Simple Summary
Melanoma patients who failed anti-PD-1 therapy have limited therapeutic options. Some studies suggested the efficacy of radiotherapy combined with anti-PD-1 monoclonal antibodies. We previously reported in small-sized studies that hypofractionated radiotherapy combined with an unmodified anti-PD-1 monotherapy regimen induced long-lasting efficacy. This study shows that the large use of hypofractionated radiotherapy combined with anti-PD-1 induced high rates of complete response (32.5% [95% CI: 26.1–38.9]) in a cohort of 206 melanoma patients. Radiated patients who had confirmed failure to anti-PD-1 monotherapy had longer progression-free and overall survival than non-radiated ones. No unexpected safety concern was observed. Although no clinical predictive factors have been identified in our study, a synergy between anti-PD1 and radiotherapy is likely. Adding hypofractionated radiotherapy in metastatic melanoma patients treated with anti-PD-1 is a safe option, which may increase the response rate and could be offered in patients with lesions threatening or not responding to anti-PD1.
Abstract
To assess the role of radiotherapy in anti-PD-1-treated melanoma patients, we studied retrospectively a cohort of 206 consecutive anti-PD-1 monotherapy-treated advanced melanoma patients (59% M1c/d, 50% ≥ 3 metastasis sites, 33% ECOG PS ≥ 1, 33% > 1st line, 32% elevated serum LDH) having widely (49%) received concurrent radiotherapy, with RECIST 1.1 evaluation of radiated and non-radiated lesions. Overall (OS) and progression-free (PFS) survivals were calculated using Kaplan–Meier. Radiotherapy was performed early (39 patients) or after 3 months (61 patients with confirmed anti-PD-1 failure). The first radiotherapy was hypofractionated extracranial radiotherapy to 1–2 targets (26 Gy-4 weekly sessions, 68 patients), intracranial radiosurgery (25 patients), or palliative. Globally, 67 (32.5% [95% CI: 26.1–38.9]) patients achieved complete response (CR), with 25 CR patients having been radiated. In patients failing anti-PD-1, PFS and OS from anti-PD-1 initiation were 16.8 [13.4–26.6] and 37.0 months [24.6–NA], respectively, in radiated patients, and 2.2 [1.5–2.6] and 4.3 months [2.6–7.1], respectively, in non-radiated patients (p < 0.001). Abscopal response was observed in 31.5% of evaluable patients who radiated late. No factors associated with response in radiated patients were found. No unusual adverse event was seen. High-dose radiotherapy may enhance CR rate above the 6–25% reported in anti-PD-1 monotherapy or ipilimumab + nivolumab combo studies in melanoma patients.
1. Introduction
Immune checkpoint inhibitors (ICI) have improved advanced melanoma patients’ treatment [1], particularly the anti-programmed death-1 (PD-1) monoclonal antibodies (mAb) nivolumab and pembrolizumab, which induced, in the first-line setting, longer progression-free survival (PFS) and overall survival (OS) than chemotherapy or ipilimumab, an anti-CTLA-4 mAb [2,3]. However, depending on treatment line [4] and length of follow-up, anti-PD-1 monotherapy was associated with a complete (CR) + partial (PR) response rate of only 27–52% and a median PFS of 3.1–6.9 months [2,3,4,5,6] in patients without active brain metastasis. Moreover, intracranial metastases, reported in 40–50% of patients, convey a poor prognosis [7]. Thus, improved strategies are required. Formal demonstration of improved PFS and OS by nivolumab and ipilimumab combination, when compared to nivolumab alone, is lacking and severe adverse events (AEs) are very frequent [6]. This combination also provided encouraging PFS and OS data and high response rates in melanoma patients with 1–4 asymptomatic intracranial metastases [8,9].
Radiotherapy (RT) exerts multiple vascular, stromal, and immunological changes in the tumor microenvironment [10]. Preclinical data suggest that RT combined with ICI may improve tumor response [11]. Some melanoma studies suggested the efficacy of RT combined with anti-PD-1 mAb, but its place in melanoma care is debated because of a limited number of prospective trials [12,13], uncontrolled design, and the use of either multisite RT [14], which precluded the evaluation of the abscopal effect, or suboptimal radiation schedules (RT delivered before anti-PD-1 initiation, insufficient dosing/session) [15,16]. Moreover, RT was frequently combined with anti-PD-1 mAb early on [16,17,18], and responses could therefore originate solely from the late efficacy of anti-PD-1.
