A Review of Medicinal Plants of the Himalayas with Anti-Proliferative Activity for the Treatment of Various Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
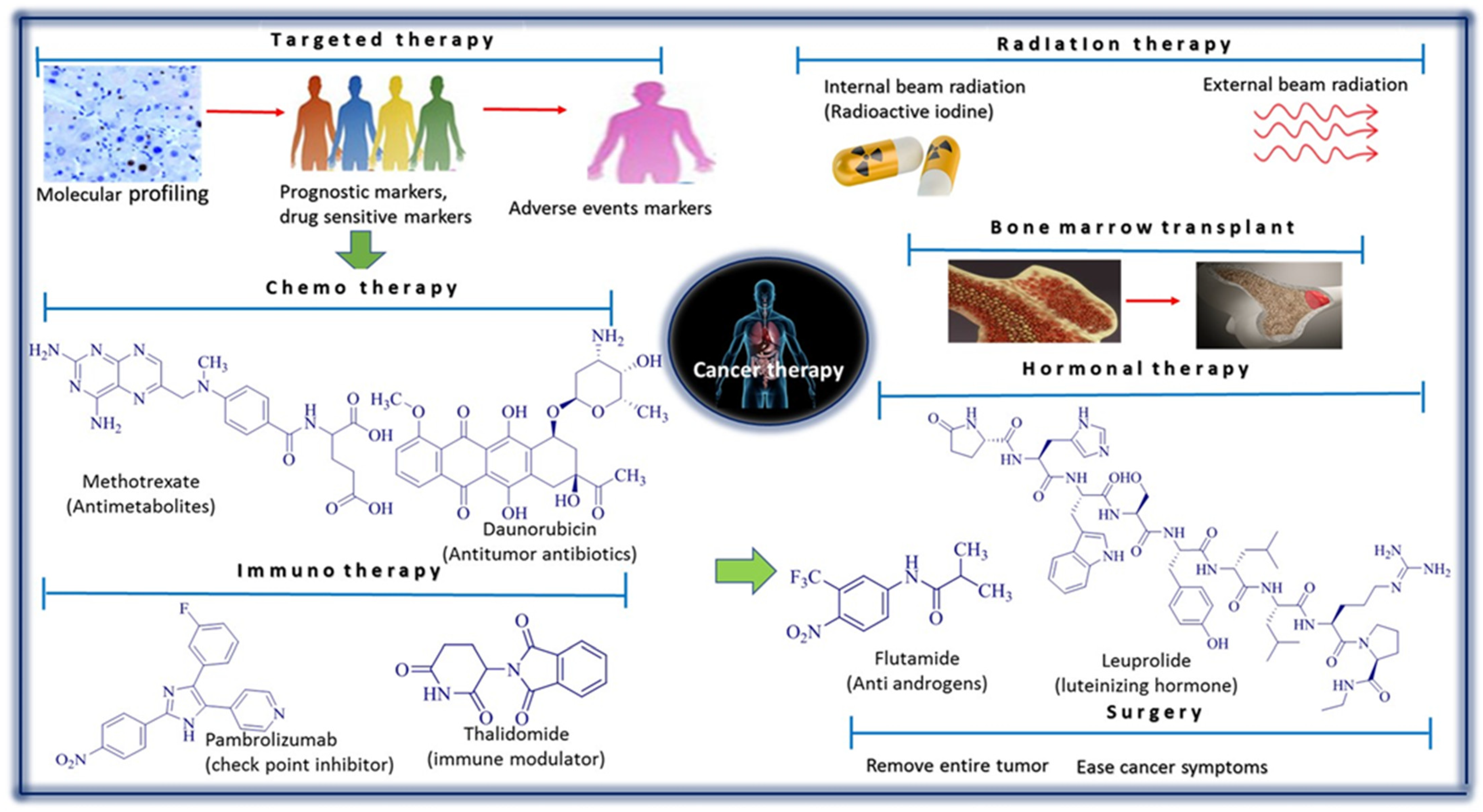
2. Major Anticancer Agents Obtained from Medicinal Plants
2.1. Vinca Alkaloids
2.2. Podophyllotoxin Derivatives
2.3. Taxanes
2.4. Camptothecin Derivatives
2.5. Homoharringtonine
2.6. Saponin Extract from Albizia Lebbeck Plant
2.7. Isoquinoline Alkaloid Extract from Annona squamosa
2.8. Shikonin and its Derivatives
2.9. Calotropin in Asclepias curassavica
2.10. Shatavarin IV in Asparagus racemosus
2.11. Stigmasterol in Bacopa monnieri Linn
2.12. Methanolic Extract of Bauhinia Racemose Plant
3. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aqil, F.; Munagala, R.; Agrawal, A.K.; Gupta, R. Anticancer phytocompounds: Experimental and clinical updates. New Look Phytomed. 2019, 1, 237–272. [Google Scholar]
- Jones, P.A.; Baylin, S.B. The epigenomics of cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Kalluri, R. Cancer without disease. Nature 2004, 427, 787. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 2005, 15, 87–96. [Google Scholar] [CrossRef]
- Varmus, H.; Kumar, H.S. Addressing the growing international challenge of cancer: A multinational perspective. Sci. Transl. Med. 2013, 5, 175cm2. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Mohammad, T.; Islam, A.; Hassan, M.I. Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochim. et Biophys. Acta BBA-Rev. Cancer 2021, 1876, 188568. [Google Scholar] [CrossRef]
- Ogle, K.S.; Swanson, G.M.; Woods, N.; Azzouz, F. Cancer and comorbidity: Redefining chronic diseases. Cancer 2000, 88, 653–663. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current challenges in cancer treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef]
- Hussain, M.; Khera, R.A.; Iqbal, J.; Khalid, M.; Hanif, M.A. Phytochemicals: Key to effective anticancer drugs. Mini-Rev. Org. Chem. 2019, 16, 141–158. [Google Scholar] [CrossRef]
- Sudhakar, A. History of cancer, ancient and modern treatment methods. J. Cancer Sci. Ther. 2009, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharipov, M.; Turaev, A.; Azizov, S.; Azizov, I.; Makhado, E.; Rahdar, A.; Kumar, D.; Pandey, S. Polymer-Based Hybrid Nanoarchitectures for Cancer Therapy Applications. Polymers 2022, 14, 3027. [Google Scholar] [CrossRef]
- Shirzad, H.; Taji, F.; Rafieian-Kopaei, M. Correlation between antioxidant activity of garlic extracts and WEHI-164 fibrosarcoma tumor growth in BALB/c mice. J. Med. Food 2011, 14, 969–974. [Google Scholar] [CrossRef]
- Moradi, M.T.; Karimi, A.; Rafieian-Kopaei, M.; Kheiri, S.; Saedi-Marghmaleki, M. The inhibitory effects of myrtle (Myrtus communis) extract on Herpes simplex virus-1 replication in Baby Hamster Kidney cells. J. Shahrekord Uuniversity Med. Sci. 2011, 12, 54–62. [Google Scholar]
- Kumar, S.; Sharma, A.K.; Lalhlenmawia, H.; Kumar, D. Natural Compounds Targeting Major Signaling Pathways in Lung Cancer. Target. Cell. Signal. Pathw. Lung Dis. 2021, 1, 821–846. [Google Scholar]
- Asgary, S.; Moshtaghian, S.J.; Setorki, M.; Kazemi, S.; Rafieian-Kopaei, M.; Adelnia, A.; Shamsi, F. Hypoglycaemic and hypolipidemic effects of pumpkin (Cucurbita pepo L.) on alloxan-induced diabetic rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2620–2626. [Google Scholar]
