Phototheranostics of Splenic Myeloid-Derived Suppressor Cells and Its Impact on Spleen Metabolism in Tumor-Bearing Mice
Abstract
Simple Summary
Abstract
1. Introduction
Purpose
2. Materials and Methods
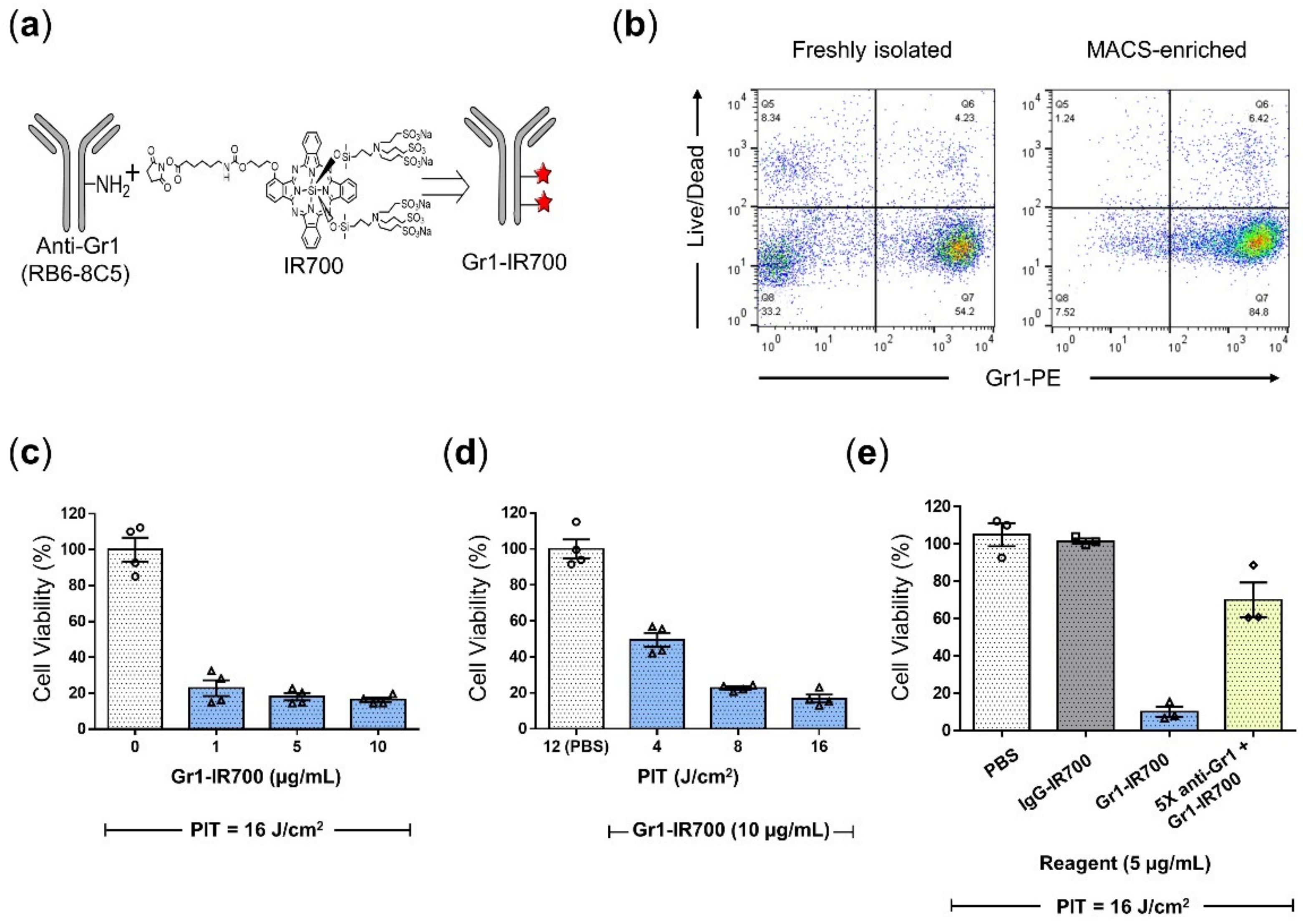
2.1. Synthesis of IR700-Conjugated Anti-Gr1 and IgG Monoclonal Antibodies
2.2. Animal Procedures
2.3. Splenic MDSC Isolation
2.4. Flow Cytometric Analysis
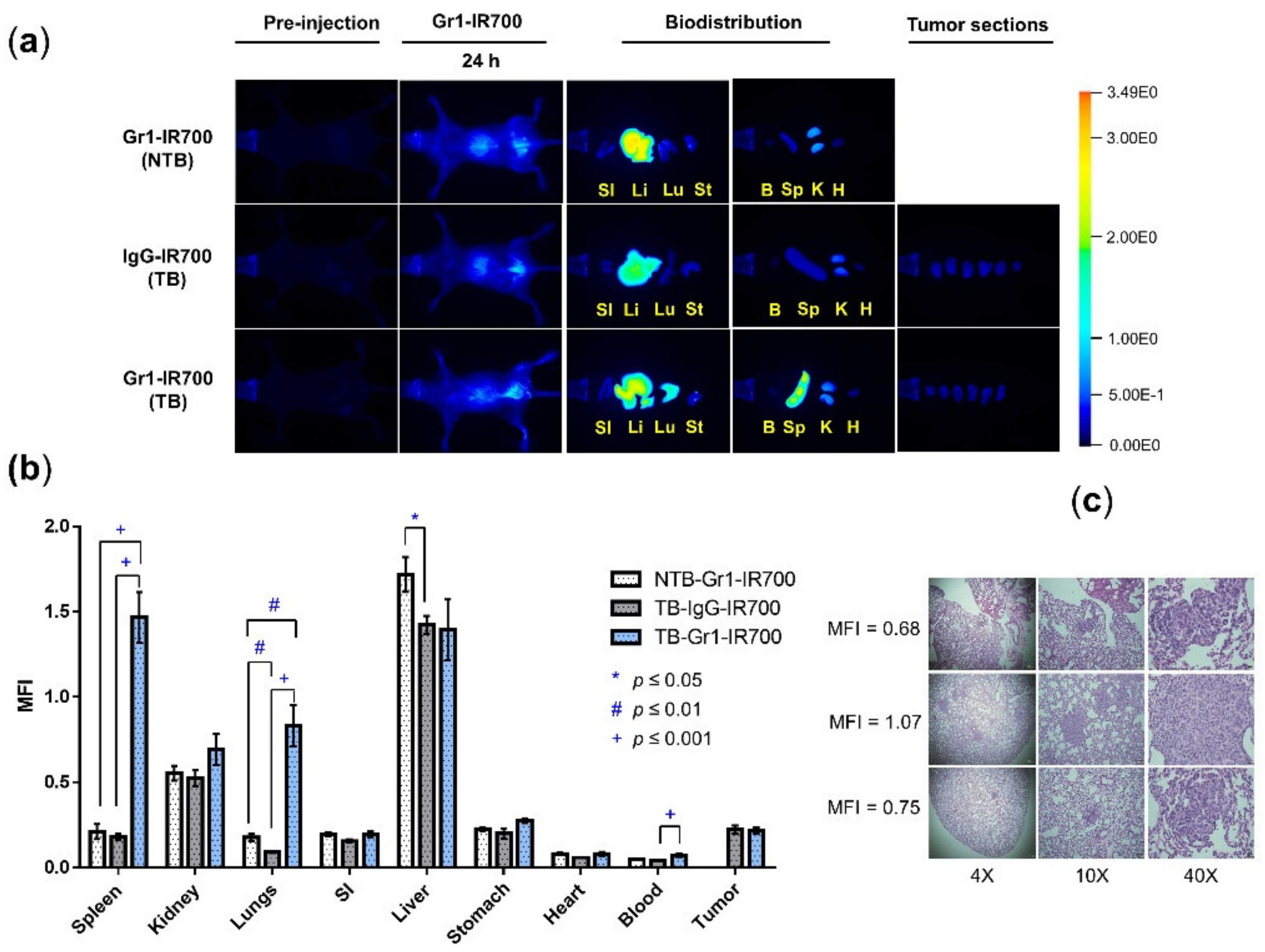
2.5. Biodistribution of Gr1-IR700
2.6. In Vitro NIR Photoimmunotherapy and Phototoxicity Assay
2.7. In Vivo NIR-PIT of Mouse Spleens
2.8. Cell Viability of NIR-PIT-Treated Mouse Spleens
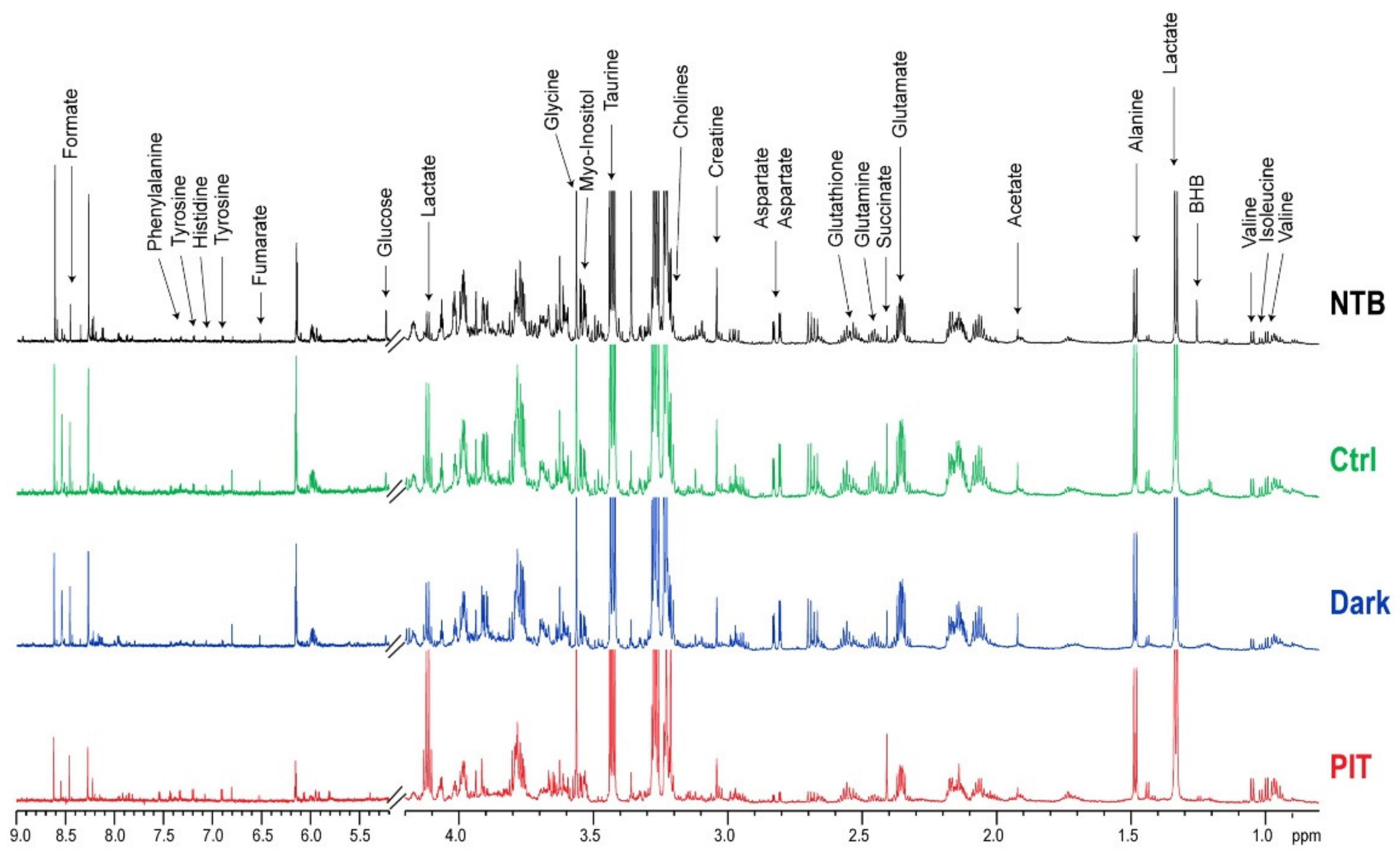
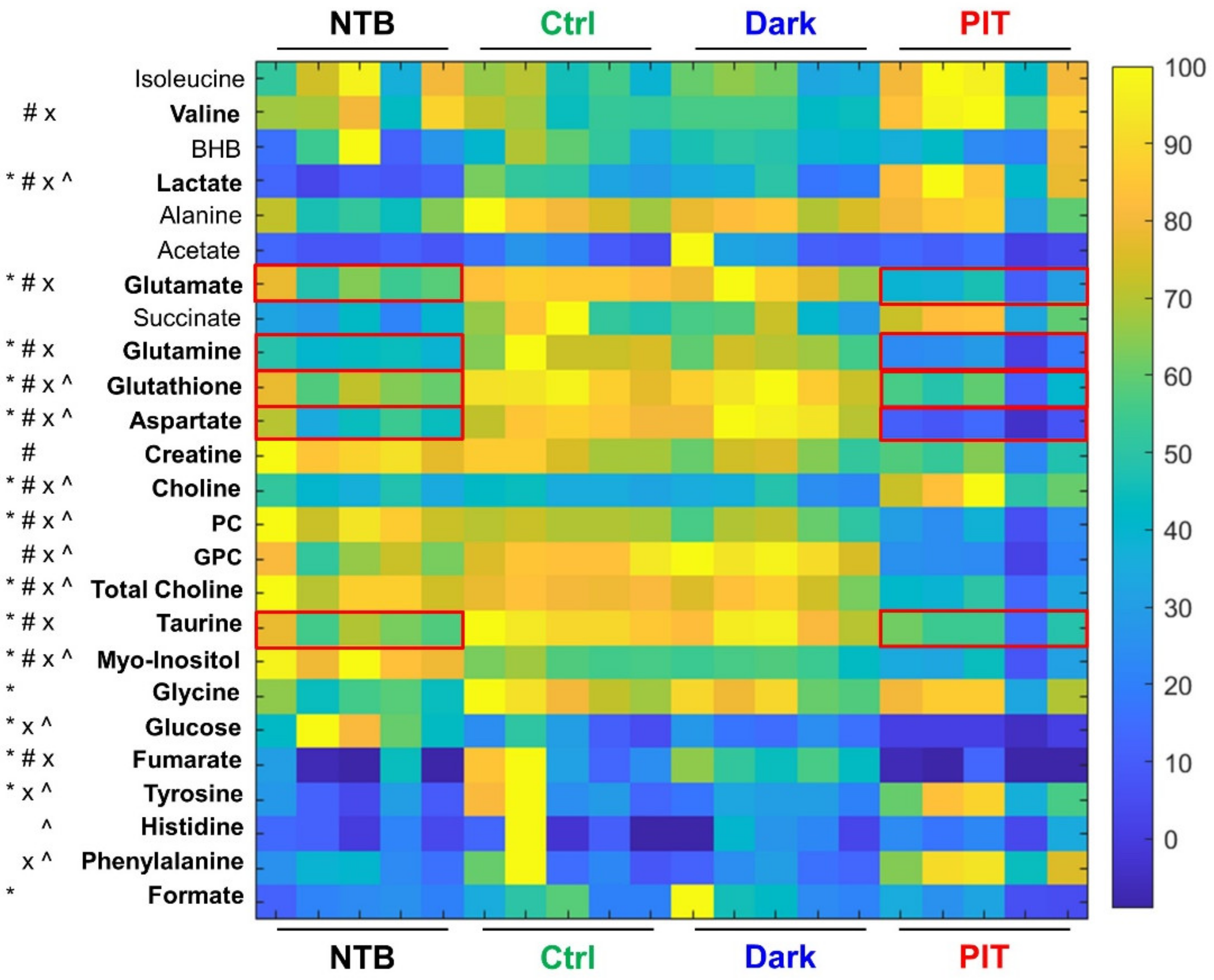
2.9. Dual-Phase Extraction and 1H MRS Analysis of NIR-PIT-Treated Mouse Spleens
2.10. Statistical Analysis
3. Results
3.1. Splenic MDSCs Significantly Increased in 4T1-Luc Tumor-Bearing Mice
3.2. Gr1-IR700-Mediated PIT Eliminated Tumor-Induced MDSCs Isolated from the Spleen
3.3. Gr1-IR700 Preferentially Accumulated in the Spleen and Lungs of Tumor-Bearing Mice
3.4. In Vivo Gr1-IR700-Mediated PIT of Tumor-Bearing Mouse Spleens Demonstrated Significant Reduction of Splenocyte Viability
3.5. In Vivo Gr1-IR700-Mediated PIT of Tumor-Bearing Mouse Spleens Resulted in Significant Metabolic Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Chen, D.; Long, H.; Zhu, B. The mechanisms of pathological extramedullary hematopoiesis in diseases. Cell. Mol. Life Sci. 2020, 77, 2723–2738. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, K.; Tian, J.; Xia, X.; Ma, J.; Tang, X.; Xu, H.; Wang, S. Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9. Adv. Sci. 2019, 6, 1901278. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Lee, O.-Y.; Shon, S.Y.; Nam, O.; Ryu, P.D.; Seo, M.W.; Lee, D.-S. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013, 15, R79. [Google Scholar] [CrossRef] [PubMed]
