In Vitro Comparative Study of Near-Infrared Photoimmunotherapy and Photodynamic Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of IR700-Conjugated mAbs
2.3. Cell Culture
2.4. Live Cell Imaging
2.5. In Vitro NIR-PIT and TS-PDT
2.6. In Vitro Cytotoxicity Assay
2.7. Annexin V/PI Staining
2.8. Detection of ICD Markers
2.9. Statistical Analysis
3. Results
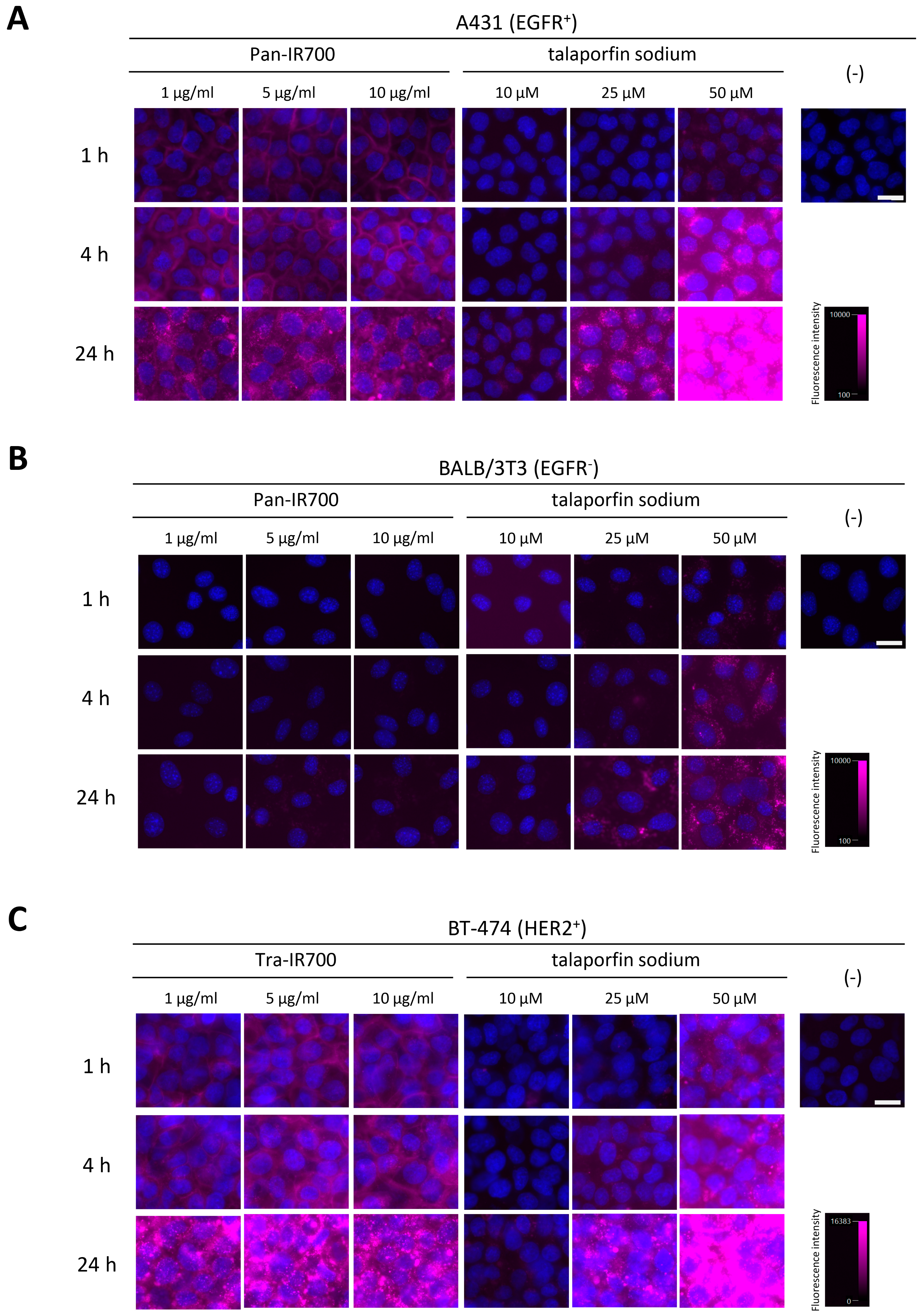
3.1. Cellular Binding/Uptake Specificity and Distribution of mAb-IR700 and Talaporfin Sodium
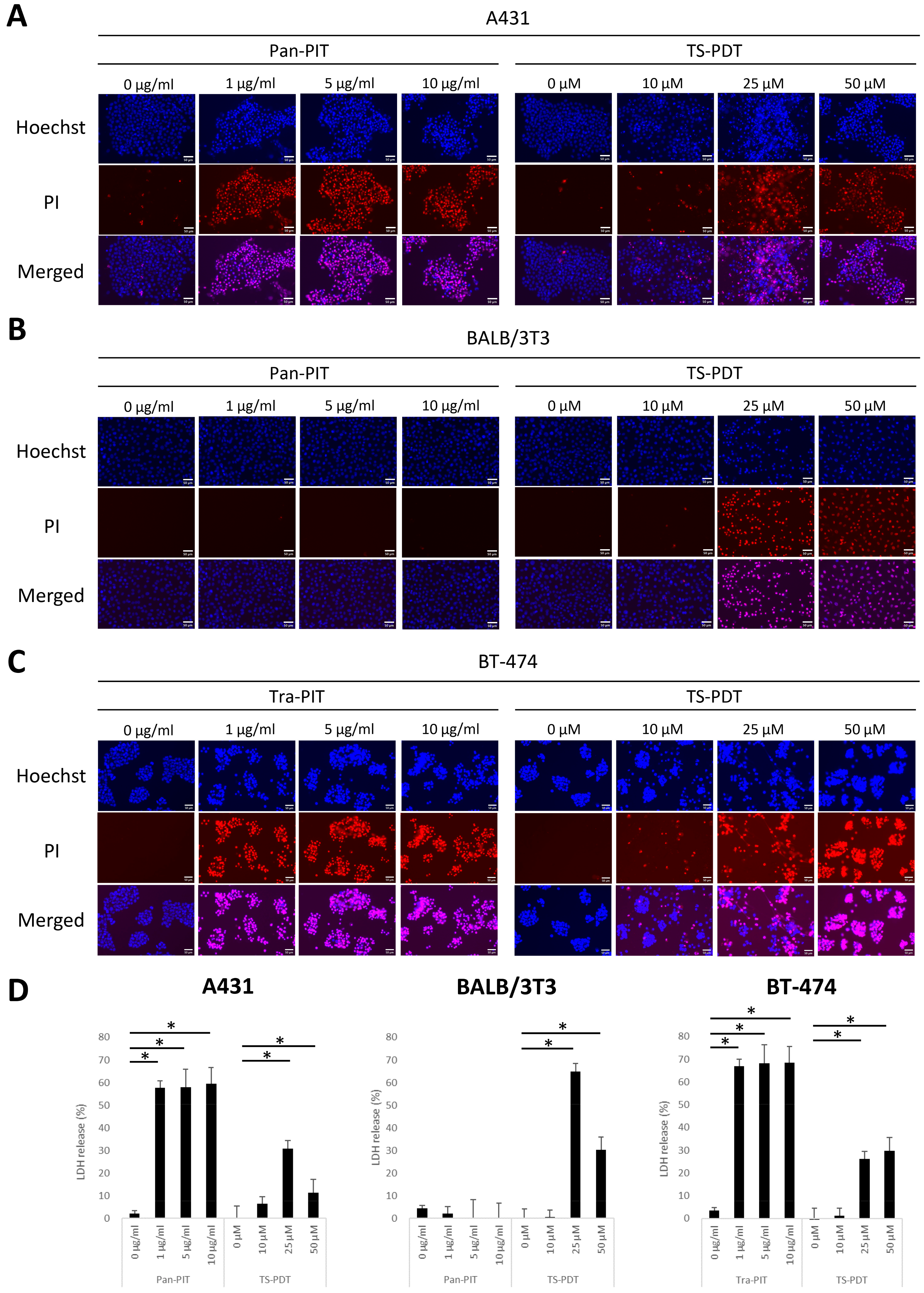
3.2. Cytotoxicity of NIR-PIT and TS-PDT
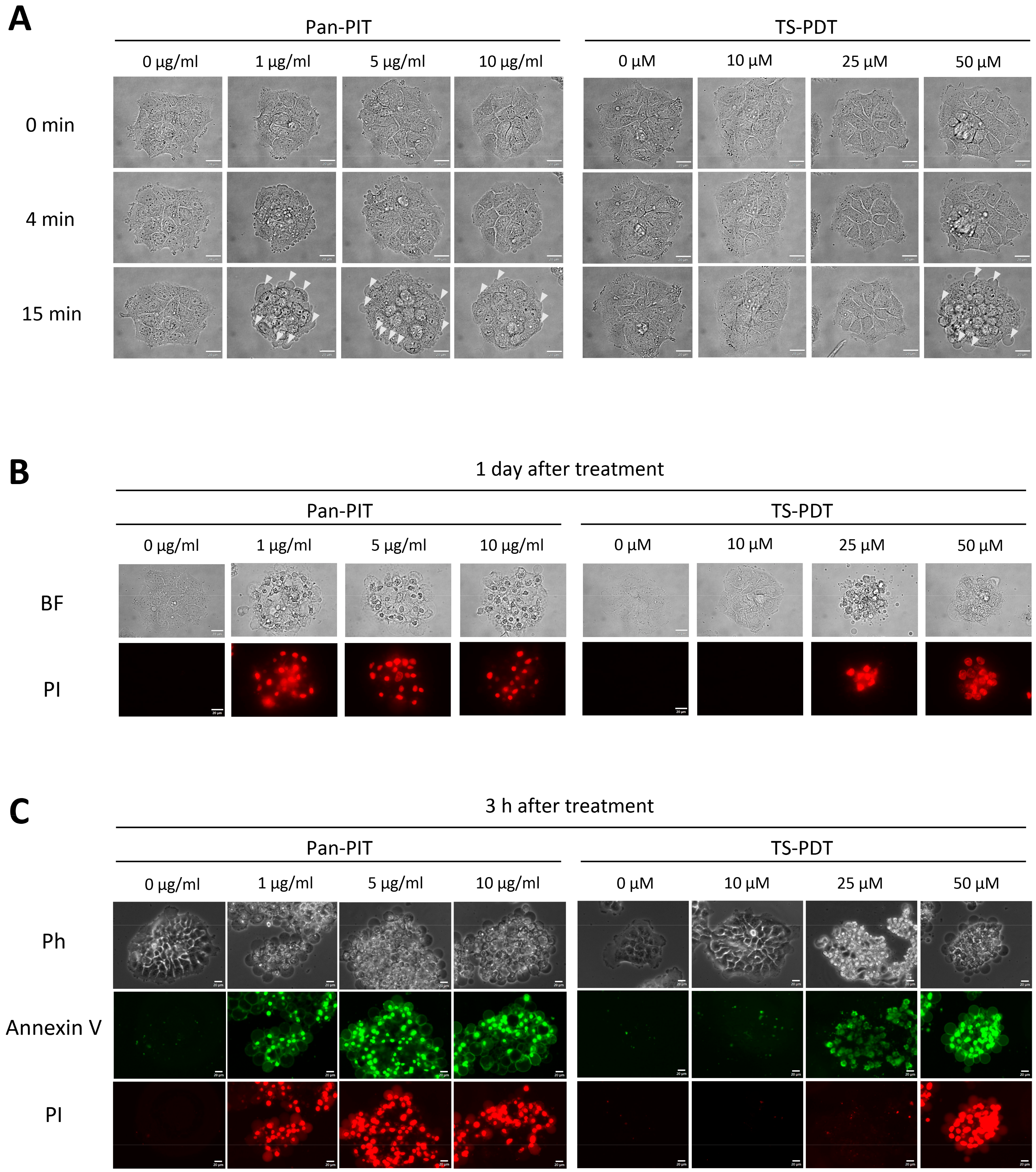
3.3. Patterns of Cell Death Induced by NIR-PIT and TS-PDT
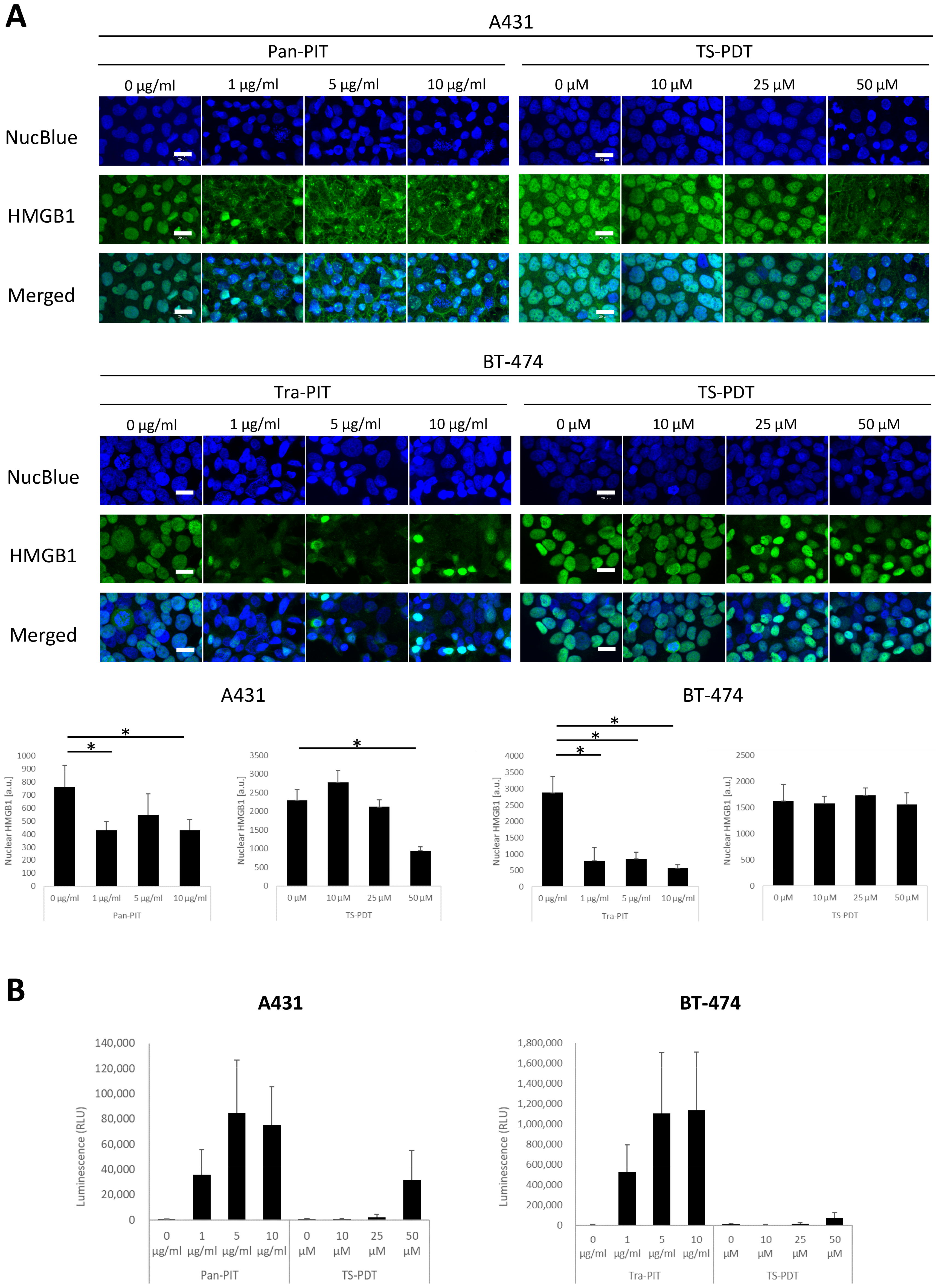
3.4. Analysis of ICD Markers following NIR-PIT and TS-PDT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Choyke, P.L.; Hisataka, K. Selective Cell Elimination from Mixed 3D Culture Using a Near Infrared Photoimmunotherapy Technique. J. Vis. Exp. 2016, 14, 53633. [Google Scholar]
- Sato, K.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy in the treatment of pleural disseminated NSCLC: Preclinical experience. Theranostics 2015, 5, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Choyke, P.L.; Kobayashi, H. Photoimmunotherapy of gastric cancer peritoneal carcinomatosis in a mouse model. PLoS ONE 2014, 9, e113276. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Hanaoka, H.; Watanabe, R.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy in the treatment of disseminated peritoneal ovarian cancer. Mol. Cancer Ther. 2015, 14, 141–150. [Google Scholar] [CrossRef]
