An International Comparison of Presentation, Outcomes and CORONET Predictive Score Performance in Patients with Cancer Presenting with COVID-19 across Different Pandemic Waves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 13 April 2022).
- Lee, R.J.; Wysocki, O.; Bhogal, T.; Shotton, R.; Tivey, A.; Angelakas, A.; Aung, T.; Banfill, K.; Baxter, M.; Boyce, H.; et al. Longitudinal characterisation of haematological and biochemical parameters in cancer patients prior to and during COVID-19 reveals features associated with outcome. ESMO Open 2021, 6, 100005. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.T.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Grivas, P.; Khaki, A.R.; Wise-Draper, T.M.; French, B.; Hennessy, C.; Hsu, C.-Y.; Shyr, Y.; Li, X.; Choueiri, T.K.; Painter, C.A.; et al. Association of Clinical Factors and Recent Anti-Cancer Therapy with COVID-19 Severity among Patients with Cancer: A Report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021, 32, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, L.; Tsourti, Z.; Gennatas, S.; Rogado, J.; Sekacheva, M.; Viñal, D.; Lee, R.; Croitoru, A.; Vitorino, M.; Khallaf, S.; et al. COVID-19 in patients with cancer: First report of the ESMO international, registry-based, cohort study (ESMO CoCARE). ESMO Open 2022, 7, 100499. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2021, 602, 654–656. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- The REMAP-CAP Investigators. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Pinato, D.J.; Aguilar-Company, J.; Ferrante, D.; Hanbury, G.; Bower, M.; Salazar, R.; Mirallas, O.; Sureda, A.; Plaja, A.; Cucurull, M.; et al. Outcomes of the SARS-CoV-2 omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: Results from the retrospective, multicentre, OnCovid registry study. Lancet Oncol. 2022, 23, 865–875. [Google Scholar] [CrossRef]
- Bhogal, T.; Khan, U.T.; Lee, R.; Stockdale, A.; Hesford, C.; Potti-Dhananjaya, V.; Jathanna, A.; Rahman, S.; Tivey, A.; Shotton, R.; et al. Haematological malignancy and nosocomial transmission are associated with an increased risk of death from COVID-19: Results of a multi-center UK cohort. Leuk. Lymphoma 2021, 62, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Zambelli, A.; Aguilar-Company, J.; Bower, M.; Sng, C.C.T.; Salazar, R.; Bertuzzi, A.; Brunet, J.; Mesia, R.; Seguí, E.; et al. Clinical Portrait of the SARS-CoV-2 Epidemic in European Patients with Cancer. Cancer Discov. 2020, 10, 1465–1474. [Google Scholar] [CrossRef]
- Lee, R.J.; Wysocki, O.; Zhou, C.; Shotton, R.; Tivey, A.; Lever, L.; Woodcock, J.; Albiges, L.; Angelakas, A.; Arnold, D.; et al. Establishment of CORONET, COVID-19 Risk in Oncology Evaluation Tool, to Identify Patients with Cancer at Low Versus High Risk of Severe Complications of COVID-19 Disease on Presentation to Hospital. JCO Clin. Cancer Inform. 2022, 6, e2100177. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Jawad, S.; Baù, L.; Alaguthurai, T.; del Molino del Barrio, I.; Laing, A.G.; Hayday, T.S.; Monin, L.; Muñoz-Ruiz, M.; McDonald, L.; Quijorna, I.F.; et al. Acute Immune Signatures and Their Legacies in Severe Acute Respiratory Syndrome Coronavirus-2 Infected Cancer Patients. Cancer Cell 2021, 39, 257–275.e6. [Google Scholar] [CrossRef]
- Fendler, A.; Au, L.; Shepherd, S.T.C.; Byrne, F.; Cerrone, M.; Boos, L.A.; Rzeniewicz, K.; Gordon, W.; Shum, B.; Gerard, C.L.; et al. Functional antibody and T cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: The CAPTURE study. Nat. Cancer 2021, 2, 1321–1337. [Google Scholar] [CrossRef]
- Hasell, J.; Ortiz-Ospina, E.; Ritchie, H.; Roser, M. “Coronavirus Pandemic (COVID-19)”. Our World in Data. 2022. Available online: https://ourworldindata.org/coronavirus (accessed on 13 April 2022).