- Muthu, C.; Ayyanar, M.; Raja, N.; Ignacimuthu, S. Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J. Ethnobiol. Ethnomedicine 2006, 2, 43. [Google Scholar] [CrossRef]
- Elujoba, A.A.; Odeleye, O.M.; Ogunyemi, C.M. Traditional medicine development for medical and dental primary health care delivery system in Africa. Afr. J. Tradit. Complementary Altern. Med. 2005, 2, 46–61. [Google Scholar] [CrossRef]
- Yadav, R.; Das, J.; Lalhlenmawia, H.; Tonk, R.K.; Singh, L.; Kumar, D. Targeting cancer using phytoconstituents-based drug delivery. In Advanced Drug Delivery Systems in the Management of Cancer; Academic Press: Cambridge, MA, USA, 2021; pp. 499–508. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Kuruppu, A.I.; Paranagama, P.; Goonasekara, C.L. Medicinal plants commonly used against cancer in traditional medicine formulae in Sri Lanka. Saudi Pharm. J. 2019, 27, 565–573. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed]
- Gezici, S.; Şekeroğlu, N. Current perspectives in the application of medicinal plants against cancer: Novel therapeutic agents. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2019, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Ahmad, Z. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Lee, K.H.; Xiao, Z. Podophyllotoxin and analogs. In Anticancer Agents from Natural Products; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; Brunner-Routledge Psychology Press: New York, NY, USA, 2005. [Google Scholar]
- Esghaei, M.; Ghaffari, H.; Esboei, B.R.; Tapeh, Z.E.; Salim, F.B.; Motevalian, M. Evaluation of anticancer activity of Camellia sinensis in the Caco-2 colorectal cancer cell line. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 1697. [Google Scholar]
- Sharangi, A.B. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—A review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Subbarayan, P.R.; Sarkar, M.; Impellizzeri, S.; Raymo, F.; Lokeshwar, B.L.; Kumar, P.; Ardalan, B. Anti-proliferative and anti-cancer properties of Achyranthes aspera: Specific inhibitory activity against pancreatic cancer cells. J. Ethnopharmacol. 2010, 131, 78–82. [Google Scholar] [CrossRef]
- Chakraborty, A.; Brantner, A.; Mukainaka, T.; Nobukuni, Y.; Kuchide, M.; Konoshima, T.; Nishino, H. Cancer chemopreventive activity of Achyranthes aspera leaves on Epstein–Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett. 2002, 177, 1–5. [Google Scholar] [CrossRef]
- Niyomtham, N.; Koontongkaew, S.; Yingyongnarongkul, B.E.; Utispan, K. Apis mellifera propolis enhances apoptosis and invasion inhibition in head and neck cancer cells. PeerJ 2021, 9, e12139. [Google Scholar] [CrossRef] [PubMed]
- Teerasripreecha, D.; Phuwapraisirisan, P.; Puthong, S.; Kimura, K.; Okuyama, M.; Mori, H.; Chanchao, C. In vitro antiproliferative/cytotoxic activity on cancer cell lines of a cardanol and a cardol enriched from Thai Apis mellifera propolis. BMC Complementary Altern. Med. 2012, 12, 27. [Google Scholar] [CrossRef]
- Kumar, R.A.; Sridevi, K.; Kumar, N.V.; Nanduri, S.; Rajagopal, S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef]
