Optimal Light Dose for hEGFR-Targeted Near-Infrared Photoimmunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Synthesis of IR700-Conjugated Cetuximab and Panitumumab
2.3. Cell Lines and Culture
2.4. Animal and Tumor Models
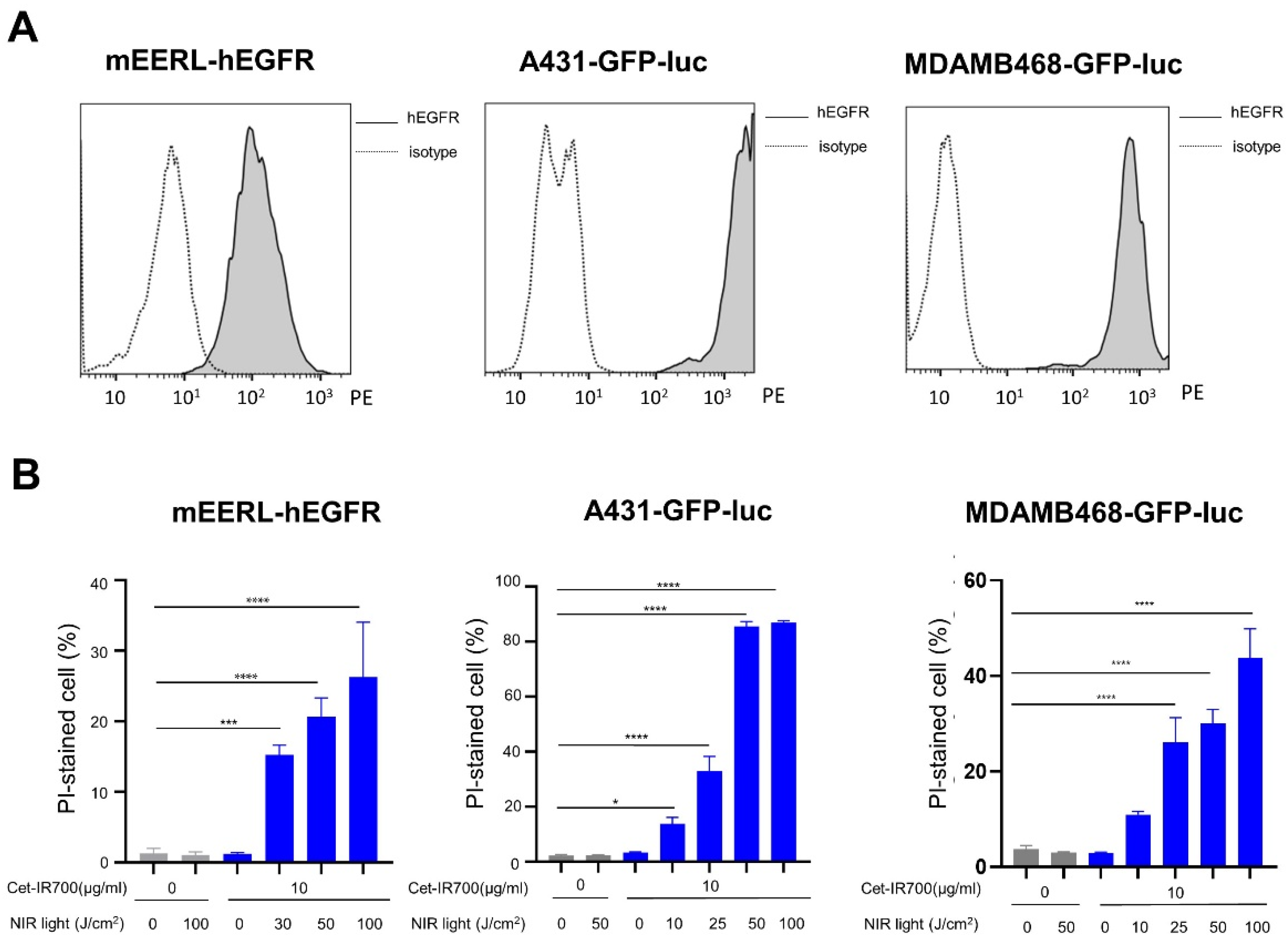
2.5. In Vitro hEGFR Expression Analysis
2.6. In Vitro NIR-PIT
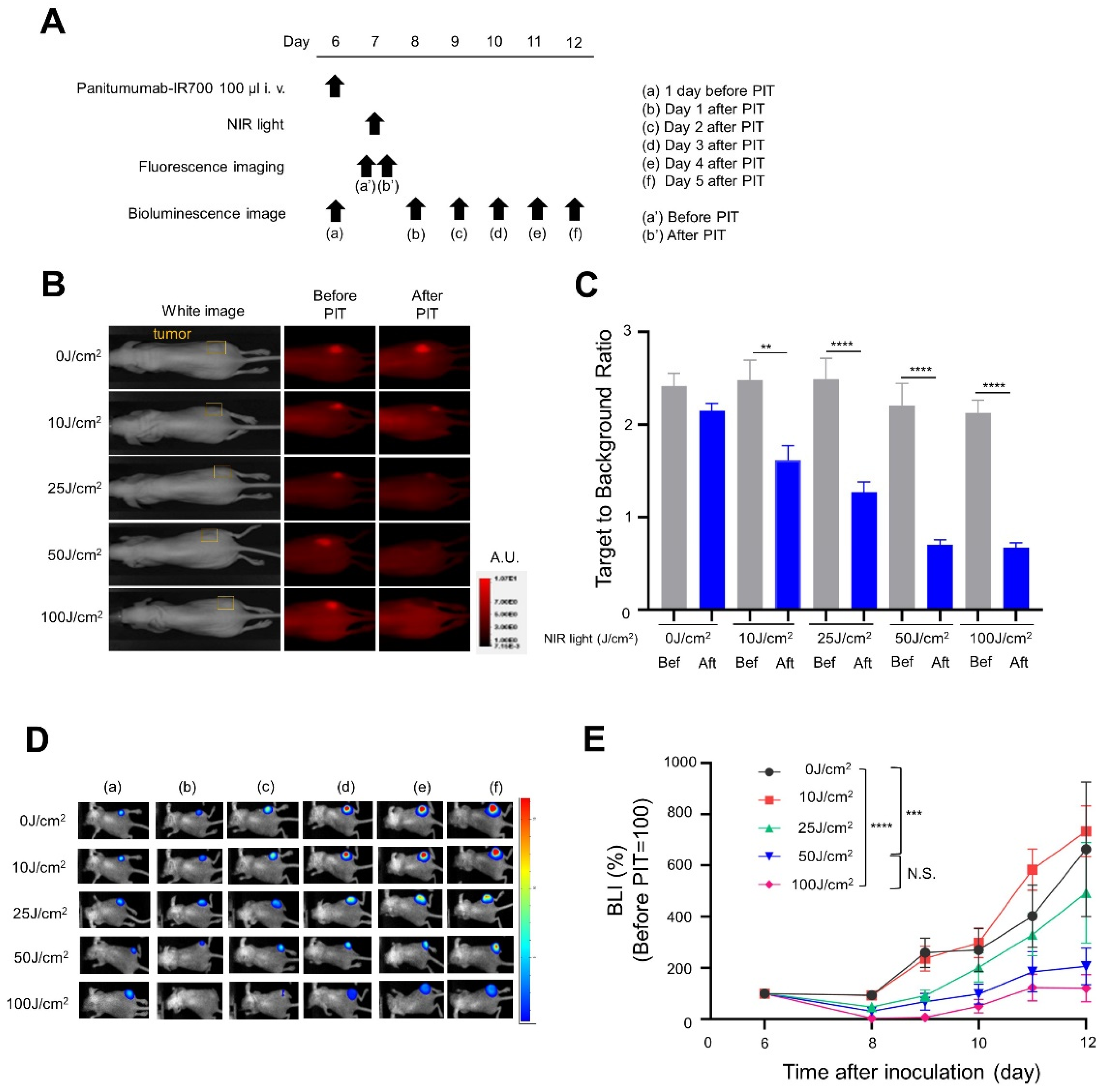
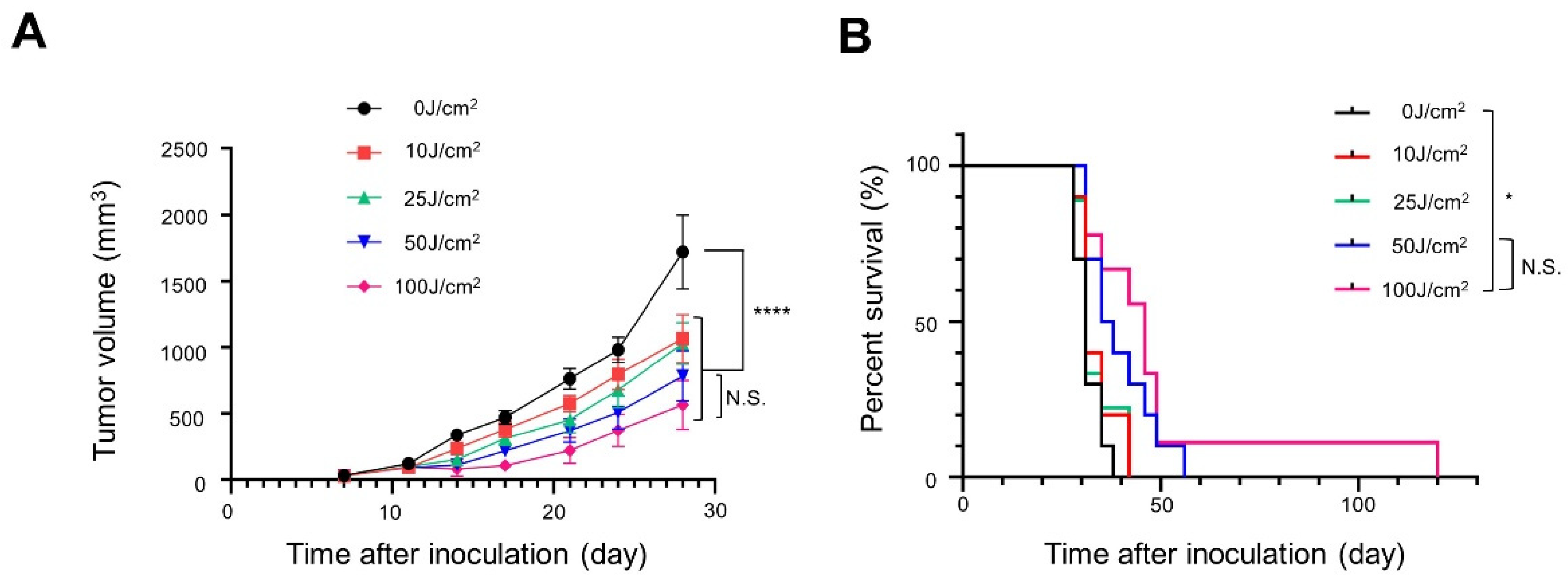
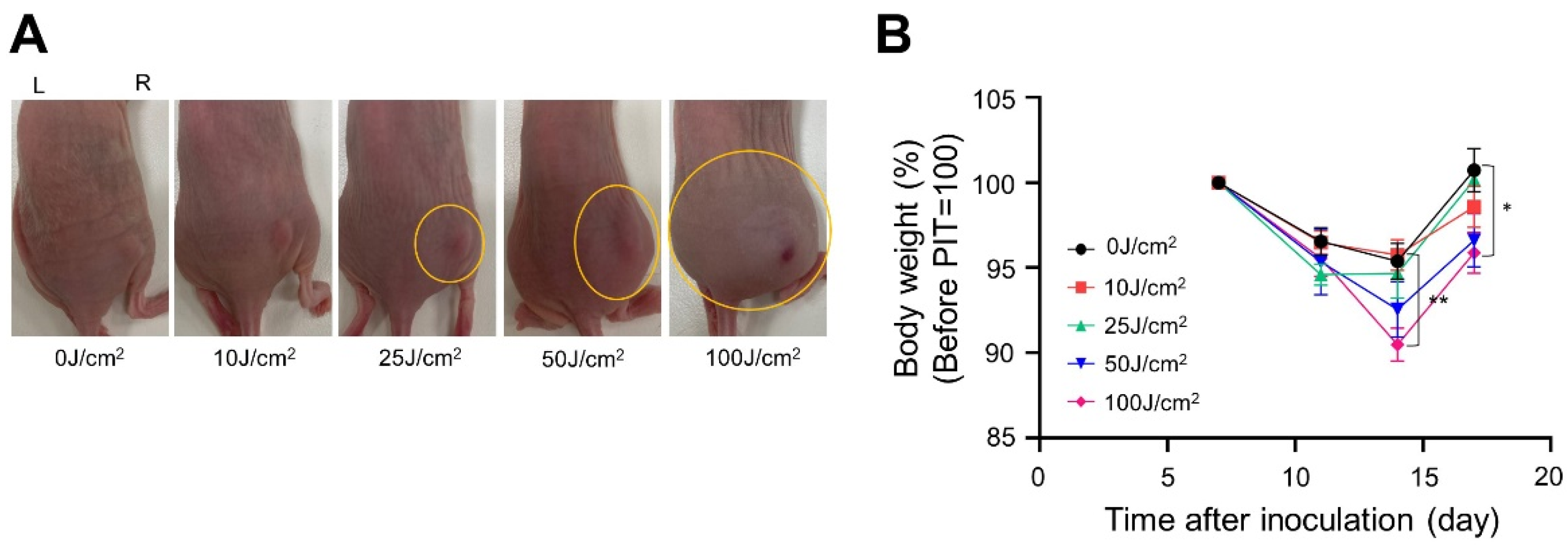
2.7. In Vivo NIR-PIT
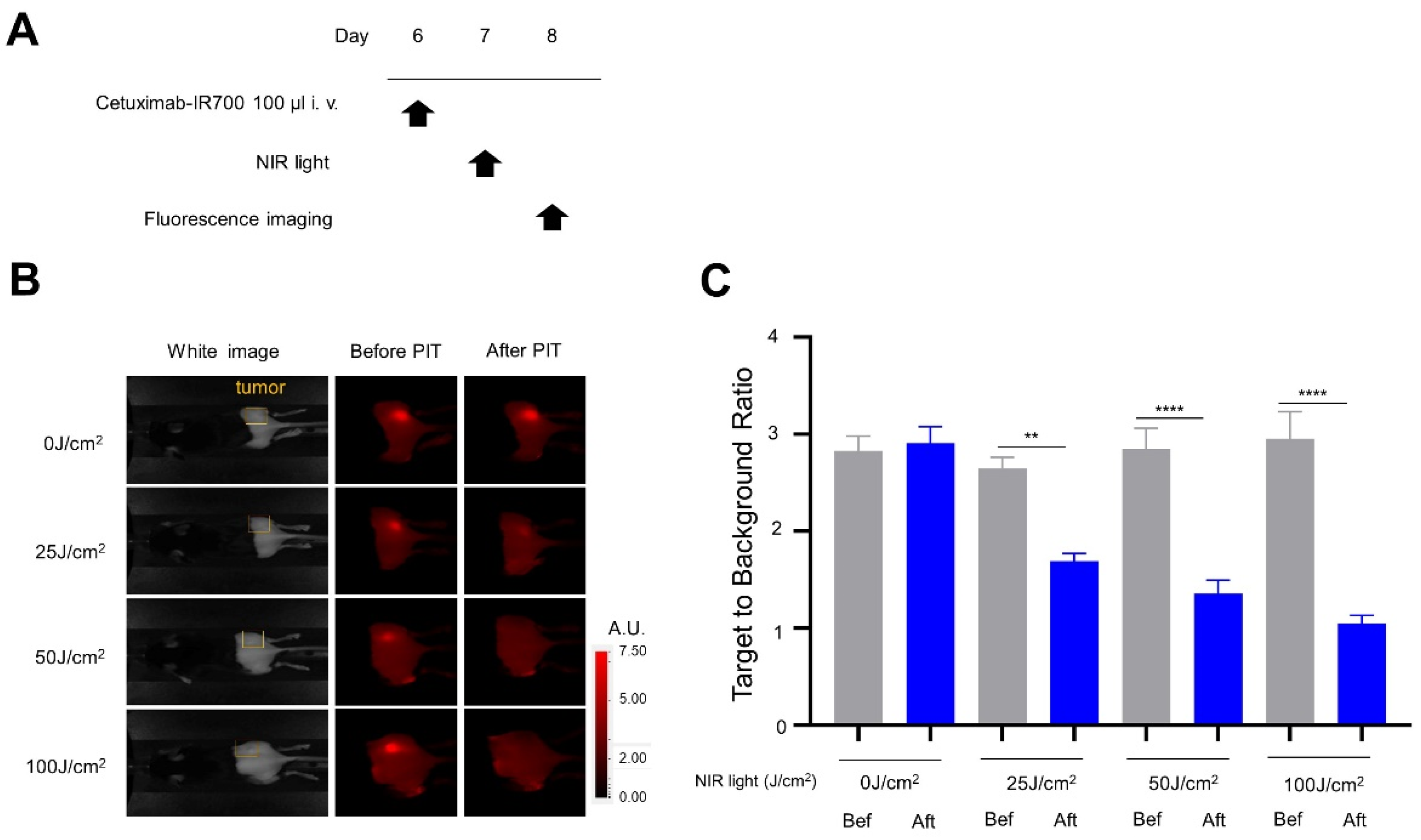
2.8. IR700 Fluorescence Imaging Study
2.9. Bioluminescence Imaging (BLI) Study
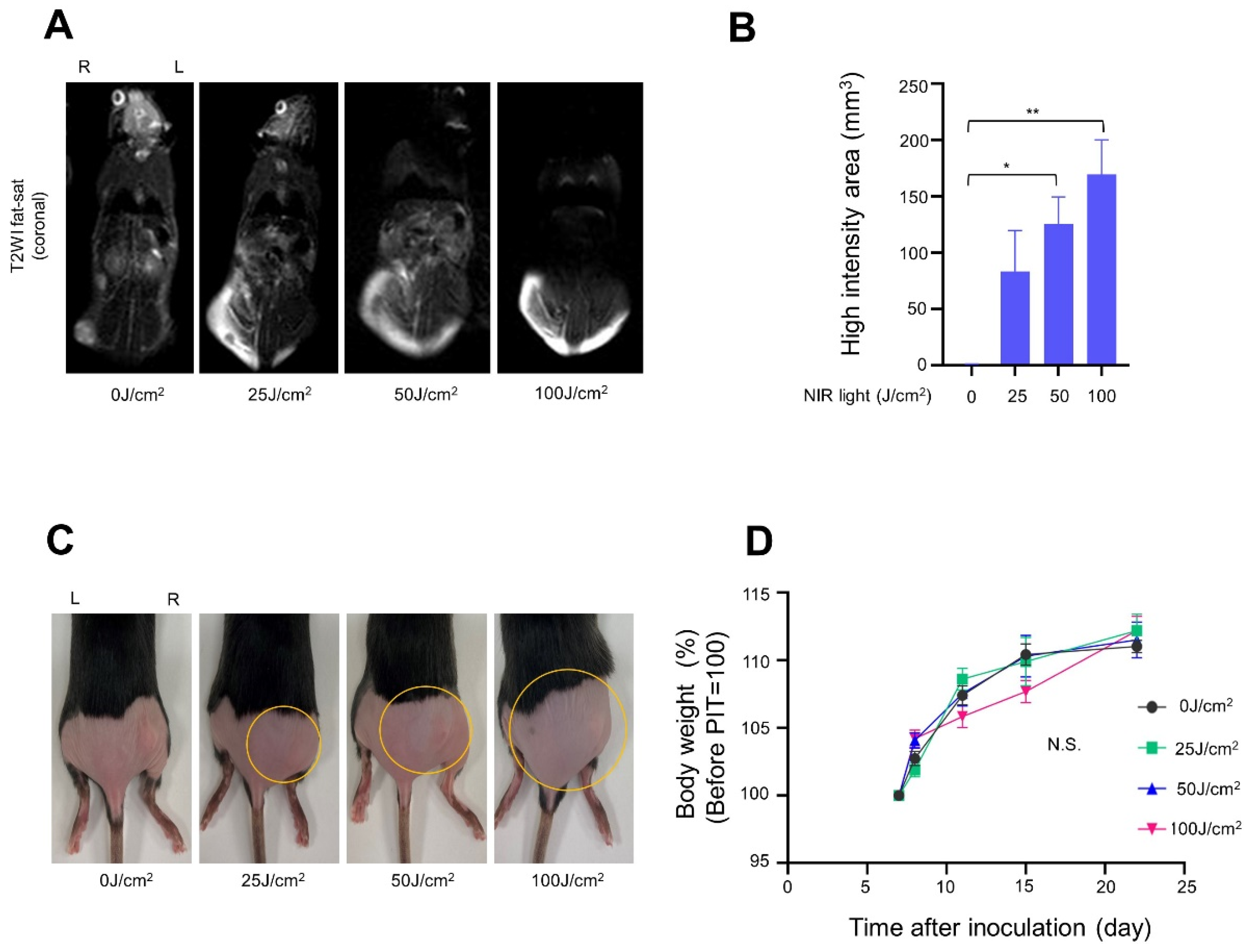
2.10. Magnetic Resonance Imaging (MRI) Study
2.11. Statistical Analysis
3. Results
3.1. In Vitro NIR-PIT with Cetuximab-IR700 (Cet-IR700)
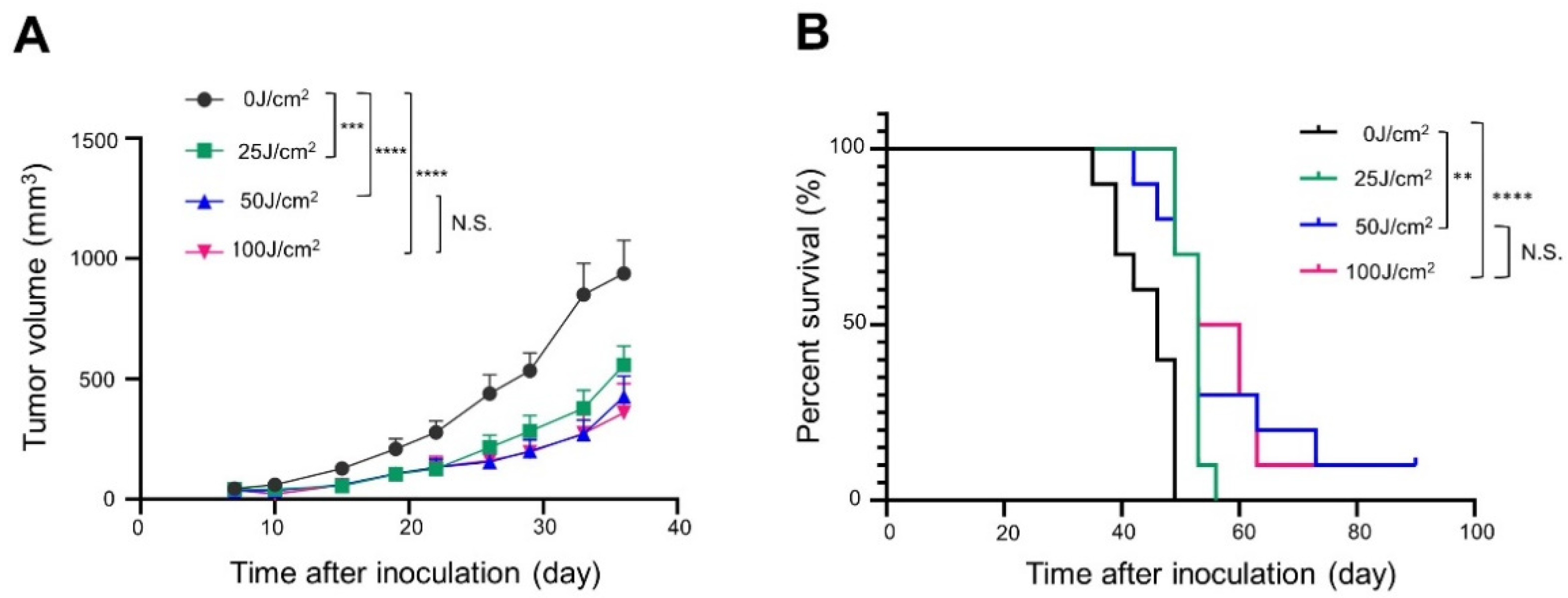
3.2. In Vivo NIR-PIT with Panitumumab-IR700 (Pan-IR700) in Immunocompromised Mice
3.3. In Vivo NIR-PIT with Cet-IR700 in Immunocompetent Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective In Vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Nagaya, T.; Sato, K.; Harada, T.; Okuyama, S.; Choyke, P.L.; Yamauchi, T.; Kobayashi, H. Alterations of filopodia by near infrared photoimmunotherapy: Evaluation with 3D low-coherent quantitative phase