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021, 22, 765. [Google Scholar] [CrossRef]
- Oosting, S.F.; van der Veldt, A.A.M.; Geurts van Kessel, C.H.; Fehrmann, R.S.N.; van Binnendijk, R.S.; Dingemans, A.M.C.; Smit, E.F.; Hiltermann, J.N.; Den Hartog, G.; Jalving, M.; et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: A prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021, 22, 1681–1691. [Google Scholar] [CrossRef]
- Lee, M.; Quinn, R.; Pradhan, K.; Fedorov, K.; Levitz, D.; Fromowitz, A.; Thakkar, A.; Shapiro, L.C.; Kabarriti, R.; Ruiz, R.E.; et al. Impact of COVID-19 on case fatality rate of patients with cancer during the Omicron wave. Cancer Cell 2022, 40, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.V.; Babady, N.E.; Mead, P.A.; Rolling, T.; Perez-Johnston, R.; Bernardes, M.; Bogler, Y.; Caldararo, M.; Figueroa-Ortiz, C.J.; Glickman, M.S.; et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020, 26, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Foulon, S.; Bayle, A.; Gachot, B.; Pommeret, F.; Willekens, C.; Stoclin, A.; Merad, M.; Griscelli, F.; Lacroix, L.; et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: Results from the Gustave Roussy cohort. Nat. Cancer 2020, 1, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, W.S.; Pham, A.; Anand, H.; Fleysher, R.; Buczek, A.; Soby, S.; Mirhaji, P.; Yee, J.; Duong, T.Q. Clinical characteristics of the first and second COVID-19 waves in the Bronx, New York: A retrospective cohort study. Lancet Reg. Health-Am. 2021, 3, 100041. [Google Scholar] [CrossRef] [PubMed]