- Kumar, A.; Shashni, S.; Kumar, P.; Pant, D.; Singh, A.; Verma, R.K. Phytochemical constituents, distributions and traditional usages of Arnebia euchroma: A review. J. Ethnopharmacol. 2021, 271, 113896. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Sehar, I.; Bhushan, S.; Gupta, B.D.; Saxena, A.K. Activation of caspases and poly (ADP-ribose) polymerase cleavage to induce apoptosis in leukemia HL-60 cells by Inula racemosa. Toxicol. Vitr. 2010, 24, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Leung, K.N. In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 2007, 252, 43–54. [Google Scholar] [PubMed]
- Prakash, O.M.; Kumar, A.; Kumar, P. Anticancer potential of plants and natural products. Am. J. Pharmacol. Sci. 2013, 1, 104–115. [Google Scholar] [CrossRef]
- Kumari, P.; Misra, K.; Sisodia, B.S.; Faridi, U.; Srivastava, S.; Luqman, S.; Kumar, J.K. A promising anticancer and antimalarial component from the leaves of Bidens pilosa. Planta Med. 2009, 75, 59–61. [Google Scholar] [CrossRef]
- Sundararajan, P.; Dey, A.; Smith, A.; Doss, A.G.; Rajappan, M.; Natarajan, S. Studies of anticancer and antipyretic activity of Bidens pilosa whole plant. Afr. Health Sci. 2006, 6, 27–30. [Google Scholar]
- Kviecinski, M.R.; Felipe, K.B.; Schoenfelder, T.; de Lemos Wiese, L.P.; Rossi, M.H.; Gonçalez, E.; Pedrosa, R.C. Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J. Ethnopharmacol. 2008, 117, 69–75. [Google Scholar] [CrossRef]
- Tang, Y.; Li, W.; Cao, J.; Zhao, Y. Bioassay-guided isolation and identification of cytotoxic compounds from Bolbostemma paniculatum. J. Ethnopharmacol. 2015, 169, 18–23. [Google Scholar] [CrossRef]
- El-Najjar, N.; Dakdouki, S.; Darwiche, N.; El-Sabban, M.; Saliba, N.A.; Gali-Muhtasib, H. Anti-colon cancer effects of Salograviolide A isolated from Centaurea ainetensis. Oncol. Rep. 2008, 19, 897–904. [Google Scholar] [CrossRef]
- Ghantous, A.; Tayyoun, A.A.; Lteif, G.A.; Saliba, N.A.; Gali-Muhtasib, H.; El-Sabban, M.; Darwiche, N. Purified Salograviolide A isolated from Centaurea ainetensis causes growth inhibition and apoptosis in neoplastic epidermal cells. Int. J. Oncol. 2008, 32, 841–849. [Google Scholar]
- Kanipandian, N.; Li, D.; Kannan, S. Induction of intrinsic apoptotic signaling pathway in A549 lung cancer cells using silver nanoparticles from Gossypium hirsutum and evaluation of in vivo toxicity. Biotechnol. Rep. 2019, 23, e00339. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Sharififar, F.; Sharifi, I.; Pournamdari, M.; Eslaminejad, T.; Khatami, M. Antioxidant, anti-proliferation and cytotoxicity activities of Gossypium hirsutum toward standard HepG2, A549, MCF-7 and U87 cancer cell lines compared to HUVEC, 3T3 normal cells. Eur. J. Med. Plants 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Sharifi, F.; Sharifi, I.; Keyhani, A.; Asadi-Khanuki, A.; Sharififar, F.; Pournamdari, M. Leishmanicidal, cytotoxic and apoptotic effects of Gossypium hirsutum bulb extract and its separated fractions on Leishmania major. J. Vector Borne Dis. 2019, 56, 330. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.C.; Barman, S.K.; Shaik, M.M. Current updates on Centella asiatica: Phytochemistry, pharmacology and traditional uses. Med. Plant Res. 2013, 3, 777–780. [Google Scholar]
- Jamil, S.S.; Nizami, Q.; Salam, M. Centella asiatica (Linn.) Urban—A review. Nat. Prod. Radiance 2007, 6, 158–170. [Google Scholar]
- Yasurin, P.; Sriariyanun, M.; Phusantisampan, T. The bioavailability activity of Centella asiatica. Appl. Sci. Eng. Prog. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, Y.; Ma, C.; Wang, X.; Li, Y.; Li, X. Antitumor activity of Annona squamosa seed oil. J. Ethnopharmacol. 2016, 193, 362–367. [Google Scholar] [CrossRef]
