Efficacy of S-1 or Capecitabine Plus Oxaliplatin Adjuvant Chemotherapy for Stage II or III Gastric Cancer after Curative Gastrectomy: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
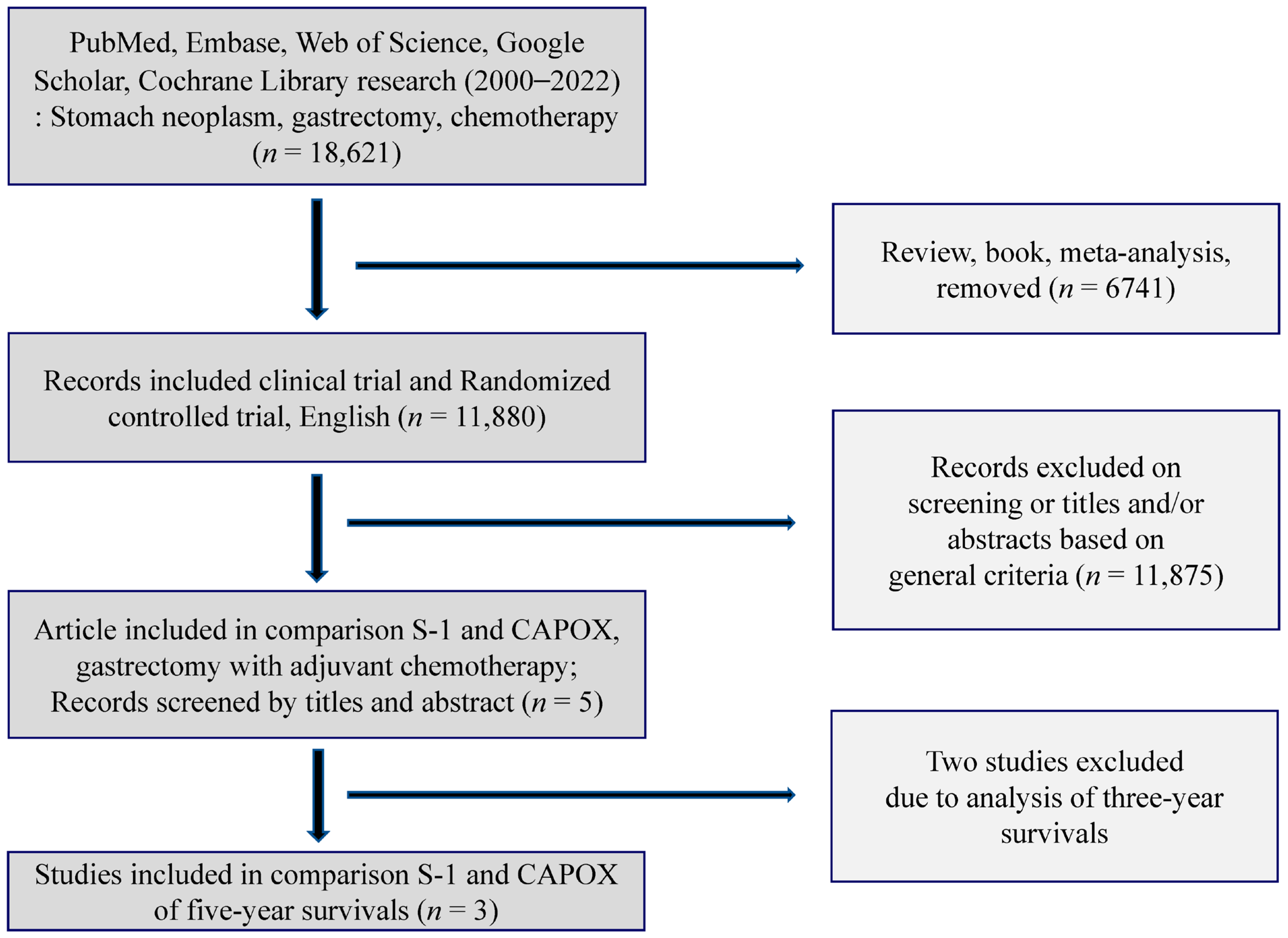
2.1. Search Scheme & Selection of Studies
2.2. Study Quality Assessment
2.3. Statistical Analysis of Data
3. Results
3.1. Literature Search and Quality of the Selected Studies
3.2. 5-Year OS of S-1 and CAPOX
3.3. 5-Year DFS of S-1 and CAPOX
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Guideline Committee of the Korean Gastric Cancer Association (KGCA); Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: An Evidence-based, Multi-disciplinary Approach. J. Gastric Cancer 2019, 19, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Almhanna, K.; Bentrem, D.J.; Chao, J.; Das, P.; Denlinger, C.S.; Fanta, P.; Farjah, F.; Fuchs, C.S.; et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2016, 14, 1286–1312. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of the People’s Republic of China. Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version). Chin. J. Cancer Res. 2019, 31, 707–737. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H. Current status of adjuvant chemotherapy for gastric cancer. World J. Gastrointest. Oncol. 2019, 11, 679–685. [Google Scholar] [CrossRef]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Niedzwiecki, D.; Mamon, H.J.; Tepper, J.E.; Ye, X.; Swanson, R.S.; Enzinger, P.C.; Haller, D.G.; Dragovich, T.; Alberts, S.R.; et al. Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy with Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer: Results From CALGB 80101 (Alliance). J. Clin. Oncol. 2017, 35, 3671–3677. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Kim, Y.-W.; Yang, H.-K.; Chung, H.; Park, Y.-K.; Lee, K.H.; Lee, K.-W.; Kim, Y.H.; Noh, S.-I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Kim, I.H.; Park, S.S.; Lee, C.M.; Kim, M.C.; Kwon, I.K.; Min, J.S.; Kim, H.-I.; Lee, H.H.; Lee, S.-I.; Chae, H. Efficacy of Adjuvant S-1 Versus XELOX Chemotherapy for Patients with Gastric Cancer After D2 Lymph Node Dissection: A Retrospective, Multi-Center Observational Study. Ann. Surg. Oncol. 2018, 25, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Park, S.J.; Lee, J.; Park, C.H.; Song, K.Y.; Lee, H.H.; Seo, H.S.; Jung, Y.J.; Park, J.M.; Lee, S.H.; et al. Efficacy of capecitabine and oxaliplatin versus S-1 as adjuvant chemotherapy in gastric cancer after D2 lymph node dissection according to lymph node ratio and N stage. BMC Cancer 2019, 19, 1232. [Google Scholar] [CrossRef]