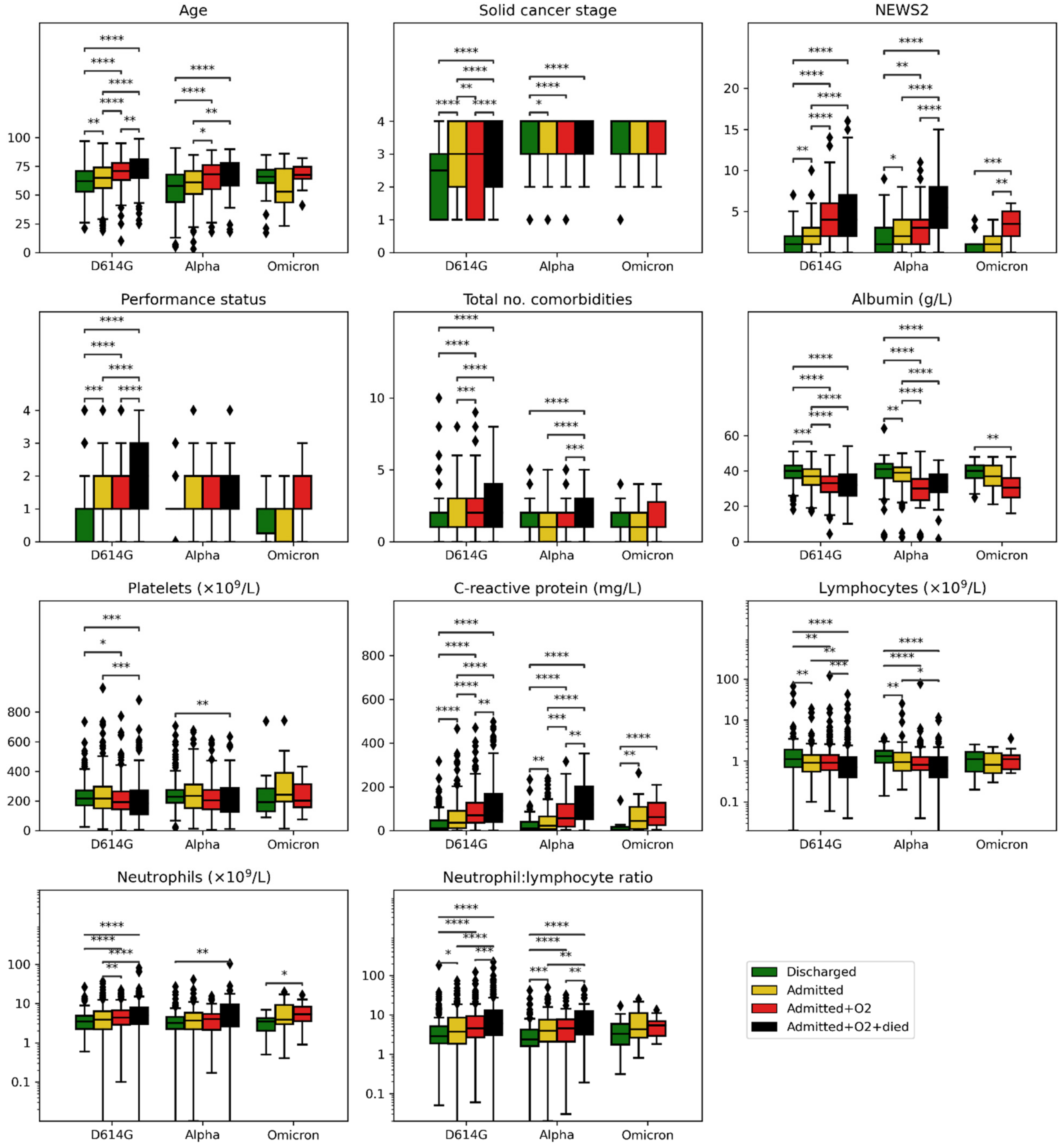
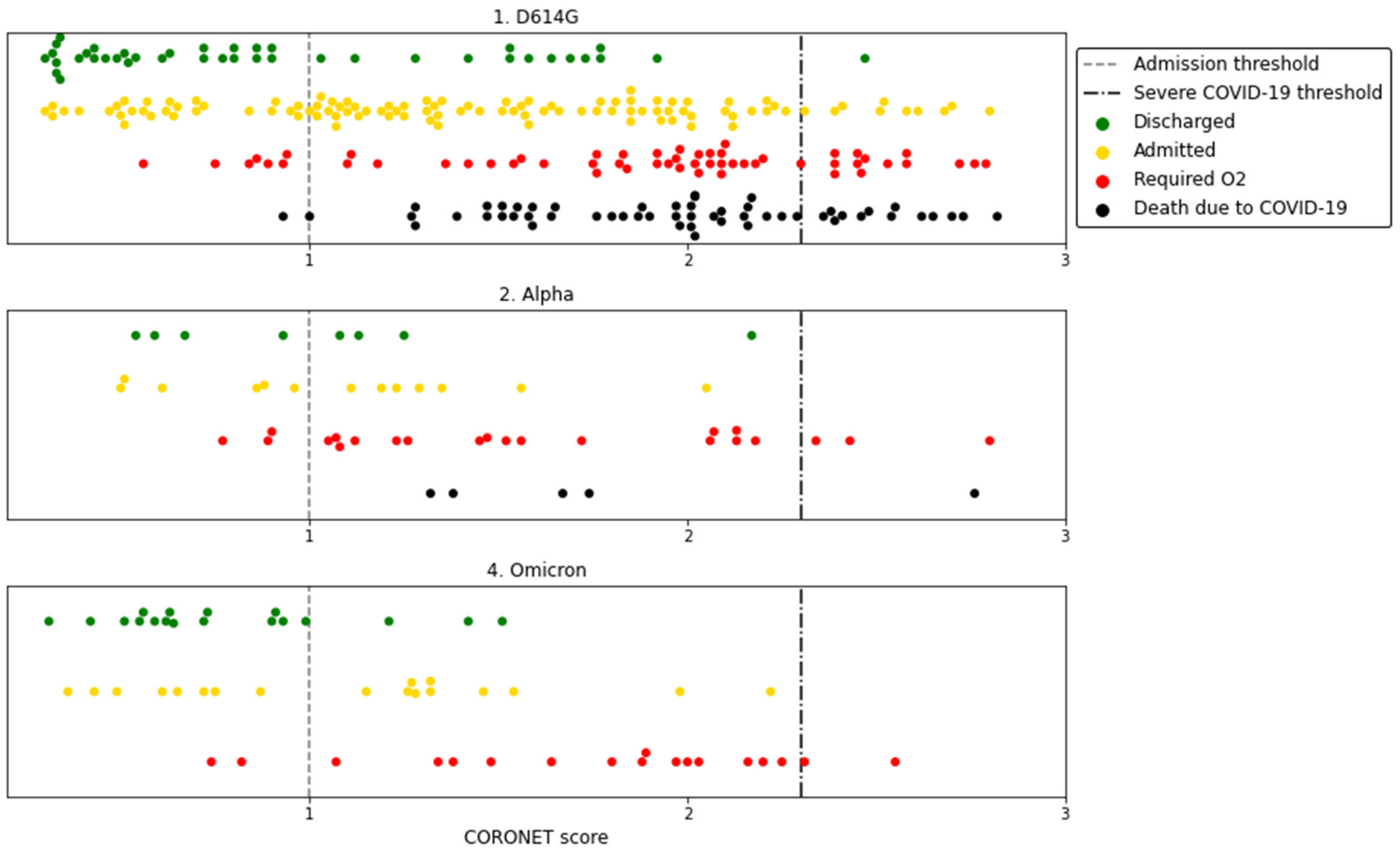
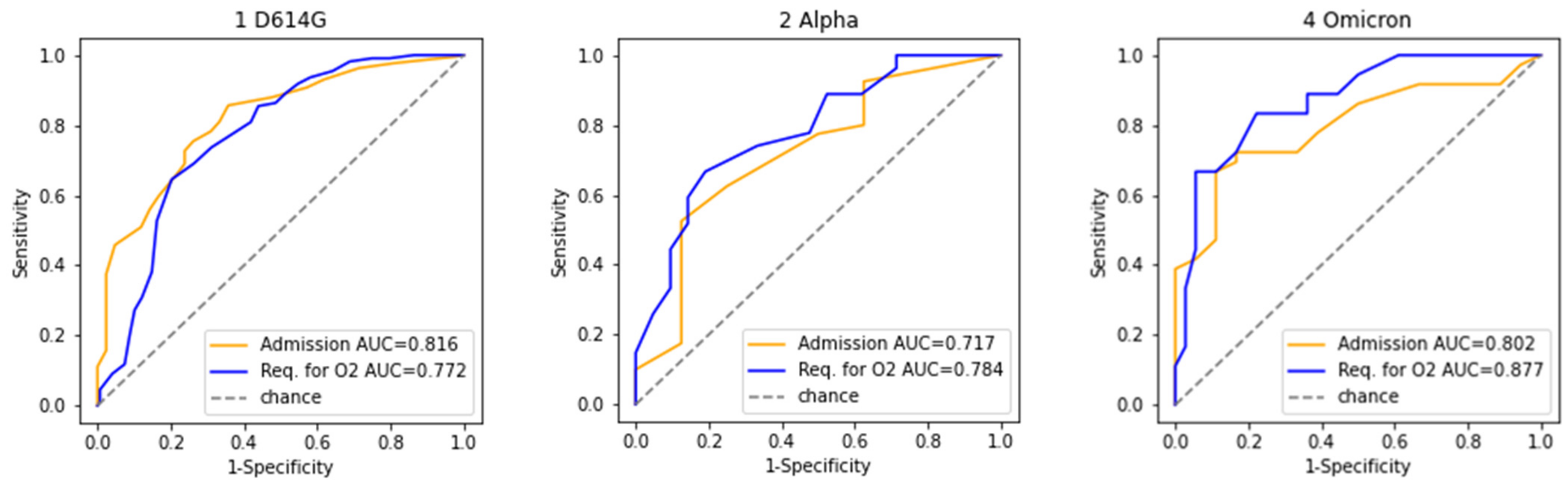
| Unknown | Overall | 1 D614G | 2 Alpha | 4 Omicron | |||
|---|---|---|---|---|---|---|---|
| n | 1968 | 1430 | 475 | 63 | |||
| Age *, median [Q1, Q3] | 0 | 67.0 [57.0, 76.0] | 68.0 [59.0, 77.0] | 61.0 [50.0, 72.0] | 66.0 [54.0, 73.0] | D614G vs. Alpha: p < 0.001, CLES ** = 0.621 | |
| Cancer type, n (%) | Breast | 141 | 325 (17.8) | 223 (17.3) | 93 (19.6) | 9 (14.3) | |
| Colorectal | 161 (8.8) | 108 (8.4) | 47 (9.9) | 6 (9.5) | |||
| Lung | 248 (13.6) | 170 (13.2) | 67 (14.1) | 11 (17.5) | |||
| Other solid cancer | 801 (43.9) | 576 (44.7) | 197 (41.6) | 28 (44.4) | |||
| Haematological malignancy | 291 (15.9) | 212 (16.4) | 70 (14.8) | 9 (14.3) | |||
| Solid cancer stage, n (%) | 1 | 539 | 197 (17.3) | 189 (24.5) | 7 (2.2) | 1 (1.9) | |
| 2 | 135 (11.9) | 103 (13.4) | 25 (7.9) | 7 (13.5) | |||
| 3 | 328 (28.8) | 193 (25.1) | 123 (38.9) | 12 (23.1) | |||
| 4 | 478 (42.0) | 285 (37.0) | 161 (50.9) | 32 (61.5) | |||
| Chemotherapy, n (%) | 314 | 653 (33.2) | 377 (26.4) | 249 (52.4) | 27 (42.9) | ||
| Immunotherapy, n (%) | 314 | 92 (4.7) | 54 (3.8) | 33 (6.9) | 5 (7.9) | ||
| Targeted Therapy, n (%) | 314 | 197 (10.0) | 121 (8.5) | 64 (13.5) | 12 (19.0) | ||
| Radiotherapy, n (%) | 486 | 106 (5.4) | 50 (3.5) | 45 (9.5) | 11 (17.5) | ||
| Vaccination, n (%) | Vaccinated 1 dose 2 doses 3 doses | 15 | - | 0 | 0 | 46 (95.8) 1 (2.2) 8 (17.4) 37 (80.4) | |
| Unvaccinated | - | 1430 (100.0) | 475 (100.0) | 2 (4.2) |
| Wave | ||||||