- Chen, X.; Guo, J.; Bao, J.; Lu, J.; Wang, Y. The anticancer properties of Salvia miltiorrhiza Bunge (Danshen): A systematic review. Med. Res. Rev. 2014, 34, 768–794. [Google Scholar] [CrossRef]
- Mirmalek, S.A.; Azizi, M.A.; Jangholi, E.; Yadollah-Damavandi, S.; Javidi, M.A.; Parsa, Y.; Alizadeh-Navaei, R. Cytotoxic and apoptogenic effect of hypericin, the bioactive component of Hypericum perforatum on the MCF-7 human breast cancer cell line. Cancer Cell Int. 2015, 16, 3. [Google Scholar] [CrossRef]
- Rady, I.; Bloch, M.B.; Chamcheu, R.C.N.; Banang Mbeumi, S.; Anwar, M.R.; Mohamed, H.; Chamcheu, J.C. Anticancer properties of graviola (Annona muricata): A comprehensive mechanistic review. Oxidative Med. Cell. Longev. 2018, 2018, 1826170. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Abou-Elella, F.; Wamunyokoli, F.; AEl-Shemy, H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac. J. Trop. Med. 2014, 7, S355–S363. [Google Scholar] [CrossRef]
- Diogo, C.V.; Félix, L.; Vilela, S.; Burgeiro, A.; Barbosa, I.A.; Carvalho, M.J.; Peixoto, F.P. Mitochondrial toxicity of the phyotochemicals daphnetoxin and daphnoretin–Relevance for possible anti-cancer application. Toxicol. Vitr. 2009, 23, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Martínez-Sánchez, S.M.; Burgos-Morón, E.; Guillén-Mancina, E.; Jiménez-Alonso, J.J.; García, F.; López-Lázaro, M. Screening for selective anticancer activity of plants from Grazalema Natural Park, Spain. Nat. Prod. Res. 2019, 33, 3454–3458. [Google Scholar] [CrossRef]
- Soni, D.; Grover, A. “Picrosides” from Picrorhiza kurroa as potential anti-carcinogenic agents. Biomed. Pharmacother. 2019, 109, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Mallick, M.; Singh, M.; Parveen, R.; Khan, W.; Ahmad, S.; Zeeshan Najm, M.; Husain, S.A. HPTLC analysis of bioactivity guided anticancer enriched fraction of hydroalcoholic extract of Picrorhiza kurroa. BioMed Res. Int. 2015, 2015, 513875. [Google Scholar] [CrossRef]
- Ganogpichayagrai, A.; Palanuvej, C.; Ruangrungsi, N. Antidiabetic and anticancer activities of Mangifera indica cv. Okrong leaves. J. Adv. Pharm. Technol. Res. 2017, 8, 19. [Google Scholar] [PubMed]
- Noratto, G.D.; Bertoldi, M.C.; Krenek, K.; Talcott, S.T.; Stringheta, P.C.; Mertens-Talcott, S.U. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J. Agric. Food Chem. 2010, 58, 4104–4112. [Google Scholar] [CrossRef]
- Bijauliya, R.K.; Alok, S.; Singh, M.; Mishra, S.B. A comprehensive review on cancer and anticancer herbal drugs. Int. J. Pharm. Sci. Res. 2017, 8, 2740–2761. [Google Scholar]
- Patel, P.R.; Raval, B.P.; Karanth, H.A.; Patel, V.R. Potent antitumor activity of Rubia cordifolia. Int. J. Phytomedicine 2010, 2, 44–46. [Google Scholar]
- Son, J.K.; Jung, S.J.; Jung, J.H.; Fang, Z.; Lee, C.S.; Seo, C.S.; Woo, M.H. Anticancer Constituents from the Roots of Rubia cordifolia L. Chem. Pharm. Bull. 2008, 56, 213–216. [Google Scholar] [CrossRef]
- Ghosh, S.; Das Sarma, M.; Patra, A.; Hazra, B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. J. Pharm. Pharmacol. 2010, 62, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mehta, A.; Baweja, S.; Ahirwal, L.; Mehta, P. Anticancer activity of Andrographis paniculata and Silybum marianum on five human cancer cell lines. J. Pharmacol. Toxicol. 2013, 8, 42–48. [Google Scholar] [CrossRef]