- Lee, C.M.; Yoo, M.W.; Son, Y.G.; Oh, S.J.; Kim, J.H.; Kim, H.I.; Park, J.M.; Hur, H.; Jee, Y.S.; Hwang, H.; et al. Long-term Efficacy of S-1 Monotherapy or Capecitabine Plus Oxaliplatin as Adjuvant Chemotherapy for Patients with Stage II or III Gastric Cancer after Curative Gastrectomy: A Propensity Score-Matched Multicenter Cohort Study. J. Gastric Cancer 2020, 20, 152–164. [Google Scholar] [CrossRef]
- Oh, S.E.; An, J.Y.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M. Comparison of Long-Term Efficacy in S-1 and Capecitabine With Oxaliplatin as Adjuvant Chemotherapy for Patients With Gastric Cancer After Curative Surgery: A Retrospective, Single-Center Observational Study. Technol. Cancer Res. Treat. 2021, 20, 15330338211039679. [Google Scholar] [CrossRef]
- Cho, J.H.; Lim, J.Y.; Cho, J.Y. Comparison of capecitabine and oxaliplatin with S-1 as adjuvant chemotherapy in stage III gastric cancer after D2 gastrectomy. PLoS ONE 2017, 12, e0186362. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, Y.-W. Surgeon’s role for gastric gastrointestinal stromal tumor in imatinib era. Korean J. Clin. Oncol. 2013, 9, 5–12. [Google Scholar] [CrossRef]
- Iacovelli, R.; Pietrantonio, F.; Maggi, C.; de Braud, F.; Di Bartolomeo, M. Combination or single-agent chemotherapy as adjuvant treatment of gastric cancer: A systematic review and meta-analysis of published trials. Crit. Rev. Oncol. Hematol. 2016, 98, 24–28. [Google Scholar] [CrossRef]
- Eom, S.S.; Choi, W.; Eom, B.W.; Park, S.H.; Kim, S.J.; Kim, Y.I.; Yoon, H.M.; Lee, J.Y.; Kim, C.G.; Kim, H.K.; et al. A Comprehensive and Comparative Review of Global Gastric Cancer Treatment Guidelines. J. Gastric Cancer 2022, 22, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.-H.; Yang, H.-K.; Kim, H.; Kim, W.H.; Kim, Y.-W.; Kook, M.-C.; Park, Y.-K.; Kim, H.-H.; Lee, H.S.; Lee, K.H.; et al. Predictive test for chemotherapy response in resectable gastric cancer: A multi-cohort, retrospective analysis. Lancet Oncol. 2018, 19, 629–638. [Google Scholar] [CrossRef]
- Yoshida, K.; Kodera, Y.; Kochi, M.; Ichikawa, W.; Kakeji, Y.; Sano, T.; Nagao, N.; Takahashi, M.; Takagane, A.; Watanabe, T.; et al. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lim, D.H.; Sohn, T.S.; Lee, J.; Zang, D.Y.; Kim, S.T.; Kang, J.H.; Oh, S.Y.; Hwang, I.G.; Ji, J.H.; et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: The ARTIST 2 trial(☆). Ann. Oncol. 2021, 32, 368–374. [Google Scholar] [CrossRef]