|---|---|---|---|---|---|---|
| Overall | 1 D614G | 2 Alpha | 4 Omicron | Significant Differences between Waves * | ||
| n | 1968 | 1430 | 475 | 63 | ||
| Outcome, n (%) | Discharged | 548 (27.8) | 336 (23.5) | 186 (39.2) | 26 (41.3) | 1 vs. 2: p < 0.001; 1 vs. 4: p = 0.002 |
| Admitted | 527 (26.8) | 379 (26.5) | 129 (27.2) | 19 (30.2) | ||
| Admitted+O2 | 454 (23.1) | 338 (23.6) | 98 (20.6) | 18 (28.6) | ||
| Admitted+O2+ died | 439 (22.3) | 377 (26.4) | 62 (13.1) | 0 | 1 vs. 2: p < 0.001 | |
| 1 D614G | 2 Alpha | 4 Omicron | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Patients Treated | No. Patients Not Treated | Missing | No. Patients Treated | No. Patients Not Treated | Missing | No. Patients Treated | No. Patients Not Treated | Missing | |
| steroids | 36 (32.4%) | 75 (67.6%) | 1319 | 88 (51.2%) | 84 (48.8%) | 303 | 19 (41.3%) | 27 (58.7%) | 17 |
| remdesivir | 1 (0.9%) | 110 (99.1%) | 1319 | 14 (8.3%) | 155 (91.7%) | 306 | 0 (0%) | 45 (100%) | 18 |
| lipinovir | 16 (14.4%) | 95 (85.6%) | 1319 | 0 (0%) | 167 (100%) | 308 | 0 (0%) | 46 (100%) | 17 |
| interferon | 0 (0%) | 108 (100%) | 1322 | 0 (0%) | 167 (100%) | 308 | 0 (0%) | 46 (100%) | 17 |
| interferon beta | 4 (3.6%) | 106 (96.4%) | 1320 | 0 (0%) | 167 (100%) | 308 | 0 (0%) | 46 (100%) | 17 |
| anticoagulation prophylaxis | 70 (64.2%) | 39 (35.8%) | 1321 | 79 (46.7%) | 90 (53.3%) | 306 | 22 (47.8%) | 24 (52.2%) | 17 |
| anticoagulation treatment dose | 6 (5.5%) | 103 (94.5%) | 1321 | 20 (11.9%) | 148 (88.1%) | 307 | 0 (0%) | 46 (100%) | 17 |
| antibiotic | 79 (71.8%) | 31 (28.2%) | 1320 | 100 (58.8%) | 70 (41.2%) | 305 | 17 (37%) | 29 (63%) | 17 |
