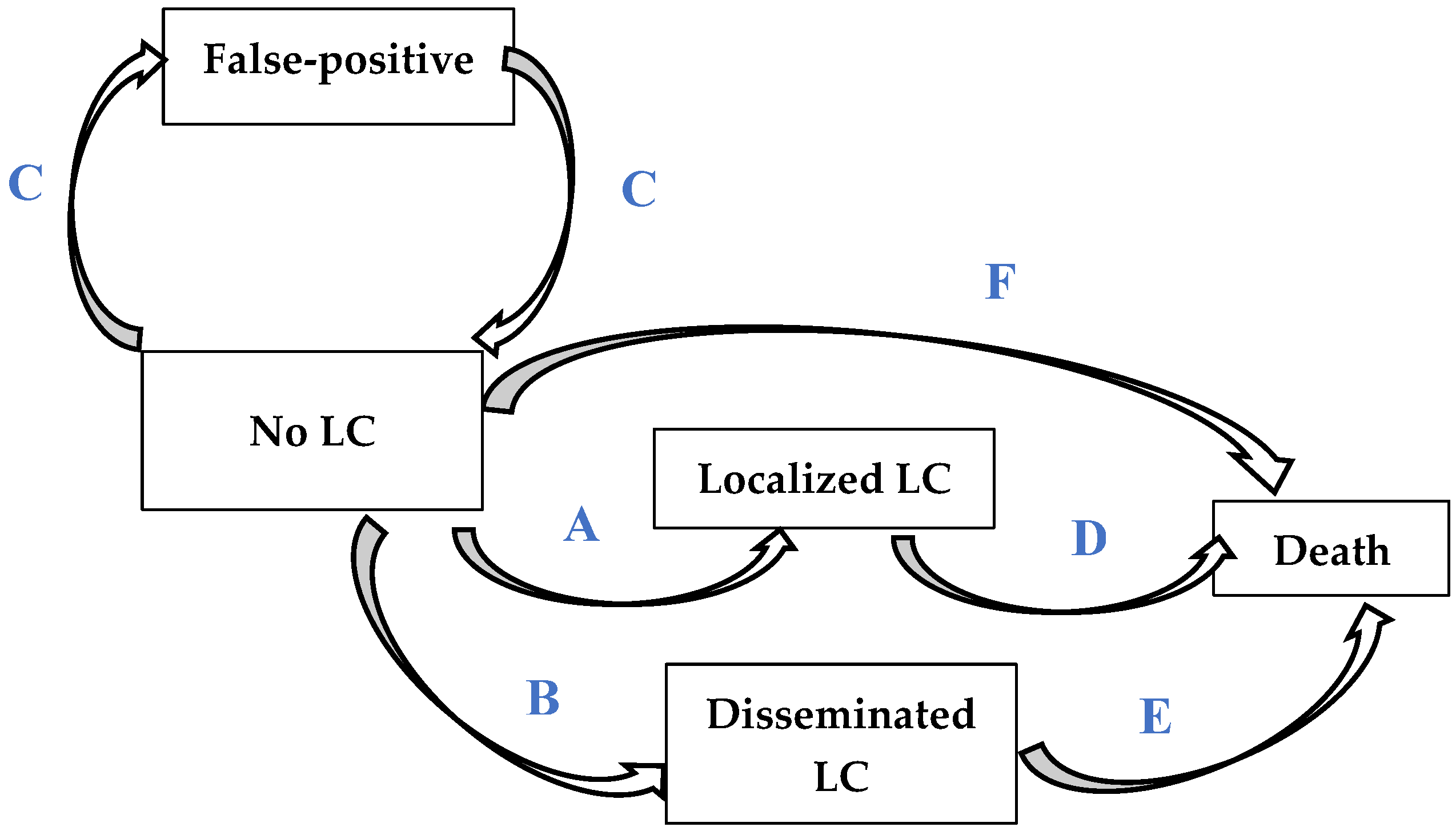
| Localized LC (A 1) | SIR 2 × 0.181 | SIR 2 × 0.171 | SIR 2 × 0.191 | Normal | [23] |
| Disseminated LC (B 1) | SIR 2 × 0.819 | SIR 2 × 0.809 | SIR 2 × 0.829 | Normal | [23] |
| Probability of transition between health states with screening | |||||
| Localized LC (As) 3 | SIR 2 × 0.702 | SIR 2 × 0.694 | SIR 2 × 0.710 | Normal | [8] |
| Disseminated LC (Bs) 3 | SIR 2 × 0.298 | SIR 2 × 0.290 | SIR 2 × 0.306 | Normal | [8] |
| HR overdiagnosis | 1.13 | – | – | – | [8] |
| Probability of false-positives (C 1) | 1.2% | – | – | – | [9] |
| Model adaptation for a 2-year interval between scans | |||||
| LDTDT-detected LCs/cancer interval | 2.8/13 4 | – | – | – | [9] |
| LCs detected every 2 vs. 1 year | 1.5/1 5 | – | – | – | [35] |
| Probability of transition between health states (2 strategies) | |||||
| Death attributed to localized LCs (D 1) | HR: 2.68 | – | – | – | [23] |
| Death attributed to disseminated LCs (E 1) | HR: 8.38 | – | – | – | [23] |
| Non-LC death (F 1) | INED 2019 death table | – | – | – | [22], Table S1 |
| Costs | |||||
| Without screening 6 | 26 € | 26 € | 73 € | Gamma | Table S2 |
| With screening 7 | 189 € | 147 € | 232 € | Gamma | Table S3 |
| Localized LC | |||||
| - Surgical | 13,390 € | 6337 € | 20,443 € | Gamma | [29] |
| - Post-surgical (/2 years) | 19,057 € | 16,770 € | 21,429 € | [30] | |
| Disseminated LC (/2 years) | 33,132 € | 29,357 € 8 | 34,305 € 9 | Gamma | [31] |
| False-positives | 2110 € | 1716 € | 2271 € | Gamma | Table S3 |
| Utility | |||||
| Localized LC | 0.825 | 0.793 | 0.857 | Beta | [26] |
| Disseminated LC | 0.573 | 0.506 | 0.640 | Beta | [26] |
| False-positives | 1.000 | 0.970 | 1.000 | Beta | [27] |
| Outputs | ARDCO Cohort Screening Every 1 y | ARDCO Cohort Screening Every 2 y | Smokers with High Asbestos Exposure Screening Every 1 y | Smokers with High Asbestos Exposure Screening Every 2 y |
