Tumor Infiltrating Lymphocytes in Multi-National Cohorts of Ductal Carcinoma In Situ (DCIS) of Breast
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical and Histological Data
2.2. Ancestry Analysis
2.3. Statistical Analysis
3. Results
3.1. Analysis for Tumor-Infiltrating Lymphocytes (TILs) in Multi-Ethnic DCIS Cases
3.1.1. Combined Analysis of All Cohorts
3.1.2. Analysis of TILs in Individual Cohorts
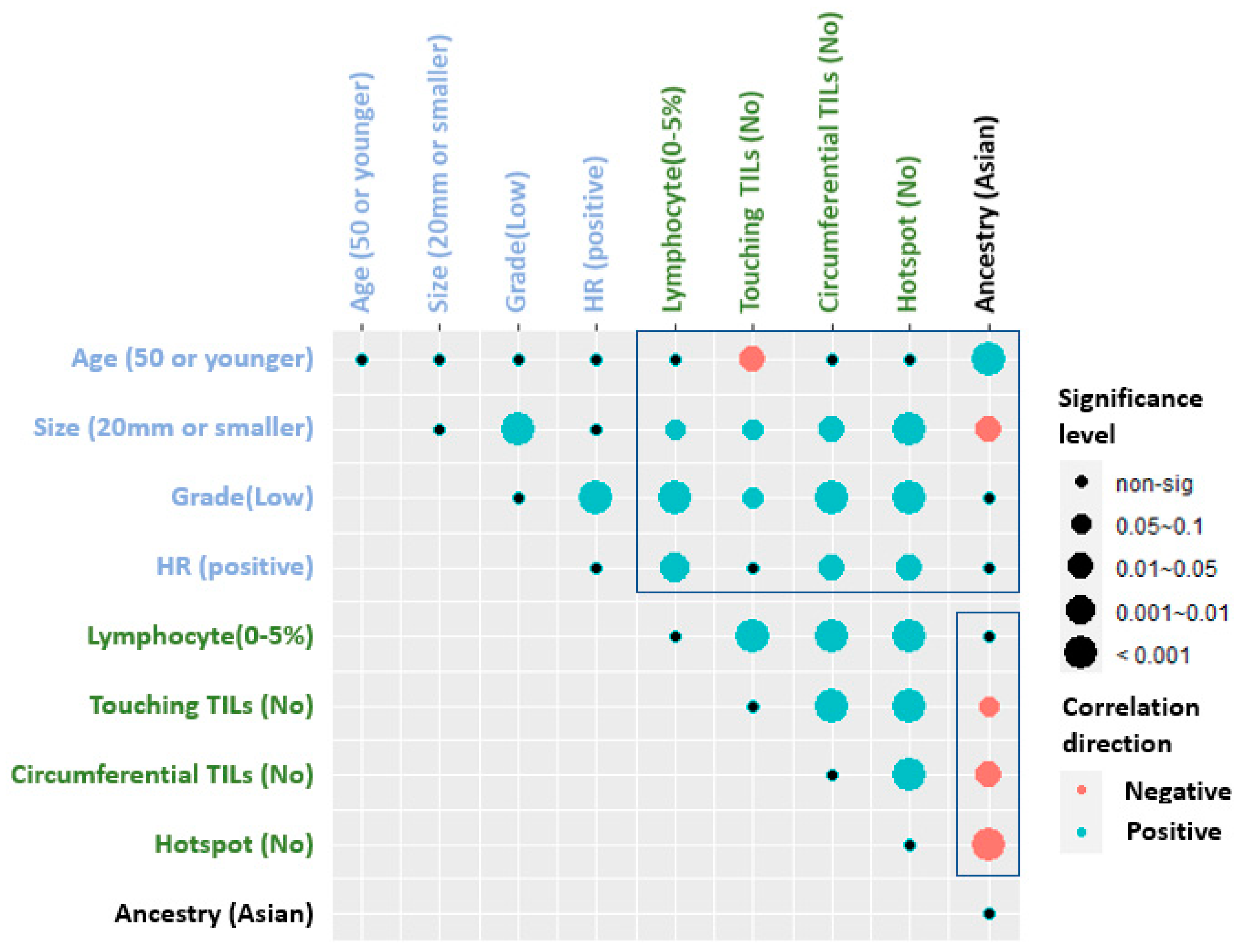
3.1.3. Correlation of TILs Assessments with Clinical and Histologic Parameters
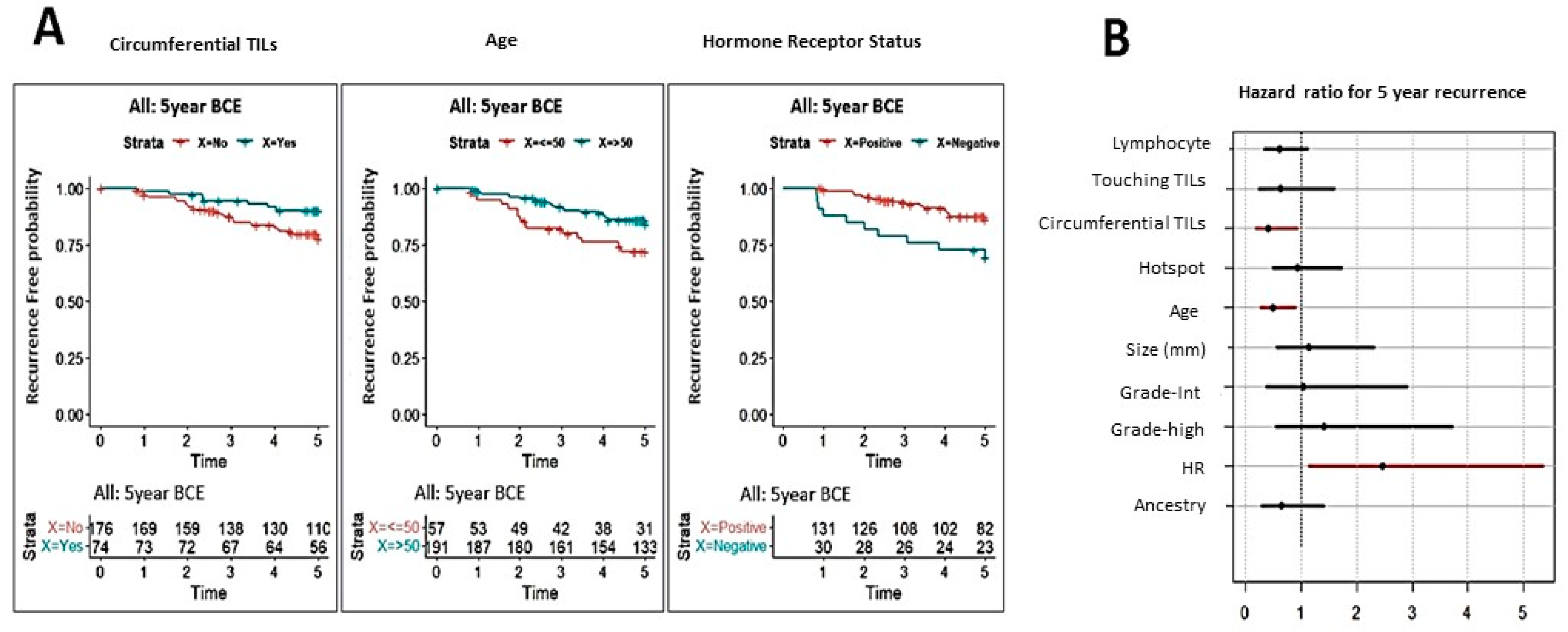
3.1.4. Correlation of TILs Assessments with Clinical Outcome
3.1.5. Impact of Ethnicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Goldstein, L.J.; Sparano, J.A.; Demaria, S.; Badve, S.S. Tumor infiltrating lymphocytes (TILs) improve prognosis in patients with triple negative breast cancer (TNBC). Oncoimmunology 2015, 4, e985930. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef]
- Trinh, A.; Gil Del Alcazar, C.R.; Shukla, S.A.; Chin, K.; Chang, Y.H.; Thibault, G.; Eng, J.; Jovanovic, B.; Aldaz, C.M.; Park, S.Y.; et al. Genomic Alterations during the In Situ to Invasive Ductal Breast Carcinoma Transition Shaped by the Immune System. Mol. Cancer Res. 2020, 19, 623–635. [Google Scholar] [CrossRef]
- Pandey, J.P.; Namboodiri, A.M.; Kistner-Griffin, E. IgG and FcgammaR genotypes and humoral immunity to mucin 1 in prostate cancer. Hum. Immunol. 2013, 74, 1030–1033. [Google Scholar] [CrossRef]
- Yeyeodu, S.T.; Kidd, L.R.; Kimbro, K.S. Protective Innate Immune Variants in Racial/Ethnic Disparities of Breast and Prostate Cancer. Cancer Immunol. Res. 2019, 7, 1384–1389. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, C.; Wang, X.; Wu, X.; Zhu, Z.; Liu, B.; Su, L. Association between TLR4 (+896A/G and +1196C/T) polymorphisms and gastric cancer risk: An updated meta-analysis. PLoS ONE 2014, 9, e109605. [Google Scholar] [CrossRef]
- Hong, C.C.; Sucheston-Campbell, L.E.; Liu, S.; Hu, Q.; Yao, S.; Lunetta, K.L.; Haddad, S.A.; Ruiz-Narvaez, E.A.; Bensen, J.T.; Cheng, T.D.; et al. Genetic Variants in Immune-Related Pathways and Breast Cancer Risk in African American Women in the AMBER Consortium. Cancer Epidemiol. Biomark. Prev. 2018, 27, 321–330. [Google Scholar] [CrossRef]