| plasma | 2 (1.8%) | 108 (98.2%) | 1320 | 5 (3%) | 162 (97%) | 308 | 0 (0%) | 46 (100%) | 17 |
| tocilizumab | 5 (4.5%) | 105 (95.5%) | 1320 | 8 (4.8%) | 159 (95.2%) | 308 | 2 (4.3%) | 44 (95.7%) | 17 |
| nebulised interferonb | 1 (0.9%) | 106 (99.1%) | 1323 | 0 (0%) | 167 (100%) | 308 | 0 (0%) | 46 (100%) | 17 |
| hydroxychloroquine | 51 (45.9%) | 60 (54.1%) | 1319 | 0 (0%) | 166 (100%) | 309 | 0 (0%) | 46 (100%) | 17 |
| aspirin | 1 (1%) | 102 (99%) | 1327 | 2 (1.2%) | 165 (98.8%) | 308 | 0 (0%) | 46 (100%) | 17 |
| baricitinib | 0 (0%) | 15 (100%) | 1415 | 0 (0%) | 49 (100%) | 426 | 0 (0%) | 46 (100%) | 17 |
| molnupiravir | - | - | 1430 | - | - | 475 | 0 (0%) | 42 (100%) | 21 |
| sotrovimab | - | - | 1430 | - | - | 475 | 0 (0%) | 42 (100%) | 21 |
| other drug | - | - | 1430 | - | - | - | - | - | 63 |
| Wave, Median [Q1, Q3] | |||||
|---|---|---|---|---|---|
| Variable | Outcome | 1 D614G | 2 Alpha | 4 Omicron | Significant Differences between Waves * |
| Age | Discharged | 62.0 [53.0, 71.0] | 58.0 [44.0, 67.8] | 66.0 [60.5, 72.0] | 1 vs. 2 p = 0.0017; 2 vs. 4 p = 0.0473 |
| Admitted | 65.0 [56.0, 74.0] | 61.0 [51.0, 71.0] | 53.0 [43.5, 73.0] | 1 vs. 2 p = 0.0273 | |
| Admitted+O2 | 71.0 [63.0, 78.0] | 68.0 [55.0, 76.0] | 67.5 [64.2, 74.5] | 1 vs. 2 p = 0.0125 | |
| Admitted+O2+died | 73.0 [65.0, 81.0] | 70.5 [58.2, 78.0] | 1 vs. 2 p = 0.0034 | ||
| Solid cancer stage | Discharged | 2.5 [1.0, 3.0] | 3.0 [3.0, 4.0] | 4.0 [3.0, 4.0] | 1 vs. 2 p = 0.0000, 1 vs. 4 p = 0.0041 |