- Sagar, S.M. Future directions for research on Silybum marianum for cancer patients. Integr. Cancer Ther. 2007, 6, 166–173. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Karunakaran, T.; Li, F. Green synthesis of gold nanoparticles from Scutellaria barbata and its anticancer activity in pancreatic cancer cell (PANC-1). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1617–1627. [Google Scholar] [CrossRef]
- Yin, X.; Zhou, J.; Jie, C.; Xing, D.; Zhang, Y. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life Sci. 2004, 75, 2233–2244. [Google Scholar] [CrossRef]
- Ye, F.; Xui, L.; Yi, J.; Zhang, W.; Zhang, D.Y. Anticancer activity of Scutellaria baicalensis and its potential mechanism. J. Altern. Complementary Med. 2002, 8, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.R.; George, V.C.; Suresh, P.K.; Kumar, R.A. Cytotoxicity, apoptosis induction and anti-metastatic potential of Oroxylum indicum in human breast cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 2729–2734. [Google Scholar] [CrossRef]
- Lalou, C.; Basak, A.; Mishra, P.; Mohanta, B.C.; Banik, R.; Dinda, B.; Khatib, A.M. Inhibition of tumor cells proliferation and migration by the flavonoid furin inhibitor isolated from Oroxylum indicum. Curr. Med. Chem. 2013, 20, 583–591. [Google Scholar]
- Li, Y.L.; Gan, G.P.; Zhang, H.Z.; Wu, H.Z.; Li, C.L.; Huang, Y.P.; Liu, J.W. A flavonoid glycoside isolated from Smilax china L. rhizome in vitro anticancer effects on human cancer cell lines. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef]
- Vyas, M. A short review on anticancer investigations of Strychnos nux-vomica. Int. J. Green Pharm. (IJGP) 2016, 10, 87–90. [Google Scholar]
- Khan, M.; Garg, A.; Srivastava, S.K.; Darokar, M.P. A cytotoxic agent from Strychnos nux-vomica and biological evaluation of its modified analogues. Med. Chem. Res. 2012, 21, 2975–2980. [Google Scholar] [CrossRef]
- Rao, P.S.; Ramanadham, M.; Prasad, M.N.V. Anti-proliferative and cytotoxic effects of Strychnos nux-vomica root extract on human multiple myeloma cell line–RPMI 8226. Food Chem. Toxicol. 2009, 47, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Bupesh, G.; Manikandan, E.; Thanigaiarul, K.; Magesh, S.; Senthilkumar, V. Enhanced antibacterial, anticancer activity from Terminalia chebula. Med. Plant Rapid Extr. Phytosynthesis Silver Nanoparticles Core-Shell Struct. J. Nanomed. Nanotechnol. 2016, 7, 355. [Google Scholar]
- Wani, K.; Shah, N.; Prabhune, A.; Jadhav, A.; Ranjekar, P.; Kaul-Ghanekar, R. Evaluating the anticancer activity and nanoparticulate nature of homeopathic preparations of Terminalia chebula. Homeopathy 2016, 105, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Owoeye, O.; Yousuf, S.; Akhtar, M.N.; Qamar, K.; Dar, A.; Farombi, E.O.; Choudhary, M.I. Another anticancer elemanolide from Vernonia amygdalina Del. Int. J. Biol. Chem. Sci. 2010, 4, 226–234. [Google Scholar] [CrossRef]
- Gresham, L.J.; Ross, J.; Izevbigie, E.B. Vernonia amygdalina: Anticancer activity, authentication, and adulteration detection. Int. J. Environ. Res. Public Health 2008, 5, 342–348. [Google Scholar] [CrossRef]