- Liu, Y.; Colditz, G.A.; Gehlert, S.; Goodman, M. Racial disparities in risk of second breast tumors after ductal carcinoma in situ. Breast Cancer Res. Treat. 2014, 148, 163–173. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group; Correa, C.; McGale, P.; Taylor, C.; Wang, Y.; Clarke, M.; Davies, C.; Peto, R.; Bijker, N.; Solin, L.; et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J. Natl. Cancer Inst. Monogr. 2010, 2010, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Pinder, S.E.; Ellis, I.O.; Forsyth, S.; Bundred, N.J.; Forbes, J.F.; Bishop, H.; Fentiman, I.S.; George, W.D. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011, 12, 21–29. [Google Scholar] [CrossRef]
- Miligy, I.; Mohan, P.; Gaber, A.; Aleskandarany, M.A.; Nolan, C.C.; Diez-Rodriguez, M.; Mukherjee, A.; Chapman, C.; Ellis, I.O.; Green, A.R.; et al. Prognostic significance of tumour infiltrating B lymphocytes in breast ductal carcinoma in situ. Histopathology 2017, 71, 258–268. [Google Scholar] [CrossRef]
- Farolfi, A.; Petracci, E.; Serra, L.; Ravaioli, A.; Bravaccini, S.; Ravaioli, S.; Tumedei, M.M.; Ulivi, P.; Canale, M.; Puccetti, M.; et al. Tumor-Infiltrating Lymphocytes (TILs) and Risk of a Second Breast Event After a Ductal Carcinoma in situ. Front. Oncol. 2020, 10, 1486. [Google Scholar] [CrossRef]
- Darvishian, F.; Ozerdem, U.; Adams, S.; Chun, J.; Pirraglia, E.; Kaplowitz, E.; Guth, A.; Axelrod, D.; Shapiro, R.; Price, A.; et al. Tumor-Infiltrating Lymphocytes in a Contemporary Cohort of Women with Ductal Carcinoma In Situ (DCIS). Ann. Surg. Oncol. 2019, 26, 3337–3343. [Google Scholar] [CrossRef]
- Beguinot, M.; Dauplat, M.M.; Kwiatkowski, F.; Lebouedec, G.; Tixier, L.; Pomel, C.; Penault-Llorca, F.; Radosevic-Robin, N. Analysis of tumour-infiltrating lymphocytes reveals two new biologically different subgroups of breast ductal carcinoma in situ. BMC Cancer 2018, 18, 129. [Google Scholar] [CrossRef]
- Agahozo, M.C.; van Bockstal, M.R.; Groenendijk, F.H.; van den Bosch, T.P.P.; Westenend, P.J.; van Deurzen, C.H.M. Ductal carcinoma in situ of the breast: Immune cell composition according to subtype. Mod. Pathol. 2020, 33, 196–205. [Google Scholar] [CrossRef]
- Gil Del Alcazar, C.R.; Huh, S.J.; Ekram, M.B.; Trinh, A.; Liu, L.L.; Beca, F.; Zi, X.; Kwak, M.; Bergholtz, H.; Su, Y.; et al. Immune Escape in Breast Cancer During In Situ to Invasive Carcinoma Transition. Cancer Discov. 2017, 7, 1098–1115. [Google Scholar] [CrossRef]