| 1st Author | Year of Publication | No of Participating Institutes | Study Design | Median Follow-Up Periods (Months) | TNM Stage | No of S-1 Cases | No of CAPOX Cases | 5 yr OS HR (S-1 vs. CAPOX, 95% CI) | p Value | 5 yr DFS, HR (S-1 vs. CAPOX, 95% CI) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shin et al. [15] | 2019 | 1 | PSM | 52.3 | II & III | 110 | 110 | 0.71 (0.40–1.26) | 0.240 | 0.65 (0.39–1.09) | 0.101 |
| II | 24 | 23 | 0.58 (0.05–6.40) | 0.655 | 0.35 (0.04–3.34) | 0.360 | |||||
| III | 86 | 87 | 0.73 (0.40–1.31) | 0.285 | 0.67 (0.40–1.13) | 0.133 | |||||
| Lee et al. [16] | 2020 | 27 | PSM | 59.0 | II & III | 429 | 155 | 0.986 (0.647–1.504) | 0.949 | 1.008 (0.728–1.395) | 0.963 |
| II | 143 | 50 | 1.662 (0.569–4.850) | 0.353 | 1.846 (0.693–4.919) | 0.220 | |||||
| III | 286 | 105 | 0.859 (0.542–1.361) | 0.517 | 0.942 (0.664–1.337) | 0.738 | |||||
| Oh et al. [17] | 2021 | 1 | Observational study | 55.0 | II & III | 761 | 634 | 1.000 (0.776–1.284) | 0.989 | 1.075 (0.869–1.333) | 0.500 |
| II | 470 | 274 | 0.471 (0.249–0.890) | 0.021 | 0.733 (0.434–1.238) | 0.245 | |||||
| III | 291 | 360 | 1.045 (0.792–1.379) | 0.756 | 1.186 (0.921–1.527) | 0.186 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-H.; Kim, R.B.; Oh, S.E.; An, J.Y.; Seo, K.W.; Min, J.-S. Efficacy of S-1 or Capecitabine Plus Oxaliplatin Adjuvant Chemotherapy for Stage II or III Gastric Cancer after Curative Gastrectomy: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3940. https://doi.org/10.3390/cancers14163940
Jeong S-H, Kim RB, Oh SE, An JY, Seo KW, Min J-S. Efficacy of S-1 or Capecitabine Plus Oxaliplatin Adjuvant Chemotherapy for Stage II or III Gastric Cancer after Curative Gastrectomy: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(16):3940. https://doi.org/10.3390/cancers14163940
Chicago/Turabian StyleJeong, Sang-Ho, Rock Bum Kim, Sung Eun Oh, Ji Yeong An, Kyung Won Seo, and Jae-Seok Min. 2022. "Efficacy of S-1 or Capecitabine Plus Oxaliplatin Adjuvant Chemotherapy for Stage II or III Gastric Cancer after Curative Gastrectomy: A Systematic Review and Meta-Analysis" Cancers 14, no. 16: 3940. https://doi.org/10.3390/cancers14163940
APA StyleJeong, S.-H., Kim, R. B., Oh, S. E., An, J. Y., Seo, K. W., & Min, J.-S. (2022). Efficacy of S-1 or Capecitabine Plus Oxaliplatin Adjuvant Chemotherapy for Stage II or III Gastric Cancer after Curative Gastrectomy: A Systematic Review and Meta-Analysis. Cancers, 14(16), 3940. https://doi.org/10.3390/cancers14163940