- Yedjou, C.; Izevbigie, E.; Tchounwou, P.B. Preclinical assessment of Vernonia amygdalina leaf extracts as DNA damaging anti-cancer agent in the management of breast cancer. Int. J. Environ. Res. Public Health 2008, 5, 337–341. [Google Scholar] [CrossRef]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef]
- Sigstedt, S.C.; Hooten, C.J.; Callewaert, M.C.; Jenkins, A.R.; Romero, A.E.; Pullin, M.J.; Steelant, W.F. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int. J. Oncol. 2008, 32, 1085–1090. [Google Scholar] [CrossRef]
- Yadav, B.; Bajaj, A.; Saxena, M.; Saxena, A.K. In vitro anticancer activity of the root, stem and leaves of Withania somnifera against various human cancer cell lines. Indian J. Pharm. Sci. 2010, 72, 659. [Google Scholar] [CrossRef]
- Rai, M.; Jogee, P.S.; Agarkar, G.; Santos, C.A.D. Anticancer activities of Withania somnifera: Current research, formulations, and future perspectives. Pharm. Biol. 2016, 54, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Pundir, S.; Shukla, M.K.; Singh, A.; Chauhan, R.; Lal, U.R.; Ali, A.; Kumar, D. A comprehensive review on angel’s trumpet (Brugmansia suaveolens). S. Afr. J. Bot. 2022, in press. [Google Scholar] [CrossRef]
- Plengsuriyakarn, T.; Viyanant, V.; Eursitthichai, V.; Tesana, S.; Chaijaroenkul, W.; Itharat, A.; Na-Bangchang, K. Cytotoxicity, toxicity, and anticancer activity of Zingiber officinale Roscoe against cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2012, 13, 4597–4606. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.A.; Ahmad, M.K.; Khan, A.R.; Fatima, N.; Khan, H.J.; Rastogi, N.; Mahdi, A.A. Anticancer and Antioxidant Activity of Zingiber Officinale Roscoe Rhizome; NISCAIR-CSIR: New Delhi, India, 2016. [Google Scholar]
- Cheng, X.L.; Liu, Q.; Peng, Y.B.; Qi, L.W.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011, 129, 1785–1792. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Nair, P.R.; Melnick, S.J.; Wnuk, S.F.; Rapp, M.; Escalon, E.; Ramachandran, C. Isolation and characterization of an anticancer catechol compound from Semecarpus anacardium. J. Ethnopharmacol. 2009, 122, 450–456. [Google Scholar] [CrossRef]
- Al-Ghazzawi, A.M. Anti-cancer activity of new benzyl isoquinoline alkaloid from Saudi plant Annona squamosa. BMC Chem. 2019, 13, 1–6. [Google Scholar] [CrossRef]
- Aizad, S.; Zubairi, S.I.; Yahaya, B.H.; Lazim, A.M. Centella asiatica Extract Potentiates Anticancer Activity in an Improved 3-D PHBV-Composite-CMC A549 Lung Cancer Microenvironment Scaffold. Arab. J. Sci. Eng. 2021, 46, 5313–5325. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical constituents and pharmacological activities of Gossypium herbaceum and Gossypium hirsutum-A. IOSR J. Pharm. 2018, 8, 64–80. [Google Scholar]
- Widowati, W.; Mozef, T.; Risdian, C.; Ratnawati, H.; Tjahjani, S.; Sandra, F. The comparison of antioxidative and proliferation inhibitor properties of Piper betle L., Catharanthus roseus [L.] G. Don, Dendrophtoe petandra L., Curcuma mangga Val. extracts on T47D cancer cell line. Int. Res. J. Biochem. Bioinform. 2011, 1, 22–28. [Google Scholar]
- Habib, M.R.; Karim, M.R. Antitumour evaluation of di-(2-ethylhexyl) phthalate (DEHP) isolated from Calotropis gigantea L. flower. Acta Pharm. 2012, 62, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K. GC-MS Analysis of Volatile Organic Constituents of Traditionally Used Medicinal Plants from the Western Ghats of India: Blumea lanceolaria (Roxb.) Druce., Heliotropium indicum L. and Triumfetta rhomboidea Jacq. J. Mex. Chem. Soc. 2020, 64, 74–82. [Google Scholar] [CrossRef]