Hypofractionated RT delivers higher doses per session than standard palliative RT and requires a reduced number of sessions. We previously reported that hypofractionated RT combined with an unmodified anti-PD-1 mAb monotherapy regimen induced long-lasting efficacy, with a CR+PR rate of 36–38% in melanoma patients who either received this combination early [19] because of life-threatening metastases or had previously failed anti-PD-1 monotherapy [20].
Herein, our objectives were to further investigate this combination in real-life conditions, including in patients with active brain metastases. Could widespread use of extracranial hypofractionated RT and/or intracranial stereotactic radiosurgery (SRS), which also delivers high doses per session, combined with anti-PD-1 monotherapy in a vast cohort of consecutive anti-PD-1 monotherapy-treated melanoma patients, enhance the CR rate above the 6–25% reported in melanoma registration trials [2,3,4,5,6] (with mainly first-line patients without active brain metastasis)? This is an important goal, as patients experiencing CR have a low recurrence probability after anti-PD-1 discontinuation [21]. Secondary objectives were (1) to compare PFS and OS in radiated and non-radiated patients in the whole population and those failing anti-PD-1 mAb; (2) to characterize the profile of radiated patients achieving CR+PR; (3) to assess safety.
2. Materials and Methods
This was a monocenter retrospective analysis of data prospectively collected according to previously published procedures [19,20] in our referral skin cancer department for anti-PD-1 mAb-treated melanoma patients not included in double-blinded trials. Nivolumab or pembrolizumab was infused intravenously according to product labels until unambiguous progressive disease (PD), unacceptable AE, or decision to discontinue treatment, but was continued beyond progression at first evaluation to allow for pseudo-progression [22], or later if ≥1 lesion could be treated locally. AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. We evaluated efficacy at least every 3 months using thoracic, abdominal, and pelvic computed tomography (CT) scans, head CT scans, or magnetic resonance imaging, carried out by melanoma-experienced radiologists, during treatment and 5 years after anti-PD-1 discontinuation. In addition, normal 18F-labeled fluorodeoxyglucose positron emission tomography (FDG-PET) scans were required to confirm CR or to address ambiguous CT images.
Images were reviewed during a weekly joint meeting with radiologists through tumor evaluations according to RECIST 1.1 [23]. CR was defined as the disappearance of all lesions (with the smallest axis of lymph nodes <10 mm), PR as a decrease by >30% of the sum of the diameters of the target lesions, PD as an increase >20% of this sum or occurrence of any new lesions, and stable disease (SD) as no sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Patients who died before the first evaluation were also qualified as PD. The abscopal effect was defined as PR or CR outside radiated fields.
Our tumor board provided indications for RT, which was performed in combination with an unchanged anti-PD-1 regimen either within the first 3 months of PD-1 blockade for rapidly progressing, symptomatic, or life-threatening lesions, or later in patients with confirmed PD on two consecutive computed tomography (CT)-scans (to rule out pseudo-progression) or with long-lasting SD. RT was standardized, with 20–26 Gy delivered in 3–5 weekly sessions for extracranial lesions and SRS in one or two sessions, delivered through a Gamma-knife, for intracranial lesions.
For this study, we analysed records of all patients with confirmed advanced melanoma who initiated anti-PD-1 monotherapy, regardless of BRAF/NRAS mutational status and previous therapies, between 1 January 2014 and 30 August 2019. Key exclusion criteria were age < 18 years and association with ipilimumab. The database was locked on 1 August 2021, and we converted all recorded American Joint Committee on Cancer (AJCC) stages to the 8th edition [24]. The primary endpoint was the CR rate. The secondary endpoints were the response rates of radiated and non-radiated lesions (to estimate the abscopal effect), as well as PFS (time from the first dose to confirmed PD after anti-PD-1 monotherapy or RT for radiated patients, or death), OS (time from the first infusion of anti-PD1 mAb to death), and safety. This study updates and expands our previous works, which included only 25 [19] and 26 patients [20].