- Pruneri, G.; Lazzeroni, M.; Bagnardi, V.; Tiburzio, G.B.; Rotmensz, N.; DeCensi, A.; Guerrieri-Gonzaga, A.; Vingiani, A.; Curigliano, G.; Zurrida, S.; et al. The prevalence and clinical relevance of tumor-infiltrating lymphocytes (TILs) in ductal carcinoma in situ of the breast. Ann. Oncol. 2017, 28, 321–328. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Pang, J.B.; Byrne, D.J.; Lakhani, S.R.; Cummings, M.C.; Campbell, I.G.; Mann, G.B.; Gorringe, K.L.; Fox, S.B. Relationship of the Breast Ductal Carcinoma In Situ Immune Microenvironment with Clinicopathological and Genetic Features. Clin. Cancer Res. 2017, 23, 5210–5217. [Google Scholar] [CrossRef]
- Toss, M.S.; Miligy, I.; Al-Kawaz, A.; Alsleem, M.; Khout, H.; Rida, P.C.; Aneja, R.; Green, A.R.; Ellis, I.O.; Rakha, E.A. Prognostic significance of tumor-infiltrating lymphocytes in ductal carcinoma in situ of the breast. Mod. Pathol. 2018, 31, 1226–1236. [Google Scholar] [CrossRef]
- Komforti, M.; Badve, S.S.; Harmon, B.; Lo, Y.; Fineberg, S. Tumour-infiltrating lymphocytes in ductal carcinoma in situ (DCIS)-assessment with three different methodologies and correlation with Oncotype DX DCIS Score. Histopathology 2020, 77, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; Zabidi, M.M.A.; Chong, B.K.; Meng, M.Y.; Ng, P.S.; Hasan, S.N.; Sandey, B.; Bahnu, S.; Rajadurai, P.; Yip, C.H.; et al. Germline APOBEC3B deletion increases somatic hypermutation in Asian breast cancer that is associated with Her2 subtype, PIK3CA mutations, and immune activation. Int. J. Cancer 2021, 148, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; Zabidi, M.M.A.; Ng, P.S.; Meng, M.Y.; Hasan, S.N.; Sandey, B.; Sammut, S.J.; Yip, C.H.; Rajadurai, P.; Rueda, O.M.; et al. The molecular landscape of Asian breast cancers reveals clinically relevant population-specific differences. Nat. Commun. 2020, 11, 6433. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Lin, P.; Higano, C.S.; Sternberg, C.N.; Sonpavde, G.; Tombal, B.; Templeton, A.J.; Fizazi, K.; Phung, D.; Wong, E.K.; et al. Development and validation of a prognostic model for overall survival in chemotherapy-naive men with metastatic castration-resistant prostate cancer. Ann. Oncol. 2018, 29, 2200–2207. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Zhang, G.; Zhang, Z.; Luo, Y.; Wang, F.; Wang, S.; Che, Y.; Zeng, Q.; Sun, N.; et al. Clinical significance and inflammatory landscapes of a novel recurrence-associated immune signature in early-stage lung adenocarcinoma. Cancer Lett. 2020, 479, 31–41. [Google Scholar] [CrossRef]