- Railkar, R.; Krane, L.S.; Li, Q.Q.; Sanford, T.; Siddiqui, M.R.; Haines, D.; Vourganti, S.; Brancato, S.J.; Choyke, P.L.; Kobayashi, H.; et al. Epidermal Growth Factor Receptor (EGFR)-targeted Photoimmunotherapy (PIT) for the Treatment of EGFR-expressing Bladder Cancer. Mol. Cancer Ther. 2017, 16, 2201–2214. [Google Scholar] [CrossRef]
- Yamashita, S.; Kojima, M.; Onda, N.; Yoshida, T.; Shibutani, M. Trastuzumab-based near-infrared photoimmunotherapy in xenograft mouse of breast cancer. Cancer Medicine. 2022, 12, 4579–4589. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Targeting Prostate Cancer with Prostate-Specific Membrane Antigen (PSMA) Antibody. Mol. Cancer Res. 2017, 15, 1153–1162. [Google Scholar] [CrossRef]
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Heim, R.; Makings, L.; Garcia-Guzman, M.; Luiken, G.A.; Kobayashi, H.; Hoffman, R.M.; Bouvet, M. Near infra-red photoimmunotherapy with anti-CEA-IR700 results in extensive tumor lysis and a significant decrease in tumor burden in orthotopic mouse models of pancreatic cancer. PLoS ONE 2015, 10, e0121989. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Future applications of and prospects for near-IR photoimmunotherapy: Benefits and differences compared with photodynamic and photothermal therapy. Immunotherapy 2021, 13, 1305–1307. [Google Scholar] [CrossRef]