- Hossain, D.M.S.; Pal, S.K.; Moreira, D.; Duttagupta, P.; Zhang, Q.; Won, H.; Jones, J.; D’Apuzzo, M.; Forman, S.; Kortylewski, M. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin. Cancer Res. 2015, 21, 3771–3782. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y.; Guo, N.; Wang, S. MDSCs: Key Criminals of Tumor Pre-metastatic Niche Formation. Front. Immunol. 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Gabrilovich, D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011, 32, 19–25. [Google Scholar] [CrossRef]
- Nagaraj, S.; Gupta, K.; Pisarev, V.; Kinarsky, L.; Sherman, S.; Kang, L.; Herber, D.L.; Schneck, J.; Gabrilovich, D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007, 13, 828–835. [Google Scholar] [CrossRef]
- Zea, A.H.; Rodriguez, P.C.; Atkins, M.B.; Hernandez, C.; Signoretti, S.; Zabaleta, J.; McDermott, D.; Quiceno, D.; Youmans, A.; O’Neill, A.; et al. Arginase-Producing Myeloid Suppressor Cells in Renal Cell Carcinoma Patients: A Mechanism of Tumor Evasion. Cancer Res. 2005, 65, 3044–3048. [Google Scholar] [CrossRef]
- Rodríguez, P.C.; Ochoa, A.C. T cell dysfunction in cancer: Role of myeloid cells and tumor cells regulating amino acid availability and oxidative stress. Semin. Cancer Biol. 2006, 16, 66–72. [Google Scholar] [CrossRef]
- Vanhaver, C.; van der Bruggen, P.; Bruger, A. MDSC in Mice and Men: Mechanisms of Immunosuppression in Cancer. J. Clin. Med. 2021, 10, 2872. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Peranzoni, E.; Zilio, S.; Marigo, I.; Dolcetti, L.; Zanovello, P.; Mandruzzato, S.; Bronte, V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 2010, 22, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Zhang, M.; Yu, Y.; Liu, X.; Cao, X. Fas Signal Promotes Lung Cancer Growth by Recruiting Myeloid-Derived Suppressor Cells via Cancer Cell-Derived PGE2. J. Immunol. 2009, 182, 3801–3808. [Google Scholar] [CrossRef] [PubMed]