- Doshi, R.P.; Chen, K.; Wang, F.; Schwartz, H.; Herzog, A.; Aseltine, R.H., Jr. Identifying risk factors for mortality among patients previously hospitalized for a suicide attempt. Sci. Rep. 2020, 10, 15223. [Google Scholar] [CrossRef] [PubMed]
- Roussel, E.; Peeters, E.; Vanthoor, J.; Bozzini, G.; Muneer, A.; Ayres, B.; Sri, D.; Watkin, N.; Bhattar, R.; Parnham, A.; et al. Predictors of local recurrence and its impact on survival after glansectomy for penile cancer: Time to challenge the dogma? BJU Int. 2021, 127, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, X.; Li, Y.; Xia, E.; Qin, Y.; Liang, S.; Xu, F.; Liang, D.; Zeng, C.; Liu, Z. Prediction and Risk Stratification of Kidney Outcomes in IgA Nephropathy. Am. J. Kidney Dis. 2019, 74, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Canner, J.K.; Mathioudakis, N.; Lippincott, C.; Sherman, R.L.; Abularrage, C.J. Incidence and Risk Factors Associated With Ulcer Recurrence Among Patients With Diabetic Foot Ulcers Treated in a Multidisciplinary Setting. J. Surg. Res. 2020, 246, 243–250. [Google Scholar] [CrossRef]
- Miller, A. Subset Selection in Regression, 2nd ed.; Chapman and Hall: London, UK; CRC: Boca Raton, FL, USA, 2002. [Google Scholar]
- Thompson, E.; Taube, J.M.; Elwood, H.; Sharma, R.; Meeker, A.; Warzecha, H.N.; Argani, P.; Cimino-Mathews, A.; Emens, L.A. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol 2016, 29, 249–258. [Google Scholar] [CrossRef]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar] [CrossRef]
- Campbell, M.J.; Baehner, F.; O’Meara, T.; Ojukwu, E.; Han, B.; Mukhtar, R.; Tandon, V.; Endicott, M.; Zhu, Z.; Wong, J.; et al. Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res. Treat. 2017, 161, 17–28. [Google Scholar] [CrossRef]
- Knopfelmacher, A.; Fox, J.; Lo, Y.; Shapiro, N.; Fineberg, S. Correlation of histopathologic features of ductal carcinoma in situ of the breast with the oncotype DX DCIS score. Mod. Pathol. 2015, 28, 1167–1173. [Google Scholar] [CrossRef]
- Thike, A.A.; Chen, X.; Koh, V.C.Y.; Binte Md Nasir, N.D.; Yeong, J.P.S.; Bay, B.H.; Tan, P.H. Higher densities of tumour-infiltrating lymphocytes and CD4(+) T cells predict recurrence and progression of ductal carcinoma in situ of the breast. Histopathology 2020, 76, 852–864. [Google Scholar] [CrossRef]
- Agahozo, M.C.; Hammerl, D.; Debets, R.; Kok, M.; van Deurzen, C.H.M. Tumor-infiltrating lymphocytes and ductal carcinoma in situ of the breast: Friends or foes? Mod. Pathol. 2018, 31, 1012–1025. [Google Scholar] [CrossRef]