- Tahara, M.; Okano, S.; Enokida, T.; Ueda, Y.; Fujisawa, T.; Shinozaki, T.; Tomioka, T.; Okano, W.; Biel, M.A.; Ishida, K.; et al. A phase I, single-center, open-label study of RM-1929 photoimmunotherapy in Japanese patients with recurrent head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2021, 26, 1812–1821. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Roberts, W.G.; Shiau, F.Y.; Nelson, J.S.; Smith, K.M.; Berns, M.W. In vitro characterization of monoaspartyl chlorin e6 and diaspartyl chlorin e6 for photodynamic therapy. J. Natl. Cancer Inst. 1988, 80, 330–336. [Google Scholar] [CrossRef]
- Saito, T.; Tsukahara, T.; Suzuki, T.; Nojima, I.; Tadano, H.; Kawai, N.; Kubo, T.; Hirohashi, Y.; Kanaseki, T.; Torigoe, T.; et al. Spatiotemporal metabolic dynamics of the photosensitizer talaporfin sodium in carcinoma and sarcoma. Cancer Sci. 2021, 112, 550–562. [Google Scholar] [CrossRef]
- Roberts, W.G.; Liaw, L.H.; Berns, M.W. In vitro photosensitization II. An electron microscopy study of cellular destruction with mono-L-aspartyl chlorin e6 and photofrin II. Lasers Surg. Med. 1989, 9, 102–108. [Google Scholar] [CrossRef]
- Reiners, J.J., Jr.; Caruso, J.A.; Mathieu, P.; Chelladurai, B.; Yin, X.M.; Kessel, D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 2002, 9, 934–944. [Google Scholar] [CrossRef]