STROBE guidelines for observational studies were followed. The Kaplan–Meier method was used to estimate survival rates. Logistic regressions were used to study relationships between CR+PR after RT and the following parameters: BRAF mutational status, LDH serum levels, number of metastatic sites, treatment-naïve status or not, Eastern Cooperative Oncology Group (ECOG) performance status, extracranial or intracranial RT, AJCC staging, oligometastatic disease (defined as ≤5 metastases) at anti-PD-1 initiation, and oligoprogression (defined as <5 metastases at progression) at first RT. A p value < 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS v. 24 (IBM Corp, Armonk, NY, USA) and R v. 3.4.3 (R Core Team, Vienna, Austria: R Foundation for Statistical Computing, 2017). The sample size calculation showed that with >200 patients treated, of whom ≥100 received concurrent radiotherapy, we had enough power to demonstrate an increase of the CR rate from 15–20% to 30%.
According to French law, this study abided by standard medical practices and did not require written informed consent nor a formal approval by a national ethics committee. Nonetheless, verbal consent was obtained from all living patients, and the protocol (File S1) was accepted by the research ethics committee of Paris-Saclay University (CER, number 257). Statistical analysis was conducted following this protocol, which was established before data collection. The study was conducted according to the principles of the Declaration of Helsinki [25]. The anonymized datasets of this study are available from the corresponding author upon request.
3. Results
During the study time frame, 206 consecutive patients initiated nivolumab (83%) or pembrolizumab (17%) monotherapy. Median duration on anti-PD-1 was 8 months (range: 1–91). Most patients had severe disease: 59% M1c+d disease, 50% ≥3 metastatic sites, 33% ECOG PS ≥1, 33% non-treatment-naïve, 32% LDH >upper limit of normal, 32% liver or 22% intracranial metastases. Median follow-up was 22 months (range: 1–79), without any lost to follow-up.
One hundred patients (49%) were very severe and received concurrent RT, either early (<3 months of PD-1 blockade for rapidly progressing, symptomatic, or life-threatening lesions (n = 39)) or late (>3 months for confirmed PD (n = 50) or more occasionally for long-lasting SD (n = 3)), or both early and late (n = 8). Table 1 shows patients’ characteristics at anti-PD-1 initiation.

Table 1.
Characteristics at anti-PD-1 initiation for radiated and non-radiated patients.
Radiated and non-radiated patients lacked statistically significant baseline differences except for liver metastases and AJCC staging. Notably, only 20% of radiated patients had oligometastatic disease and 21% had oligoprogression. The first combined RT was hypofractionated RT targeting 1–2 extracranial lesions (n = 68), SRS for intracranial metastases (n = 25), or standard palliative RT (Table 2). A total of 39 patients later received a second RT for persistent PD, targeting a previously non-radiated target lesion at a median of 7.2 months [range: 2–17] after the first one (Table 2).

Table 2.
Characteristics of the radiotherapy series in the 100 radiated and anti-PD-1-treated patients.
Overall, 67 out of 206 patients (32.5% [95% CI: 26.1–38.9]) achieved CR (including seven with surgical resection of a residual lesion), and anti-PD-1 was discontinued in 64 patients with CR, of whom 14 (22%) had relapsed after a median of 37 months [range: 8–75] off anti-PD-1. RT had been performed in 25 patients achieving CR (10 and 15 with early or late RT, respectively), while 42 patients with CR were not radiated. We also observed 34 PR (17%), 15 SD (7%), and 90 PD (44%).
Table 3 shows the response rates in radiated and non-radiated areas for radiated patients. Of note, eight (33%) of the radiated patients achieving CR and six (55%) of those with PR required two sessions of RT. Among the 61 patients with late RT (because of anti-PD-1 failure), 54 had evaluable lesions outside radiated areas: abscopal response was observed in non-radiated lesions in 17 patients (31.5%).

Table 3.
Best response after radiotherapy and anti-PD-1 monoclonal antibodies combination in melanoma patients who failed anti-PD-1 monotherapy or with rapidly progressing disease at anti-PD-1 initiation.