microscopy. Biomed. Opt. Express 2016, 7, 2738–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, F.; Nagaya, T.; Okuyama, S.; Maruoka, Y.; Choyke, P.L.; Yamauchi, T.; Kobayashi, H. Dynamic changes in the cell membrane on three dimensional low coherent quantitative phase microscopy (3D LC-QPM) after treatment with the near infrared photoimmunotherapy. Oncotarget 2017, 8, 104295–104302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef] [Green Version]
- Okada, R.; Kato, T.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Fujimura, D.; Okuyama, S.; Furumoto, H.; Fukushima, H.; Choyke, P.L.; et al. Selection of antibody and light exposure regimens alters therapeutic effects of EGFR-targeted near-infrared photoimmunotherapy. Cancer Immunol. Immunother. CII 2022, 71, 1877–1887. [Google Scholar] [CrossRef]
- Fujimura, D.; Inagaki, F.; Okada, R.; Rosenberg, A.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Conjugation Ratio, Light Dose, and pH Affect the Stability of Panitumumab-IR700 for Near-Infrared Photoimmunotherapy. ACS Med. Chem. Lett. 2020, 11, 1598–1604. [Google Scholar] [CrossRef]
- Ogata, F.; Nagaya, T.; Nakamura, Y.; Sato, K.; Okuyama, S.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Near-infrared photoimmunotherapy: A comparison of light dosing schedules. Oncotarget 2017, 8, 35069–35075. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Sato, K.; Hanaoka, H.; Watanabe, R.; Harada, T.; Choyke, P.L.; Kobayashi, H. The effects of conjugate and light dose on photo-immunotherapy induced cytotoxicity. BMC Cancer 2014, 14, 389. [Google Scholar] [CrossRef] [Green Version]
- Mitsunaga, M.; Nakajima, T.; Sano, K.; Choyke, P.L.; Kobayashi, H. Near-infrared theranostic photoimmunotherapy (PIT): Repeated exposure of light enhances the effect of immunoconjugate. Bioconjugate Chem. 2012, 23, 604–609. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Nagaya, T.; Sato, K.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Interstitial near-infrared photoimmunotherapy: Effective treatment areas and light doses needed for use with fiber optic diffusers. Oncotarget 2018, 9, 11159–11169. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Nagaya, T.; Ogata, F.; Maruoka, Y.; Sato, K.; Nakamura, Y.; Choyke, P.L.; Kobayashi, H. Avoiding thermal injury during near-infrared photoimmunotherapy (NIR-PIT): The importance of NIR light power density. Oncotarget 2017, 8, 113194–113201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Watanabe, R.; Hanaoka, H.