- Kato, H.; Furukawa, K.; Sato, M.; Okunaka, T.; Kusunoki, Y.; Kawahara, M.; Fukuoka, M.; Miyazawa, T.; Yana, T.; Matsui, K.; et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 103–111. [Google Scholar] [CrossRef]
- Akimoto, J. Photodynamic Therapy for Malignant Brain Tumors. Neurol. Med. Chir. Tokyo 2016, 56, 151–157. [Google Scholar] [CrossRef]
- Yano, T.; Minamide, T.; Takashima, K.; Nakajo, K.; Kadota, T.; Yoda, Y. Clinical Practice of Photodynamic Therapy Using Talaporfin Sodium for Esophageal Cancer. J. Clin. Med. 2021, 10, 2785. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Super enhanced permeability and retention (SUPR) effects in tumors following near infrared photoimmunotherapy. Nanoscale 2016, 8, 12504–12509. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Harada, M.; Takakura, H.; Ando, K.; Goto, Y.; Tsuneda, T.; Ogawa, M.; Taketsugu, T. Theoretical and Experimental Studies on the Near-Infrared Photoreaction Mechanism of a Silicon Phthalocyanine Photoimmunotherapy Dye: Photoinduced Hydrolysis by Radical Anion Generation. Chempluschem 2020, 85, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldan-Varona, P.; Rodriguez-Cobo, L.; Lopez-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef]
- Nagaya, T.; Friedman, J.; Maruoka, Y.; Ogata, F.; Okuyama, S.; Clavijo, P.E.; Choyke, P.L.; Allen, C.; Kobayashi, H. Host Immunity Following Near-Infrared Photoimmunotherapy Is Enhanced with PD-1 Checkpoint Blockade to Eradicate Established Antigenic Tumors. Cancer Immunol. Res. 2019, 7, 401–413. [Google Scholar] [CrossRef]