For the whole cohort, median PFS and OS were 16.3 [95% CI: 9.4–NA] and 23.7 [16.8–35.9] months, respectively, without significant difference in non-radiated and radiated patients (Figure 1). When restricting analysis to the 61 patients radiated for anti-PD-1 failure and to the 51 non-radiated patients also with anti-PD-1 failure, median PFS and OS were 16.8 [13.4–26.6] and 37.0 [24.6–NA] months, respectively, versus 2.2 [1.5–2.6] and 4.3 months [2.6–7.1], respectively (p < 0.001) (Figure 2). We also studied the role of hypofractionated extracranial radiotherapy: when restricting the analysis to the 75 patients who received hypofractionated radiotherapy and to the 106 non-radiated patients, median PFS and OS were 16.2 [12.2–26.6] and 31.0 [23.7–41.9] months, respectively, in radiated patients, versus not achieved and 20.4 [11.2–52.7] months, respectively (p = 0.054 for OS, 0.16 for PFS) (Figure 3). Overall, PFS and OS curves seemed to have reached a plateau in radiated patients. File S2 shows PFS and OS curves from date of RT in radiated patients.
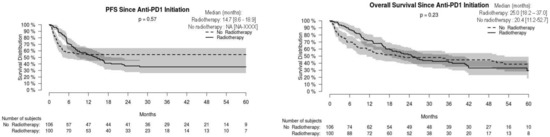
Figure 1.
Progression-free (PFS) and overall (OS) survival in radiated and non-radiated anti-PD-1-treated melanoma patients.
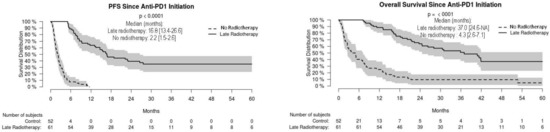
Figure 2.
Progression-free (PFS) and overall (OS) survival in radiated and non-radiated melanoma patients with anti-PD-1 treatment failure.
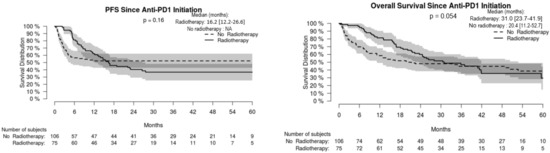
Figure 3.
Progression-free (PFS) and overall (OS) survival in radiated melanoma patients having received hypofractionated radiotherapy and non-radiated melanoma patients.
In radiated patients, the following parameters collected at anti-PD-1 initiation were not associated with achieving CR+PR: BRAF mutational status, AJCC staging, naïve versus non-naïve status, ECOG performance status (0 versus ≥1), LDH serum level (normal versus >normal), presence of <3 metastatic sites versus ≥3 or of oligometastatic disease. Use of extracranial RT versus SRS, early versus late RT, and oligoprogression were also not associated with CR+PR.
A total of 29.1%, 9.7%, 1%, and 0.5% of patients experienced grade 1–2, 3, 4, or 5 AEs, respectively, without unusual ones. RT-related AEs included 5% asthenia or digestive symptoms, 4% grade 2 dermatitis, 1% RT-induced pneumonitis, 1% radiation-induced brain necrosis.
4. Discussion
We report herein a retrospective analysis of data collected prospectively in a cohort of 206 consecutive anti-PD-1 monotherapy-treated melanoma patients with 49% of patients receiving hypofractionated RT combined with an unmodified anti-PD-1 regimen. We observed a 32.5% [95% CI: 26.1–38.9] CR rate, which is above figures obtained in melanoma patients receiving anti-PD-1 monotherapy [2,3,4,5,6] or combined ipilimumab + nivolumab [26], despite numerous patients with active intracranial metastasis and/or non-treatment-naïve status being included. The high percentage of AJCC M1c and M1d patients, with high LDH serum levels, ECOG performance status >1, or liver metastasis, which all represent poor prognostic factors [27], also highlights real-life patients’ severity. Benefits of RT were observed particularly in patients failing anti-PD-1 blockade. Anti-PD-1 treatment could be withdrawn in 97% of patients with CR. Thus, combining anti-PD-1 with RT could represent an opportunity for patients failing anti-PD-1 blockade.