- Hurez, V.; Daniel, B.J.; Sun, L.; Liu, A.-J.; Ludwig, S.M.; Kious, M.J.; Thibodeaux, S.R.; Pandeswara, S.; Murthy, K.; Livi, C.B.; et al. Mitigating Age-Related Immune Dysfunction Heightens the Efficacy of Tumor Immunotherapy in Aged Mice. Cancer Res. 2012, 72, 2089–2099. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Zhu, L.; Harris-White, M.; Kar, U.; Huang, M.; Johnson, M.F.; Lee, J.M.; Elashoff, D.; Strieter, R.; Dubinett, S.; et al. Myeloid Suppressor Cell Depletion Augments Antitumor Activity in Lung Cancer. PLoS ONE 2012, 7, e40677. [Google Scholar] [CrossRef]
- Ma, C.; Kapanadze, T.; Gamrekelashvili, J.; Manns, M.P.; Korangy, F.; Greten, T.F. Anti-Gr-1 antibody depletion fails to eliminate hepatic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukoc. Biol. 2012, 92, 1199–1206. [Google Scholar] [CrossRef]
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rébé, C.; Ghiringhelli, F. 5-Fluorouracil Selectively Kills Tumor-Associated Myeloid-Derived Suppressor Cells Resulting in Enhanced T Cell–Dependent Antitumor Immunity. Cancer Res. 2010, 70, 3052–3061. [Google Scholar] [CrossRef]
- Bauer, R.; Udonta, F.; Wroblewski, M.; Ben-Batalla, I.; Santos, I.M.; Taverna, F.; Kuhlencord, M.; Gensch, V.; Päsler, S.; Vinckier, S.; et al. Blockade of Myeloid-Derived Suppressor Cell Expansion with All-Trans Retinoic Acid Increases the Efficacy of Antiangiogenic Therapy. Cancer Res. 2018, 78, 3220–3232. [Google Scholar] [CrossRef]
- Flores-Toro, J.A.; Luo, D.; Gopinath, A.; Sarkisian, M.R.; Campbell, J.J.; Charo, I.F.; Singh, R.; Schall, T.J.; Datta, M.; Jain, R.K.; et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc. Natl. Acad. Sci. USA 2020, 117, 1129–1138. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Qin, J.; Zhou, H.; Majumdar, A.P.N.; Peng, F. Blockade of CCR5-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy in gastric cancer. Immunopharmacol. Immunotoxicol. 2018, 40, 91–97. [Google Scholar] [CrossRef]
- Qin, H.; Lerman, B.; Sakamaki, I.; Wei, G.; Cha, S.C.; Rao, S.S.; Qian, J.; Hailemichael, Y.; Nurieva, R.; Dwyer, K.C.; et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat. Med. 2014, 20, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Harada, M.; Takakura, H.; Ando, K.; Goto, Y.; Tsuneda, T.; Ogawa, M.; Taketsugu, T. Theoretical and Experimental Studies on the Near-Infrared Photoreaction Mechanism of a Silicon Phthalocyanine Photoimmunotherapy Dye: Photoinduced Hydrolysis by Radical Anion Generation. ChemPlusChem 2020, 85, 1953. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Okada, R.; Goto, Y.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Daar, D.; Turkbey, B.; Choyke, P.L.; et al. Electron Donors Rather Than Reactive Oxygen Species Needed for Therapeutic Photochemical Reaction of Near-Infrared Photoimmunotherapy. ACS Pharmacol. Transl. Sci. 2021, 4, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Bosiljcic, M.; Cederberg, R.A.; Hamilton, M.J.; LePard, N.E.; Harbourne, B.T.; Collier, J.L.; Halvorsen, E.C.; Shi, R.; Franks, S.E.; Kim, A.Y.; et al. Targeting myeloid-derived suppressor cells in combination with primary mammary tumor resection reduces metastatic growth in the lungs. Breast Cancer Res. 2019, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- DuPre’, S.A.; Redelman, D.; Hunter, K.W., Jr. The mouse mammary carcinoma 4T1: Characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int. J. Exp. Pathol. 2007, 88, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Chafe, S.C.; Lou, Y.; Sceneay, J.; Vallejo, M.; Hamilton, M.J.; McDonald, P.C.; Bennewith, K.L.; Möller, A.; Dedhar, S. Carbonic Anhydrase IX Promotes Myeloid-Derived Suppressor Cell Mobilization and Establishment of a Metastatic Niche by Stimulating G-CSF Production. Cancer Res. 2015, 75, 996–1008. [Google Scholar] [CrossRef]
- Yan, H.H.; Pickup, M.; Pang, Y.; Gorska, A.E.; Li, Z.; Chytil, A.; Geng, Y.; Gray, J.W.; Moses, H.L.; Yang, L. Gr-1+CD11b+ Myeloid Cells Tip the Balance of Immune Protection to Tumor Promotion in the Premetastatic Lung. Cancer Res. 2010, 70, 6139–6149. [Google Scholar] [CrossRef]
- Ouzounova, M.; Lee, E.; Piranlioglu, R.; El Andaloussi, A.; Kolhe, R.; Demirci, M.F.; Marasco, D.; Asm, I.; Chadli, A.; Hassan, K.A.; et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 2017, 8, 14979. [Google Scholar] [CrossRef]