- Debaprotim, D.; Suvakanta, D.; Jashabir, C. Evaluation of Anticancer Activity of Mikania micrantha Kunth (Asteraceae) Against Ehrlich Ascites Carcinoma in Swiss Albino Mice. Int. J. Pharm. Res. Allied Sci. 2014, 3, 9–18. [Google Scholar]
- SS, A.; Joseph, B. Anticancer Effect of Phytochemicals from Cyanthillium cinereum against Cancer Target Matrix Metallopeptidase. Int. J. Adv. Res. Eng. Technol. (IJARET) 2020, 11, 65–78. [Google Scholar]
- Pouyfung, P.; Choonate, S.; Wongnoppavich, A.; Rongnoparut, P.; Chairatvit, K. Anti-proliferative effect of 8α-tigloyloxyhirsutinolide-13-O-acetate (8αTGH) isolated from Vernonia cinerea on oral squamous cell carcinoma through inhibition of STAT3 and STAT2 phosphorylation. Phytomedicine 2019, 52, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.T.; Truong, T.M.; Nguyen, T.T.H.; Nguyen, H.D.; Zhao, Y.; Le, V.M. Phytochemical screening and anticancer activity of the aerial parts extract of Xanthium strumarium L. on HepG2 cancer cell line. Clin. Phytoscience 2021, 7, 14. [Google Scholar] [CrossRef]
- Zubair, M.S.; Anam, S.; Khumaidi, A.; Susanto, Y.; Hidayat, M.; Ridhay, A. Molecular docking approach to identify potential anticancer compounds from Begonia (Begonia sp). In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1755, p. 080005. [Google Scholar]
- Kim, J.H.; Lee, S.J.; Han, Y.B.; Moon, J.J.; Kim, J.B. Isolation of isoguanosine from Croton tiglium and its antitumor activity. Arch. Pharmacal Res. 1994, 17, 115–118. [Google Scholar] [CrossRef]
- Sharma, N.; Samarakoon, K.W.; Gyawali, R.; Park, Y.H.; Lee, S.J.; Oh, S.J.; Jeong, D.K. Evaluation of the antioxidant, anti-inflammatory, and anticancer activities of Euphorbia hirta ethanolic extract. Molecules 2014, 19, 14567–14581. [Google Scholar] [CrossRef]
- Kumar, A.; Patil, M.; Kumar, P.; Bhatti, R.C.; Kaur, R.; Sharma, N.K.; Singh, A.N. Mallotus philippensis (Lam.) Müll. Arg.: A review on its pharmacology and phytochemistry. J. Herbmed Pharmacol. 2020, 10, 31–50. [Google Scholar] [CrossRef]
- Desai, T.H.; Joshi, S.V. Anticancer activity of saponin isolated from Albizia lebbeck using various in vitro models. J. Ethnopharmacol. 2019, 231, 494–502. [Google Scholar] [CrossRef]
- Karia, P.; Patel, K.V.; Rathod, S.S. Breast cancer amelioration by Butea monosperma in-vitro and in-vivo. J. Ethnopharmacol. 2018, 217, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Chitra, V.; Sharma, S.; Kayande, N. Evaluation of anticancer activity of Vitex negundo in experimental animals: An in vitro and in vivo study. Int. J. Pharm. Tech. Res. 2009, 1, 1485–1489. [Google Scholar]
- Das, P.K.; Goswami, S.; Chinniah, A.; Panda, N.; Banerjee, S.; Sahu, N.P.; Achari, B. Woodfordia fruticosa: Traditional uses and recent findings. J. Ethnopharmacol. 2007, 110, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Malar, T.J.; Antonyswamy, J.; Vijayaraghavan, P.; Kim, Y.O.; Al-Ghamdi, A.A.; Elshikh, M.S.; Kim, H.J. In-vitro phytochemical and pharmacological bio-efficacy studies on Azadirachta indica A. Juss and Melia azedarach Linn for anticancer activity. Saudi J. Biol. Sci. 2020, 27, 682–688. [Google Scholar] [CrossRef]