| Admitted | 3.0 [2.0, 4.0] | 4.0 [3.0, 4.0] | 4.0 [3.0, 4.0] | 1 vs. 2 p = 0.0001 | |
| Admitted+O2 | 3.0 [1.0, 4.0] | 4.0 [3.0, 4.0] | 4.0 [3.0, 4.0] | 1 vs. 2 p = 0.0000, 1 vs. 4 p = 0.0146 | |
| Admitted+O2+died | 4.0 [2.0, 4.0] | 4.0 [3.0, 4.0] | 1 vs. 2 p = 0.0125 | ||
| Performance status | Discharged | 1.0 [0.0, 1.0] | 1.0 [1.0, 1.0] | 1.0 [0.2, 1.0] | |
| Admitted | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 1.0 [0.0, 1.0] | ||
| Admitted+O2 | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 2.0 [1.0, 2.0] | ||
| Admitted+O2+died | 2.0 [1.0, 3.0] | 1.0 [1.0, 2.0] | 1 vs. 2 p = 0.0075 | ||
| Total no. comorbidities | Discharged | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | |
| Admitted | 1.0 [1.0, 3.0] | 1.0 [0.0, 2.0] | 1.0 [0.0, 2.0] | ||
| Admitted+O2 | 2.0 [1.0, 3.0] | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.8] | 1 vs. 2 p = 0.0014 | |
| Admitted+O2+died | 2.0 [1.0, 4.0] | 2.0 [1.0, 3.0] | |||
| NEWS2 | Discharged | 1.0 [0.0, 2.0] | 1.0 [0.0, 3.0] | 1.0 [0.0, 1.0] | |
| Admitted | 2.0 [1.0, 3.0] | 2.0 [1.0, 4.0] | 1.0 [0.0, 2.0] | ||
| Admitted+O2 | 4.0 [2.0, 6.0] | 3.0 [1.0, 4.0] | 3.5 [2.0, 5.0] | 1 vs. 2 p = 0.0063 | |
| Admitted+O2+died | 4.0 [2.0, 7.0] | 5.5 [3.0, 8.0] | |||
| Albumin | Discharged | 40.0 [36.0, 43.0] | 41.0 [36.0, 44.0] | 40.0 [36.0, 43.2] | |
| Admitted | 37.0 [32.0, 41.0] | 39.0 [34.0, 42.0] | 37.0 [31.5, 43.0] | ||
| Admitted+O2 | 33.0 [28.0, 37.0] | 30.0 [23.5, 35.5] | 30.5 [25.0, 36.0] | 1 vs. 2 p = 0.0029 | |
| Admitted+O2+died | 32.0 [26.0, 38.0] | 31.0 [28.0, 38.0] | |||