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Comparative effectiveness of light emitting diodes (LEDs) and Lasers in near infrared photoimmunotherapy. Oncotarget 2016, 7, 14324–14335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsunaga, M.; Nakajima, T.; Sano, K.; Kramer-Marek, G.; Choyke, P.L.; Kobayashi, H. Immediate In Vivo target-specific cancer cell death after near infrared photoimmunotherapy. BMC Cancer 2012, 12, 345. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Okada, R.; Goto, Y.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Daar, D.; Turkbey, B.; Choyke, P.L.; et al. Electron Donors Rather than Reactive Oxygen Species Needed for Therapeutic Photochemical Reaction of Near-Infrared Photoimmunotherapy. ACS Pharmacol. Transl. Sci. 2021, 4, 1689–1701. [Google Scholar] [CrossRef]
- Okada, R.; Furusawa, A.; Vermeer, D.W.; Inagaki, F.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Spanos, W.C.; Allen, C.T.; et al. Near-infrared photoimmunotherapy targeting human-EGFR in a mouse tumor model simulating current and future clinical trials. EBioMedicine 2021, 67, 103345. [Google Scholar] [CrossRef]
- Hoover, A.C.; Spanos, W.C.; Harris, G.F.; Anderson, M.E.; Klingelhutz, A.J.; Lee, J.H. The role of human papillomavirus 16 E6 in anchorage-independent and invasive growth of mouse tonsil epithelium. Arch. Otolaryngol.—Head Neck Surg. 2007, 133, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanos, W.C.; Hoover, A.; Harris, G.F.; Wu, S.; Strand, G.L.; Anderson, M.E.; Klingelhutz, A.J.; Hendriks, W.; Bossler, A.D.; Lee, J.H. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. J. Virol. 2008, 82, 2493–2500. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.; Lee, D.W.; Elzey, B.D.; Anderson, M.E.; Hostager, B.S.; Lee, J.H. Preclinical models of HPV+ and HPV- HNSCC in mice: An immune clearance of HPV+ HNSCC. Head Neck 2009, 31, 911–918. [Google Scholar] [CrossRef]
- Mermod, M.; Hiou-Feige, A.; Bovay, E.; Roh, V.; Sponarova, J.; Bongiovanni, M.; Vermeer, D.W.; Lee, J.H.; Petrova, T.V.; Rivals, J.P.; et al. Mouse model of postsurgical primary tumor recurrence and regional lymph node metastasis progression in HPV-related head and neck cancer. Int. J. Cancer 2018, 142, 2518–2528. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Fujimura, D.; Inagaki, F.; Okada, R.; Maruoka, Y.; Wakiyama, H.; Kato, T.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Real-time IR700 Fluorescence Imaging during Near-infrared Photoimmunotherapy Using a Clinically-approved Camera for Indocyanine Green. Cancer Diagn. Progn. 