- Tanaka, M.; Kataoka, H.; Yano, S.; Sawada, T.; Akashi, H.; Inoue, M.; Suzuki, S.; Inagaki, Y.; Hayashi, N.; Nishie, H.; et al. Immunogenic cell death due to a new photodynamic therapy (PDT) with glycoconjugated chlorin (G-chlorin). Oncotarget 2016, 7, 47242–47251. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, G.; Le, V.; Zhang, A.; Chen, S.; Liang, X.; Liu, J. Photodynamic-therapy Activates Immune Response by disrupting Immunity Homeostasis of Tumor Cells, which Generates Vaccine for Cancer Therapy. Int. J. Biol. Sci. 2016, 12, 120–132. [Google Scholar] [CrossRef]
- Nakajima, T.; Sato, K.; Hanaoka, H.; Watanabe, R.; Harada, T.; Choyke, P.L.; Kobayashi, H. The effects of conjugate and light dose on photo-immunotherapy induced cytotoxicity. BMC Cancer 2014, 14, 389. [Google Scholar] [CrossRef]
- Nakajima, K.; Ogawa, M. Phototoxicity in near-infrared photoimmunotherapy is influenced by the subcellular localization of antibody-IR700. Photodiagnosis Photodyn. Ther. 2020, 31, 101926. [Google Scholar] [CrossRef]
- Usuda, J.; Tsunoda, Y.; Ichinose, S.; Ishizumi, T.; Ohtani, K.; Maehara, S.; Ono, S.; Tsutsui, H.; Ohira, T.; Okunaka, T.; et al. Breast cancer resistant protein (BCRP) is a molecular determinant of the outcome of photodynamic therapy (PDT) for centrally located early lung cancer. Lung Cancer 2010, 67, 198–204. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanaka, M.; Sasaki, M.; Ichikawa, H.; Nishie, H.; Kataoka, H. Vascular Shutdown by Photodynamic Therapy Using Talaporfin Sodium. Cancers 2020, 12, 2369. [Google Scholar] [CrossRef]