Limitations of our study include its retrospective nature, the absence of an independent evaluation of images, and the absence of formal comparison with patients treated beyond PD with anti-PD-1 mAb alone [28]. Moreover, evaluations retained for radiated patients were those performed after radiation, which induced a systematic bias. Thus, the potential benefits elicited by this combination are not formally established. However, to the best of our knowledge, this represents the largest series of combined RT and anti-PD-1 blockade, and we are confident with the CR we observed, as our recurrence rate of 22% after a median of 37 months off therapy compares favorably with the 14% observed after a median 18 months of follow-up in patients achieving CR [21].
Our results obtained in patients radiated late because of anti-PD-1 failure are likely not due to late efficacy of ICI and suggest a true synergy of the combination, as (1) we included patients after the exclusion of pseudo-progression [22]; (2) the median delay between anti-PD-1 mAb initiation and the first day of RT was 6.1 months in these patients, whereas most anti-PD-1-treated patients who later achieve CR demonstrate PR at the 3-month evaluation [29]; (3) an abscopal effect was observed in 31.5% of these patients, a feature which was infrequently reported before the ICI era [30]; and (4) there are robust preclinical data (cf. infra).
The absence of a statistically significant difference in PFS and OS between radiated and non-radiated patients in the whole population despite similar characteristics at inclusion for most prognostic criteria (Table 1) can be explained by our indications of RT, which required initial life-threatening or symptomatic metastases (39% of radiated patients), later anti-PD-1 initial monotherapy failure (53%), or a combination of both situations (8%), leading to a poorer prognosis in the radiated population. This reinforces the value of our findings.
Previous [12,13,14,15,16,17,18,19,20] and present results favor the hypothesis that hypofractionated RT delivering fractions of ≥6 Gy represents a suitable extracranial RT regimen in anti-PD-1-treated melanoma patients. In cell culture and animal models, radiation-induced immunogenic cell death occurred through the release of damage-associated molecular patterns, such as double-stranded DNA, with the secretion of type-1 interferons (IFN) through the GMP–AMP synthase/stimulator of IFN genes (cGAS/STING) pathway [11]. DNA exonuclease 3′ repair exonuclease 1 (Trex1), induced by radiation doses > 12 Gy, degraded double-stranded DNA, abolished type-1 IFN release, and finally radiation immunogenicity [31]. Fractionated RT was more effective in inducing immune-mediated effects than a single ablative dose and may overcome RT-induced adaptive resistance [32]. Repeated radiations at doses (8 Gyx3) above the threshold of Trex1 induction greatly amplified type-1 IFN production, resulting in recruitment and activation of Batf3-dependent dendritic cells, which are essential for priming of CD8+ T cells that induce tumor rejection in the context of ICI [31]. Notably, when restricting the analysis to the patients who received hypofractionated extracranial radiotherapy and to the non-radiated patients, OS tended to be longer in radiated patients versus non-radiated ones, with a p-value of 0.054. Optimal dosing and fractionation strategy for each cancer type has not yet been determined, but larger doses per fraction, as in our series, were associated with enhanced abscopal effects [10]. Moreover, a trial of ipilimumab combined with hypofractionated RT (6 Gyx5 or 9 Gyx3) in patients with non-small-cell lung cancer (for which ipilimumab had failed to demonstrate any efficacy) provided a proof-of-concept, with radiological responses observed along with type-1 IFN release and radiation-induced transcriptional upregulation of neo-antigens [33]. Solid data on dose-ranging for combined ICI and SRS in melanoma patients are lacking, but the tumor microenvironment is likely different in the brain. Finally, in addition to hypofractionated RT at a high fraction per session, repetition of RT while on anti-PD-1 mAb may also contribute to our findings, as repetition of RT on a different target occurred in 40% of our radiated patients achieving CR or PR. Treating wider tumor areas with RT combined with ICI has been shown to improve mucosal melanoma control [34].
This large series confirms the tolerability of combined RT and anti-PD-1 mAb [12,17,18,19,34], as no unusual new nor higher frequency of anti-PD-1-related or RT-induced AEs were observed.
We did not find significant clinical predictive factors for CR+PR during combination therapy but did not assay potential biomarkers relevant for investigating combined ICI and RT [11,32] as none of these have been validated.
Although we included consecutive patients during a long period in a real-life setting, the external validity of our results should be demonstrated in other settings using similar hypofractionated RT regimens. This approach should also be investigated in anti-PD-1+anti-CTLA-4 or anti-PD-1+anti-LAG3–3 [35] refractory patients.