- Zhao, F.; Obermann, S.; von Wasielewski, R.; Haile, L.; Manns, M.P.; Korangy, F.; Greten, T.F. Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology 2009, 128, 141–149. [Google Scholar] [CrossRef]
- Cassetta, L.; Bruderek, K.; Skrzeczynska-Moncznik, J.; Osiecka, O.; Hu, X.; Rundgren, I.M.; Lin, A.; Santegoets, K.; Horzum, U.; Godinho-Santos, A.; et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J. Immunother. Cancer 2020, 8, e001223. [Google Scholar] [CrossRef]
- Brandau, S.; Trellakis, S.; Bruderek, K.; Schmaltz, D.; Steller, G.; Elian, M.; Suttmann, H.; Schenck, M.; Welling, J.; Zabel, P.; et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J. Leukoc. Biol. 2011, 89, 311–317. [Google Scholar] [CrossRef]
- Toor, S.M.; Khaja, A.S.S.; El Salhat, H.; Bekdache, O.; Kanbar, J.; Jaloudi, M.; Elkord, E. Increased Levels of Circulating and Tumor-Infiltrating Granulocytic Myeloid Cells in Colorectal Cancer Patients. Front. Immunol. 2016, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2008, 58, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Schmielau, J.; Finn, O.J. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001, 61, 4756–4760. [Google Scholar] [PubMed]
- Movahedi, K.; Guilliams, M.; Van den Bossche, J.; Bergh, R.V.D.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell–suppressive activity. Blood 2008, 111, 4233–4244. [Google Scholar] [CrossRef]
- Bergenfelz, C.; Leandersson, K. The Generation and Identity of Human Myeloid-Derived Suppressor Cells. Front. Oncol. 2020, 10, 109. [Google Scholar] [CrossRef]
- Alshetaiwi, H.; Pervolarakis, N.; McIntyre, L.L.; Ma, D.; Nguyen, Q.; Rath, J.A.; Nee, K.; Hernandez, G.; Evans, K.; Torosian, L.; et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. 2020, 5, eaay6017. [Google Scholar] [CrossRef]
- Maruoka, Y.; Wakiyama, H.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy for cancers: A translational perspective. eBioMedicine 2021, 70, 103501. [Google Scholar] [CrossRef]