| C-reactive protein | Discharged | 12.0 [4.6, 47.0] | 9.5 [3.0, 39.8] | 4.3 [3.1, 17.0] | |
| Admitted | 36.0 [12.0, 90.4] | 22.0 [6.4, 65.2] | 43.6 [7.8, 108.9] | ||
| Admitted+O2 | 70.0 [36.3, 127.0] | 57.1 [18.5, 122.4] | 62.4 [25.7, 128.1] | ||
| Admitted+O2+died | 91.0 [40.0, 168.0] | 118.8 [52.8, 201.7] | |||
| Lymphocytes | Discharged | 1.1 [0.7, 1.9] | 1.3 [0.9, 1.8] | 1.1 [0.6, 1.6] | |
| Admitted | 0.9 [0.5, 1.4] | 0.9 [0.6, 1.6] | 0.8 [0.5, 1.6] | ||
| Admitted+O2 | 0.9 [0.6, 1.4] | 0.8 [0.6, 1.2] | 1.1 [0.6, 1.4] | ||
| Admitted+O2+died | 0.7 [0.4, 1.2] | 0.6 [0.4, 1.2] | |||
| NLR | Discharged | 2.9 [1.9, 5.1] | 2.4 [1.6, 4.2] | 3.3 [1.7, 5.9] | |
| Admitted | 3.7 [1.9, 8.6] | 3.9 [2.1, 7.5] | 4.3 [2.6, 11.1] | ||
| Admitted+O2 | 4.5 [2.7, 9.3] | 4.6 [2.1, 7.6] | 5.4 [2.9, 6.8] | ||
| Admitted+O2+died | 5.9 [3.1, 13.0] | 6.6 [3.1, 12.3] | |||
| Neutrophils | Discharged | 3.5 [2.2, 4.9] | 3.2 [2.2, 4.5] | 3.5 [2.0, 4.2] | |
| Admitted | 3.9 [2.2, 6.3] | 3.7 [2.2, 5.8] | 3.9 [3.0, 9.2] | ||
| Admitted+O2 | 4.4 [2.9, 6.8] | 4.0 [2.1, 5.4] | 5.3 [3.6, 8.3] | ||
| Admitted+O2+died | 5.0 [3.0, 7.9] | 5.3 [2.6, 9.6] | |||
| Platelets | Discharged | 217.0 [170.0, 271.0] | 229.0 [188.0, 275.0] | 193.0 [130.5, 285.5] | |
| Admitted | 216.0 [149.2, 296.0] | 234.0 [151.8, 311.5] | 243.0 [196.5, 389.0] | ||
| Admitted+O2 | 192.0 [143.0, 264.0] | 204.0 [143.0, 275.2] | 202.5 [157.5, 312.8] | ||