5. Conclusions
We suggest a synergy between anti-PD1 and RT, which could be due to tumor neoantigens release after irradiation stimulating the anti-tumor immune response [33]. High-dose hypofractionated RT may enhance anti-PD-1 efficacy by enhancing the CR rate above 30% in melanoma patients, thus allowing safe anti-PD-1 cessation. Controlled studies are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14174069/s1, File S1: Study protocol [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]; File S2: PFS and OS curves from date of RT in radiated patients.
Author Contributions
Conceptualization, P.S.; Data curation, R.M. and A.B. (Alain Beauchet); Formal analysis, P.S. and A.B. (Alain Beauchet); Investigation, R.M., A.R., B.B., Y.O., J.O., C.-A.V., A.B. (Astrid Blom), C.L. and E.F.-B.; Methodology, P.S. and A.B. (Alain Beauchet); Project administration, E.F.-B.; Resources, P.S.; Supervision, E.F.-B.; Validation, A.R.; Visualization, A.B. (Alain Beauchet); Writing—original draft, P.S., R.M. and A.B. (Astrid Blom); Writing—review & editing, A.R., B.B., Y.O., J.O., C.-A.V., A.B. (Astrid Blom), C.L. and E.F.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocol (File S1) was accepted by the research ethics committee of Paris-Saclay University (CER, number 257). The study was conducted according to the principles of the declaration of Helsinki.
Informed Consent Statement
According to French Law, this study abided by standard medical practices and did not require written informed consent nor a formal approval by a national ethics committee. However, consent was obtained orally from all living patients.
Data Availability Statement
This study analyses data originating from patients’ records and completely open access to data was not included in our submission to the Ethics committee. The anonymised datasets of this study in xlsx format are therefore available from the corresponding author upon reasonable request.
Conflicts of Interest
P.S. has received outside of this study personal fees from Bristol-Myers Squibb, MSD, Merck-Serono, Pfizer, Roche-Genentech, Pierre Fabre, and Novartis; has received nonfinancial support from Bristol-Myers Squibb, MSD, Roche-Genentech, and Novartis. EFB has received personal fees from Pierre Fabre, Bristol-Myers Squibb, MSD and Novartis. All remaining authors have declared no conflicts of interest.
References
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Rutkowski, P.; Hassel, J.C.; McNeil, C.M.; Kalinka, E.A.; et al. Five-Year Outcomes with Nivolumab in Patients with Wild-Type BRAF Advanced Melanoma. J. Clin. Oncol. 2020, 38, 3937–3946. [Google Scholar] [CrossRef] [PubMed]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Ugurel, S.; Röhmel, J.; Ascierto, P.A.; Becker, J.C.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Livingstone, E.; Long, G.V.; et al. Survival of patients with advanced metastatic melanoma: The impact of MAP kinase pathway inhibition and immune checkpoint inhibition—Update 2019. Eur. J. Cancer 2020, 130, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Minor, D.; D’Angelo, S.; Neyns, B.; Smylie, M.; Miller, W.H., Jr.; Gutzmer, R.; Linette, G.; Chmielowski, B.; Lao, C.D.; et al. Overall Survival in Patients with Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J. Clin. Oncol. 2018, 36, 383–390. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Saberian, C.; Davies, M.A. Re-thinking therapeutic development for CNS metastatic disease. Exp. Dermatol. 2022, 31, 74–81. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Hodi, F.S.; Algazi, A.P.; Hamid, O.; Lao, C.D.; Moschos, S.J.; Atkins, M.B.; Lewis, K.; Postow, M.A.; et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): Final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 1692–1704. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.E.; Vanpouille-Box, C.; Melero, I.; Formenti, S.C.; Demaria, S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018, 39, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Mick, R.; Huang, A.C.; George, S.M.; Farwell, M.D.; Lukens, J.N.; Berman, A.T.; Mitchell, T.C.; Bauml, J.; Schuchter, L.M.; et al. A phase I trial of pembrolizumab with hypofractionated radiotherapy in patients with metastatic solid tumours. Br. J. Cancer 2018, 119, 1200–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundahl, N.; Seremet, T.; Van Dorpe, J.; Neyns, B.; Ferdinande, L.; Meireson, A.; Brochez, L.; Kruse, V.; Ost, P. Phase 2 Trial of Nivolumab Combined with Stereotactic Body Radiation Therapy in Patients with Metastatic or Locally Advanced Inoperable Melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Doyen, J.; Picard, A.; Naghavi, A.O.; Thyss, A.; Passeron, T.; Lacour, J.P.; Montaudie, H. Clinical outcomes of metastatic melanoma treated with checkpoint inhibitors and multisite radiotherapy. JAMA Dermatol. 2017, 153, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Aboudaram, A.; Modesto, A.; Chaltiel, L.; Gomez-Roca, C.; Boulinguez, S.; Sibaud, V.; Delord, J.P.; Chira, C.; Delannes, M.; Moyal, E.; et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: A safe and effective combination. Melanoma Res. 2017, 27, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Liniker, E.; Menzies, A.M.; Kong, B.Y.; Cooper, A.; Ramanujam, S.; Lo, S.; Kefford, R.F.; Fogarty, G.B.; Guminski, A.; Wang, T.W.; et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology 2016, 5, e1214788. [Google Scholar] [CrossRef]
- Yin, G.; Guo, W.; Huang, Z.; Chen, X. Efficacy of radiotherapy combined with immune checkpoint inhibitors in patients with melanoma: A systemic review and meta-analysis. Melanoma Res. 2022, 32, 71–78. [Google Scholar] [CrossRef]
- Ribeiro Gomes, J.; Schmerling, R.A.; Haddad, C.K.; Racy, D.J.; Ferrigno, R.; Gil, E.; Zanuncio, P.; Buzaid, A.C. Analysis of the abscopal effect with anti-pd1 therapy in patients with metastatic solid tumors. J. Immunother. 2016, 39, 367–372. [Google Scholar] [CrossRef]
- Roger, A.; Finet, A.; Boru, B.; Beauchet, A.; Mazeron, J.J.; Otzmeguine, Y.; Blom, A.; Longvert, C.; de Maleissye, M.F.; Fort, M.; et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. Oncoimmunology 2018, 7, e1442166. [Google Scholar] [CrossRef]
- Funck-Brentano, E.; Baghad, B.; Fort, M.; Aouidad, I.; Roger, A.; Beauchet, A.; Otmezguine, Y.; Blom, A.; Longvert, C.; Boru, B.; et al. Efficacy of late concurrent hypofractionated radiotherapy in advanced melanoma patients failing anti-PD-1 monotherapy. Int. J. Cancer 2020, 147, 1707–1714. [Google Scholar] [CrossRef]
- Jansen, Y.J.L.; Rozeman, E.A.; Mason, R.; Goldinger, S.M.; Geukes Foppen, M.H.; Hoejbergs, L.; Schmidt, H.; van Thienen, J.V.; Haanen, J.; Tiainen, L.; et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: Clinical outcomes in advanced melanoma. Ann. Oncol. 2019, 30, 1154–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodi, F.S.; Hwu, W.J.; Kefford, R.; Weber, J.S.; Daud, A.; Hamid, O.; Patnaik, A.; Ribas, A.; Robert, C.; Gangadhar, T.C.; et al. Evaluation of immune-related response criteria and recist v1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 2016, 34, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the american joint committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [Green Version]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Waninger, J.J.; Ma, V.T.; Journey, S.; Skvarce, J.; Chopra, Z.; Tezel, A.; Bryant, A.K.; Mayo, C.; Sun, Y.; Sankar, K.; et al. Validation of the American Joint Committee on Cancer Eighth Edition Staging of Patients With Metastatic Cutaneous Melanoma Treated With Immune Checkpoint Inhibitors. JAMA Netw. Open 2021, 4, e210980. [Google Scholar] [CrossRef]
- Long, G.V.; Weber, J.S.; Larkin, J.; Atkinson, V.; Grob, J.J.; Schadendorf, D.; Dummer, R.; Robert, C.; Marquez-Rodas, I.; McNeil, C.; et al. Nivolumab for patients with advanced melanoma treated beyond progression: Analysis of 2 phase 3 clinical trials. JAMA Oncol. 2017, 3, 1511–1519. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Hamid, O.; Daud, A.; Wolchok, J.D.; Joshua, A.M.; Hwu, W.J.; Weber, J.S.; Gangadhar, T.C.; Joseph, R.W.; et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J. Clin. Oncol. 2018, 36, 1668–1674. [Google Scholar] [CrossRef]