- Chalouni, C.; Doll, S. Fate of Antibody-Drug Conjugates in Cancer Cells. J. Exp. Clin. Cancer Res. 2018, 37, 20. [Google Scholar] [CrossRef]
- Ohashi, S.; Kikuchi, O.; Tsurumaki, M.; Nakai, Y.; Kasai, H.; Horimatsu, T.; Miyamoto, S.; Shimizu, A.; Chiba, T.; Muto, M. Preclinical validation of talaporfin sodium-mediated photodynamic therapy for esophageal squamous cell carcinoma. PLoS ONE 2014, 9, e103126. [Google Scholar] [CrossRef]
- Kanda, T.; Sugihara, T.; Takata, T.; Mae, Y.; Kinoshita, H.; Sakaguchi, T.; Hasegawa, T.; Kurumi, H.; Ikebuchi, Y.; Murakami, T.; et al. Low-density lipoprotein receptor expression is involved in the beneficial effect of photodynamic therapy using talaporfin sodium on gastric cancer cells. Oncol. Lett. 2019, 17, 3261–3266. [Google Scholar] [CrossRef]
- Ogawa, E.; Ito, A.; Arai, T. Dependence of damage within 10 min to myocardial cells by a photodynamic reaction with a high concentration of talaporfin sodium outside cells in vitro on parameters of laser irradiation. Photodiagnosis Photodyn. Ther. 2016, 15, 1–5. [Google Scholar] [CrossRef]
- Miki, Y.; Akimoto, J.; Yokoyama, S.; Homma, T.; Tsutsumi, M.; Haraoka, J.; Hirano, K.; Beppu, M. Photodynamic therapy in combination with talaporfin sodium induces mitochondrial apoptotic cell death accompanied with necrosis in glioma cells. Biol. Pharm. Bull. 2013, 36, 215–221. [Google Scholar] [CrossRef]