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS Nano 2016, 10, 3918–3935. [Google Scholar] [CrossRef]
- Xue, Z.; Li, X.; Li, Y.; Jiang, M.; Liu, H.; Zeng, S.; Hao, J. X-ray-Activated Near-Infrared Persistent Luminescent Probe for Deep-Tissue and Renewable in Vivo Bioimaging. ACS Appl. Mater. Interfaces 2017, 9, 22132–22142. [Google Scholar] [CrossRef]
- Kishimoto, S.; Oshima, N.; Yamamoto, K.; Munasinghe, J.; Ardenkjaer-Larsen, J.H.; Mitchell, J.B.; Choyke, P.L.; Krishna, M.C. Molecular imaging of tumor photoimmunotherapy: Evidence of photosensitized tumor necrosis and hemodynamic changes. Free Radic. Biol. Med. 2017, 116, 1–10. [Google Scholar] [CrossRef]
- Wei, D.; Tao, Z.; Shi, Q.; Wang, L.; Liu, L.; She, T.; Yi, Q.; Wen, X.; Liu, L.; Li, S.; et al. Selective Photokilling of Colorectal Tumors by Near-Infrared Photoimmunotherapy with a GPA33-Targeted Single-Chain Antibody Variable Fragment Conjugate. Mol. Pharm. 2020, 17, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2012, 46, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Baliou, S.; Kyriakopoulos, A.; Spandidos, D.; Zoumpourlis, V. Role of taurine, its haloamines and its lncRNA TUG1 in both inflammation and cancer progression. On the road to therapeutics? (Review). Int. J. Oncol. 2020, 57, 631–664. [Google Scholar] [CrossRef]
- Boutté, A.M.; McDonald, W.H.; Shyr, Y.; Yang, L.; Lin, P.C. Characterization of the MDSC Proteome Associated with Metastatic Murine Mammary Tumors Using Label-Free Mass Spectrometry and Shotgun Proteomics. PLoS ONE 2011, 6, e22446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singer, K.; Cheng, W.-C.; Kreutz, M.; Ho, P.-C.; Siska, P.J. Immunometabolism in cancer at a glance. Dis. Model. Mech. 2018, 11, dmm034272. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Y.; Wu, W.; Feng, Y. Taurine promotes the production of CD4+CD25+FOXP3+ Treg cells through regulating IL-35/STAT1 pathway in a mouse allergic rhinitis model. Allergy Asthma Clin. Immunol. 2021, 17, 59. [Google Scholar] [CrossRef]