- Chicas-Sett, R.; Morales-Orue, I.; Rodriguez-Abreu, D.; Lara-Jimenez, P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: A systematic review. Clin. Transl. Radiat. Oncol. 2018, 9, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Otsuka, A.; Yoshimura, M.; Nonomura, Y.; Kaku, Y.; Matsumoto, S.; Muto, M. Efficacy and safety of concurrent immunoradiotherapy in patients with metastatic melanoma after progression on nivolumab. Cancer Chemother. Pharmacol. 2018, 81, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 18, 463–482. [Google Scholar] [CrossRef] [Green Version]
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: Three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019, 5, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, J.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Goyal, S.; Silk, A.W.; Tian, S.; Mehnert, J.; Danish, S.; Ranjan, S.; Kaufman, H.L. Clinical Management of Multiple Melanoma Brain Metastases: A Systematic Review. JAMA Oncol. 2015, 1, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.A.; Liu, P.; McIntyre, S.; Kim, K.B.; Papadopoulos, N.; Hwu, W.J.; Hwu, P.; Bedikian, A. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011, 117, 1687–1696. [Google Scholar] [CrossRef]
- Sampson, J.H.; Carter, J.H., Jr.; Friedman, A.H.; Seigler, H.F. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J. Neurosurg. 1998, 88, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanigan, J.C.; Jilaveanu, L.B.; Faries, M.; Sznol, M.; Ariyan, S.; Yu, J.B.; Knisely, J.P.S.; Chiang, V.L.; Kluger, H.M. Melanoma brain metastases: Is it time to reassess the bias? Curr. Probl. Cancer 2011, 35, 200–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taillibert, S.; Le Rhun, E. Epidemiology of brain metastases. Cancer Radiother. 2015, 19, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Hadjinicolaou, A.V.; Chiarion Sileni, V.; Rossi, C.R.; Mocellin, S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev. 2018, 2, CD011123. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Weaver, B.D.; Goodman, J.R.; Jensen, R. Concurrent radiosurgery and systemic therapies for melanoma brain metastases: A systematic review. Cureus 2019, 11, e6147. [Google Scholar] [CrossRef] [Green Version]
- Minniti, G.; Anzellini, D.; Reverberi, C.; Cappellini, G.C.A.; Marchetti, L.; Bianciardi, F.; Bozzao, A.; Osti, M.; Gentile, P.C.; Esposito, V. Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: Evaluation of brain control and toxicity. J. Immunother. Cancer 2019, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Nardin, C.; Mateus, C.; Texier, M.; Lanoy, E.; Hibat-Allah, S.; Ammari, S.; Robert, C.; Dhermain, F. Tolerance and outcomes of stereotactic radiosurgery combined with anti-programmed cell death-1 (pembrolizumab) for melanoma brain metastases. Melanoma Res. 2018, 28, 111–119. [Google Scholar] [CrossRef]
- Comito, F.; Leslie, I.; Boos, L.; Furness, A.; Pickering, L.; Turajlic, S.; Larkin, J. Oligoprogression after checkpoint inhibition in metastatic melanoma treated with locoregional therapy: A single-center retrospective analysis. J. Immunother. 2020, 43, 250–255. [Google Scholar] [CrossRef]
- Moreau, S.; Saiag, P.; Aegerter, P.; Bosset, D.; Longvert, C.; Hélias-Rodzewicz, Z.; Marin, C.; Peschaud, F.; Chagnon, S.; Zimmermann, U.; et al. Prognostic value of BRAF V600 mutations in melanoma patients after resection of metastatic lymph nodes. Ann. Surg. Oncol. 2012, 19, 4314–4321. [Google Scholar] [CrossRef]
- Hélias-Rodzewicz, Z.; Funck-Brentano, E.; Terrones, N.; Beauchet, A.; Zimmermann, U.; Marin, C.; Saiag, P.; Emile, J.-F. Variation of mutant allele frequency in NRAS Q61 mutated melanomas. BMC Dermatol. 2017, 17, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).