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014, 3, e955691. [Google Scholar] [CrossRef]
- Isoda, Y.; Piao, W.; Taguchi, E.; Iwano, J.; Takaoka, S.; Uchida, A.; Yoshikawa, K.; Enokizono, J.; Arakawa, E.; Tomizuka, K.; et al. Development and evaluation of a novel antibody-photon absorber conjugate reveals the possibility of photoimmunotherapy-induced vascular occlusion during treatment in vivo. Oncotarget 2018, 9, 31422–31431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, S.; Kojima, M.; Onda, N.; Shibutani, M. In Vitro Comparative Study of Near-Infrared Photoimmunotherapy and Photodynamic Therapy. Cancers 2023, 15, 3400. https://doi.org/10.3390/cancers15133400
Yamashita S, Kojima M, Onda N, Shibutani M. In Vitro Comparative Study of Near-Infrared Photoimmunotherapy and Photodynamic Therapy. Cancers. 2023; 15(13):3400. https://doi.org/10.3390/cancers15133400
Chicago/Turabian StyleYamashita, Susumu, Miho Kojima, Nobuhiko Onda, and Makoto Shibutani. 2023. "In Vitro Comparative Study of Near-Infrared Photoimmunotherapy and Photodynamic Therapy" Cancers 15, no. 13: 3400. https://doi.org/10.3390/cancers15133400
APA StyleYamashita, S., Kojima, M., Onda, N., & Shibutani, M. (2023). In Vitro Comparative Study of Near-Infrared Photoimmunotherapy and Photodynamic Therapy. Cancers, 15(13), 3400. https://doi.org/10.3390/cancers15133400