| Metabolites | Chemical Shift (ppm) | Concentration (mM) | Percent Change (%), p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NTB | Ctrl | Dark | PIT | Ctrl vs. PIT | Dark vs. PIT | NTB vs. PIT | Ctrl vs. Dark | ||||||
| Isoleucine | 1.02 | 0.101 | 0.084 | 0.079 | 0.116 | 38 | 0.078 | 47 | 0.052 | 15 | 0.459 | −6 | 0.668 |
| Valine | 1.05 | 0.170 | 0.144 | 0.129 | 0.204 | 41 | 0.018 | 57 | 0.003 | 20 | 0.190 | −10 | 0.275 |
| BHB | 1.20 | 0.127 | 0.151 | 0.136 | 0.125 | −17 | 0.426 | −8 | 0.708 | −2 | 0.969 | −10 | 0.399 |
| Lactate | 1.33 | 1.066 | 3.163 | 2.367 | 5.048 | 60 | 0.024 | 113 | 0.004 | 374 | 1 × 10−4 | −25 | 0.160 |
| Alanine | 1.48 | 0.666 | 0.932 | 0.891 | 0.798 | −14 | 0.312 | −10 | 0.442 | 20 | 0.317 | −4 | 0.524 |
| Acetate | 1.92 | 0.068 | 0.089 | 0.163 | 0.065 | −27 | 0.214 | −60 | 0.143 | −5 | 0.771 | 82 | 0.264 |
| Glutamate | 2.35 | 3.247 | 4.391 | 4.264 | 1.962 | −55 | 3 × 10−5 | −54 | 4 × 10−4 | −40 | 0.008 | −3 | 0.652 |
| Succinate | 2.41 | 0.104 | 0.196 | 0.149 | 0.187 | −5 | 0.794 | 26 | 0.233 | 80 | 0.011 | −24 | 0.162 |
| Glutamine | 2.46 | 0.922 | 1.516 | 1.311 | 0.500 | −67 | 7 × 10−5 | −62 | 5 × 10−5 | −46 | 0.001 | −13 | 0.136 |
| Glutathione | 2.54 | 3.801 | 4.979 | 4.905 | 2.629 | −47 | 0.001 | −46 | 0.002 | −31 | 0.037 | −1 | 0.809 |
| Aspartate | 2.80 | 0.437 | 0.675 | 0.721 | 0.120 | −82 | 7 × 10−8 | −83 | 1 × 10−6 | −72 | 2 × 10−4 | 7 | 0.347 |
| Creatine | 3.04 | 0.380 | 0.334 | 0.291 | 0.227 | −32 | 0.010 | −22 | 0.083 | −40 | 0.001 | −13 | 0.100 |
| Choline | 3.21 | 0.155 | 0.142 | 0.129 | 0.249 | 75 | 0.004 | 93 | 0.004 | 61 | 0.010 | −9 | 0.433 |
| PC | 3.23 | 1.903 | 1.606 | 1.437 | 0.680 | −58 | 3 × 10−5 | −53 | 5 × 10−4 | −64 | 5 × 10−5 | −10 | 0.072 |
| GPC | 3.24 | 1.261 | 1.550 | 1.670 | 0.478 | −69 | 2 × 10−6 | −71 | 3 × 10−6 | −62 | 9 × 10−5 | 8 | 0.203 |
| Total Choline | 3.23 | 3.345 | 3.215 | 3.088 | 1.585 | −51 | 8 × 10−5 | −49 | 5 × 10−4 | −53 | 3 × 10−4 | −4 | 0.446 |
| Taurine | 3.43 | 9.036 | 12.408 | 11.58 | 6.820 | −45 | 0.001 | −41 | 0.004 | −25 | 0.087 | −7 | 0.253 |