2021, 1, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Takashima, K.; Koga, Y.; Anzai, T.; Migita, K.; Yamaguchi, T.; Ishikawa, A.; Sakashita, S.; Yasunaga, M.; Yano, T. Evaluation of Fluorescence Intensity and Antitumor Effect Using Real-time Imaging in Photoimmunotherapy. Pharmaceuticals 2022, 15, 223. [Google Scholar] [CrossRef]
- Tahara, M.; Okano, S.; Enokida, T.; Ueda, Y.; Fujisawa, T.; Shinozaki, T.; Tomioka, T.; Okano, W.; Biel, M.A.; Ishida, K.; et al. A phase I, single-center, open-label study of RM-1929 photoimmunotherapy in Japanese patients with recurrent head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2021, 26, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, D.M.; Johnson, J.M.; Curry, J.M.; Kochuparambil, S.T.; McDonald, D.; Mott, F.; Fidler, M.J.; Stenson, K.; Vasan, N.R.; Razaq, M.A.; et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck 2021, 43, 3875–3887. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.F.; Granger, D.N. Blood cells and endothelial barrier function. Tissue Barriers 2015, 3, e978720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zhang, Y.; Cao, Y.; Shi, Z.; Lin, Y.; Chen, Y.; Zhao, H.; Liu, X. Glycyrrhetinic acid alleviates acute lung injury by PI3K/AKT suppressing macrophagic Nlrp3 inflammasome activation. Biochem. Biophys. Res. Commun. 