| Admitted+O2+died | 183.5 [110.0, 270.0] | 181.0 [127.0, 287.0] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocki, O.; Zhou, C.; Rogado, J.; Huddar, P.; Shotton, R.; Tivey, A.; Albiges, L.; Angelakas, A.; Arnold, D.; Aung, T.; et al. An International Comparison of Presentation, Outcomes and CORONET Predictive Score Performance in Patients with Cancer Presenting with COVID-19 across Different Pandemic Waves. Cancers 2022, 14, 3931. https://doi.org/10.3390/cancers14163931
Wysocki O, Zhou C, Rogado J, Huddar P, Shotton R, Tivey A, Albiges L, Angelakas A, Arnold D, Aung T, et al. An International Comparison of Presentation, Outcomes and CORONET Predictive Score Performance in Patients with Cancer Presenting with COVID-19 across Different Pandemic Waves. Cancers. 2022; 14(16):3931. https://doi.org/10.3390/cancers14163931
Chicago/Turabian StyleWysocki, Oskar, Cong Zhou, Jacobo Rogado, Prerana Huddar, Rohan Shotton, Ann Tivey, Laurence Albiges, Angelos Angelakas, Dirk Arnold, Theingi Aung, and et al. 2022. "An International Comparison of Presentation, Outcomes and CORONET Predictive Score Performance in Patients with Cancer Presenting with COVID-19 across Different Pandemic Waves" Cancers 14, no. 16: 3931. https://doi.org/10.3390/cancers14163931
APA StyleWysocki, O., Zhou, C., Rogado, J., Huddar, P., Shotton, R., Tivey, A., Albiges, L., Angelakas, A., Arnold, D., Aung, T., Banfill, K., Baxter, M., Barlesi, F., Bayle, A., Besse, B., Bhogal, T., Boyce, H., Britton, F., Calles, A., ... on behalf of the ESMO Co-Care. (2022). An International Comparison of Presentation, Outcomes and CORONET Predictive Score Performance in Patients with Cancer Presenting with COVID-19 across Different Pandemic Waves. Cancers, 14(16), 3931. https://doi.org/10.3390/cancers14163931