|---|---|---|---|---|
| Number of subjects in the simulation | N = 14,218 | N = 14,218 | N = 14,218 | N = 14,218 |
| Localized LC: Usual care | 169 (150–187) | 169 (149–187) | 513 (462–567) | 513 (462–566) |
| Localized LC: Intervention scenario | 740 (725–756) | 641 (627–654) | 2198 (2144–2248) | 1931 (1897–1970) |
| Disseminated LC: Usual care | 761 (743–777) | 761 (745–777) | 2304 (2256–2352) | 2304 (2245–2358) |
| Disseminated LC: Intervention scenario | 316 (300–331) | 289 (274–302) | 926 (874–969) | 867 (824–912) |
| Total Number of False Positive results | 4993 (4988–4998) | 2551 (2550–2554) | 4560 (4545–4574) | 2357 (2351–2363) |
| Per capita | ||||
| Total Costs: Usual care (€) | 5493 € (5136–7403) | 5493 € (5121–7256) | 15,264 € (12,931–19,454) | 15,264 € (12,835–19,770) |
| Total Costs: Intervention scenario (€) | 12,408 € (9049–18,811) | 9653 € (6705–14,390) | 28,310 € (19,518–45,422) | 24,330 € (16,629–39,851) |
| Total QALYs: Usual care | 17.6911 (17.6758–17.7066) | 17.6911 (17.6757–17.7055) | 17.1491 (17.1001–17.1951) | 17.1491 (17.1063–17.1921) |
| Total QALYs: Intervention scenario | 17.7314 (17.699–17.759) | 17.7560 (17.7293–17.7798) | 17.2931 (17.2064–17.3733) | 17.3491 (17.2715–17.4256) |
| Total incremental cost (€) | 6915 € (3671–11,774) | 4161 € (1346–7542) | 13,046 € (5652–26,757) | 9066 € (2697–20,488) |
| QALYs gained | 0.0403 (0.0094–0.0652) | 0.0650 (0.0399–0.0879) | 0.1440 (0.0717–0.2155) | 0.2000 (0.1288–0.2601) |
| ICER (€/QALY) | 171,575 €/QALY (74,669–644,761) | 64,023 €/QALY (20,460–143,220) | 90,624 €/QALY (35,405–276,018) | 45,331 €/QALY (14,992–115,809) |
| Characteristic | Incremental Cost Effectiveness Ratio (€/QALY) | |||||
|---|---|---|---|---|---|---|
| Annual Scan | Biennial Scan | |||||
| Age at screening start | 50 years | 55 years | 60 years | 50 years | 55 years | 60 years |