- Liu, Y.; West, R.; Weber, J.D.; Colditz, G.A. Race and risk of subsequent aggressive breast cancer following ductal carcinoma in situ. Cancer 2019, 125, 3225–3233. [Google Scholar] [CrossRef] [PubMed]
- Taparra, K.; Fukui, J.; Killeen, J.; Sumida, K.; Loo, L.W.M.; Hernandez, B.Y. Racial and Ethnic Disparities in Rates of Invasive Second Breast Cancer Among Women With Ductal Carcinoma In Situ in Hawai’i. JAMA Netw. Open 2021, 4, e2128977. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 256) | |
|---|---|
| Age | |
| N-Miss | 3 |
| <=50 | 60 (23.7%) |
| >50 | 193 (76.3%) |
| Size (mm) | |
| N-Miss | 50 |
| <=20 | 131 (63.6%) |
| >20 | 75 (36.4%) |
| Grade | |
| N-Miss | 13 |
| Low | 32 (13.2%) |
| Intermediate | 90 (37.0%) |
| High | 121 (49.8%) |
| HR | |
| N-Miss | 86 |
| Positive | 137 (80.6%) |
| Negative | 33 (19.4%) |
| Recurrence | |
| N | 189 (73.8%) |
| Y | 67 (26.2%) |
| Lymphocyte | |
| 0–5% | 101 (39.5%) |
| >5% | 155 (60.5%) |
| Touching TILs | |
| 0 | 214 (83.6%) |
| >0 | 42 (16.4%) |
| Circumferential TILs | |
| No | 181 (70.7%) |
| Yes | 75 (29.3%) |
| Hotspot | |
| No | 160 (62.5%) |
| Dense | 96 (37.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badve, S.S.; Cho, S.; Lu, X.; Cao, S.; Ghose, S.; Thike, A.A.; Tan, P.H.; Ocal, I.T.; Generali, D.; Zanconati, F.; et al. Tumor Infiltrating Lymphocytes in Multi-National Cohorts of Ductal Carcinoma In Situ (DCIS) of Breast. Cancers 2022, 14, 3916. https://doi.org/10.3390/cancers14163916
Badve SS, Cho S, Lu X, Cao S, Ghose S, Thike AA, Tan PH, Ocal IT, Generali D, Zanconati F, et al. Tumor Infiltrating Lymphocytes in Multi-National Cohorts of Ductal Carcinoma In Situ (DCIS) of Breast. Cancers. 2022; 14(16):3916. https://doi.org/10.3390/cancers14163916
Chicago/Turabian StyleBadve, Sunil S., Sanghee Cho, Xiaoyu Lu, Sha Cao, Soumya Ghose, Aye Aye Thike, Puay Hoon Tan, Idris Tolgay Ocal, Daniele Generali, Fabrizio Zanconati, and et al. 2022. "Tumor Infiltrating Lymphocytes in Multi-National Cohorts of Ductal Carcinoma In Situ (DCIS) of Breast" Cancers 14, no. 16: 3916. https://doi.org/10.3390/cancers14163916
APA StyleBadve, S. S., Cho, S., Lu, X., Cao, S., Ghose, S., Thike, A. A., Tan, P. H., Ocal, I. T., Generali, D., Zanconati, F., Harris, A. L., Ginty, F., & Gökmen-Polar, Y. (2022). Tumor Infiltrating Lymphocytes in Multi-National Cohorts of Ductal Carcinoma In Situ (DCIS) of Breast. Cancers, 14(16), 3916. https://doi.org/10.3390/cancers14163916