2020, 532, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Bernardo, M.; Saito, K.; Koyasu, S.; Mitchell, J.B.; Choyke, P.L.; Krishna, M.C. Evaluation of oxygen dependence on in vitro and In Vivo cytotoxicity of photoimmunotherapy using IR-700-antibody conjugates. Free. Radic. Biol. Med. 2015, 85, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Harada, M.; Takakura, H.; Ando, K.; Goto, Y.; Tsuneda, T.; Ogawa, M.; Taketsugu, T. Theoretical and Experimental Studies on the Near-Infrared Photoreaction Mechanism of a Silicon Phthalocyanine Photoimmunotherapy Dye: Photoinduced Hydrolysis by Radical Anion Generation. Chempluschem 2020, 85, 1959–1963. [Google Scholar] [CrossRef]
- Nagaya, T.; Friedman, J.; Maruoka, Y.; Ogata, F.; Okuyama, S.; Clavijo, P.E.; Choyke, P.L.; Allen, C.; Kobayashi, H. Host Immunity Following Near-Infrared Photoimmunotherapy Is Enhanced with PD-1 Checkpoint Blockade to Eradicate Estab-lished Antigenic Tumors. Cancer Immunol. Res. 2019, 7, 401–413. [Google Scholar] [CrossRef]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Fujimura, D.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Combined CD44- and CD25-Targeted Near-Infrared Photoimmunotherapy Selectively Kills Cancer and Regulatory T Cells in Syngeneic Mouse Cancer Models. Cancer Immunol. Res. 2020, 8, 345–355. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furumoto, H.; Okada, R.; Kato, T.; Wakiyama, H.; Inagaki, F.; Fukushima, H.; Okuyama, S.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Optimal Light Dose for hEGFR-Targeted Near-Infrared Photoimmunotherapy. Cancers 2022, 14, 4042. https://doi.org/10.3390/cancers14164042
Furumoto H, Okada R, Kato T, Wakiyama H, Inagaki F, Fukushima H, Okuyama S, Furusawa A, Choyke PL, Kobayashi H. Optimal Light Dose for hEGFR-Targeted Near-Infrared Photoimmunotherapy. Cancers. 2022; 14(16):4042. https://doi.org/10.3390/cancers14164042
Chicago/Turabian StyleFurumoto, Hideyuki, Ryuhei Okada, Takuya Kato, Hiroaki Wakiyama, Fuyuki Inagaki, Hiroshi Fukushima, Shuhei Okuyama, Aki Furusawa, Peter L. Choyke, and Hisataka Kobayashi. 2022. "Optimal Light Dose for hEGFR-Targeted Near-Infrared Photoimmunotherapy" Cancers 14, no. 16: 4042. https://doi.org/10.3390/cancers14164042
APA StyleFurumoto, H., Okada, R., Kato, T., Wakiyama, H., Inagaki, F., Fukushima, H., Okuyama, S., Furusawa, A., Choyke, P. L., & Kobayashi, H. (2022). Optimal Light Dose for hEGFR-Targeted Near-Infrared Photoimmunotherapy. Cancers, 14(16), 4042. https://doi.org/10.3390/cancers14164042