| Asbestos exposure | ||||||
| Any | 170,485 | 171,575 | 187,957 | 66,386 | 64,023 | 69,005 |
| High | 152,324 | 146,952 | 155,982 | 61,387 | 58,743 | 60,170 |
| Intermediate | 173,469 | 174,300 | 193,499 | 67,196 | 65,241 | 70,090 |
| Any and smoker | 117,769 | 114,854 | 117,955 | 52,179 | 49,195 | 51,099 |
| High and smoker | 103,039 | 90,624 | 90,809 | 47,661 | 45,331 | 41,597 |
| Pleural plaques | 167,606 | 157,823 | 157,215 | 65,916 | 60,790 | 61,333 |
| Asbestosis | 112,202 | 99,531 | 101,620 | 50,067 | 48,011 | 44,366 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gendarme, S.; Pairon, J.-C.; Andujar, P.; Laurent, F.; Brochard, P.; Delva, F.; Clin, B.; Gislard, A.; Paris, C.; Thaon, I.; et al. Cost-Effectiveness of an Organized Lung Cancer Screening Program for Asbestos-Exposed Subjects. Cancers 2022, 14, 4089. https://doi.org/10.3390/cancers14174089
Gendarme S, Pairon J-C, Andujar P, Laurent F, Brochard P, Delva F, Clin B, Gislard A, Paris C, Thaon I, et al. Cost-Effectiveness of an Organized Lung Cancer Screening Program for Asbestos-Exposed Subjects. Cancers. 2022; 14(17):4089. https://doi.org/10.3390/cancers14174089
Chicago/Turabian StyleGendarme, Sébastien, Jean-Claude Pairon, Pascal Andujar, François Laurent, Patrick Brochard, Fleur Delva, Bénédicte Clin, Antoine Gislard, Christophe Paris, Isabelle Thaon, and et al. 2022. "Cost-Effectiveness of an Organized Lung Cancer Screening Program for Asbestos-Exposed Subjects" Cancers 14, no. 17: 4089. https://doi.org/10.3390/cancers14174089
APA StyleGendarme, S., Pairon, J.-C., Andujar, P., Laurent, F., Brochard, P., Delva, F., Clin, B., Gislard, A., Paris, C., Thaon, I., Goussault, H., Canoui-Poitrine, F., & Chouaïd, C. (2022). Cost-Effectiveness of an Organized Lung Cancer Screening Program for Asbestos-Exposed Subjects. Cancers, 14(17), 4089. https://doi.org/10.3390/cancers14174089