| Myo-Inositol | 3.55 | 2.777 | 1.964 | 1.770 | 1.131 | −42 | 0.002 | −36 | 0.009 | −59 | 6 × 10−5 | −10 | 0.082 |
| Glycine | 3.56 | 0.649 | 0.951 | 0.877 | 0.836 | −12 | 0.371 | −5 | 0.757 | 29 | 0.136 | −8 | 0.464 |
| Glucose | 5.24 | 0.140 | 0.063 | 0.055 | 0.016 | −75 | 0.015 | −71 | 9 × 10−5 | −89 | 4 × 10−4 | −13 | 0.633 |
| Fumarate | 6.52 | 0.010 | 0.033 | 0.034 | 0.003 | −92 | 0.015 | −92 | 1 × 10−5 | −75 | 0.281 | 3 | 0.930 |
| Tyrosine | 6.90 | 0.034 | 0.078 | 0.046 | 0.098 | 26 | 0.461 | 110 | 5 × 10−3 | 187 | 0.002 | −40 | 0.214 |
| Histidine | 7.08 | 0.031 | 0.051 | 0.041 | 0.048 | −6 | 0.930 | 16 | 0.703 | 55 | 0.128 | −19 | 0.795 |
| Phenylalanine | 7.43 | 0.030 | 0.040 | 0.022 | 0.066 | 65 | 0.133 | 196 | 5 × 10−4 | 119 | 0.002 | −44 | 0.242 |
| Formate | 8.46 | 0.066 | 0.101 | 0.122 | 0.066 | −35 | 0.143 | −46 | 0.137 | 0 | 0.987 | 21 | 0.570 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnett, J.D.; Jin, J.; Penet, M.-F.; Kobayashi, H.; Bhujwalla, Z.M. Phototheranostics of Splenic Myeloid-Derived Suppressor Cells and Its Impact on Spleen Metabolism in Tumor-Bearing Mice. Cancers 2022, 14, 3578. https://doi.org/10.3390/cancers14153578
Barnett JD, Jin J, Penet M-F, Kobayashi H, Bhujwalla ZM. Phototheranostics of Splenic Myeloid-Derived Suppressor Cells and Its Impact on Spleen Metabolism in Tumor-Bearing Mice. Cancers. 2022; 14(15):3578. https://doi.org/10.3390/cancers14153578
Chicago/Turabian StyleBarnett, James D., Jiefu Jin, Marie-France Penet, Hisataka Kobayashi, and Zaver M. Bhujwalla. 2022. "Phototheranostics of Splenic Myeloid-Derived Suppressor Cells and Its Impact on Spleen Metabolism in Tumor-Bearing Mice" Cancers 14, no. 15: 3578. https://doi.org/10.3390/cancers14153578
APA StyleBarnett, J. D., Jin, J., Penet, M.-F., Kobayashi, H., & Bhujwalla, Z. M. (2022). Phototheranostics of Splenic Myeloid-Derived Suppressor Cells and Its Impact on Spleen Metabolism in Tumor-Bearing Mice. Cancers, 14(15), 3578. https://doi.org/10.3390/cancers14153578