- Jung, I.L. Soluble extract from Moringa oleifera leaves with a new anticancer activity. PLoS ONE 2014, 9, e95492. [Google Scholar] [CrossRef]
- Yadav, D.K.; Singh, N.; Dev, K.; Sharma, R.; Sahai, M.; Palit, G.; Maurya, R. Anti-ulcer constituents of Annona squamosa twigs. Fitoterapia 2011, 82, 666–675. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, Q.; Marques, M.; Witcher, M. Anticancer properties of Phyllanthus emblica (Indian gooseberry). Oxidative Med. Cell. Longev. 2015, 2015, 950890. [Google Scholar] [CrossRef]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Kudos, M.B.A.; Sulaiman, M.W.A.W.; Sengupta, P.; Susanti, D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef]
- Hirata, T.; Fujii, M.; Akita, K.; Yanaka, N.; Ogawa, K.; Kuroyanagi, M.; Hongo, D. Identification and physiological evaluation of the components from Citrus fruits as potential drugs for anti-corpulence and anticancer. Bioorganic Med. Chem. 2009, 17, 25–28. [Google Scholar] [CrossRef]
- Juyal, D.; Thawani, V.; Thaledi, S.; Joshi, M. Ethnomedical properties of Taxus wallichiana zucc. (Himalayan yew). J. Tradit. Complementary Med. 2014, 4, 159–161. [Google Scholar] [CrossRef]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M.C. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985, 29, 197–202. [Google Scholar] [CrossRef]
- Biba, V.S.; Amily, A.; Sangeetha, S.; Remani, P. Anticancer, antioxidant and antimicrobial activity of Annonaceae family. World J. Pharm. Pharm. Sci. 2014, 3, 1595–1604. [Google Scholar]
- Mitra, S.K.; Prakash, N.S.; Sundaram, R. Shatavarins (containing Shatavarin IV) with anticancer activity from the roots of Asparagus racemosus. Indian J. Pharmacol. 2012, 44, 732. [Google Scholar] [PubMed]
- Ghosh, T.; Maity, T.K.; Singh, J. Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Orient. Pharm. Exp. Med. 2011, 11, 41–49. [Google Scholar] [CrossRef]
- Rahman, M.A.; Akhtar, J.; Arshad, M. Evaluation of cytotoxic potential and apoptotic effect of a methanolic extract of Bauhinia racemosa Lam. against a human cancer cell line, HeLa. Eur. J. Integr. Med. 2016, 8, 513–518. [Google Scholar] [CrossRef]
- Kumar, E.P.; Elshurafa, A.A.; Elango, K.; Subburaju, T.; Suresh, B. Anti–Tumour Effect of Berberis Asiatica Roxb. Ex. Dc. On Dalton’s Lymphoma Ascite. Anc. Sci. Life 1998, 17, 290. [Google Scholar]
- Harmila, K.J.; Monisha, S.M.; Beevi, A.A.; Deebarathi, V. Antibacterial, antioxidant, anticancer effects and GCMS analysis of Berberis aristata. Biomedicine 2020, 40, 286–293. [Google Scholar]
- Nazir, N.; Rahman, A.; Uddin, F.; Khan Khalil, A.A.; Zahoor, M.; Nisar, M.; Mostafa, G.A. Quantitative Ethnomedicinal Status and Phytochemical Analysis of Berberis lyceum Royle. Agronomy 2021, 11, 130. [Google Scholar] [CrossRef]
- Gurav, S.; Gurav, N. A Comprehensive review: Bergenia ligulata Wall—A controversial clinical candidate. Int. J. Pharm. Sci. Rev. Res. 2014, 5, 1630–1642. [Google Scholar]
- Pardesi Goldee, S.; Chhaya, G.; Vaidya Madhav, D.; Hasni Hamid, Y.; More Babita, H.; Bhuskat Pallavi, P. Preliminary Studies on Antimitotic and Anti Cancer Activity of Calotropis gigantea. Pharmacologyonline 2008, 1, 38–47. [Google Scholar]
- Choedon, T.; Mathan, G.; Arya, S.; Kumar, V.L.; Kumar, V. Anticancer and cytotoxic properties of the latex of Calotropis procera in a transgenic mouse model of hepatocellular carcinoma. World J. Gastroenterol. WJG 2006, 12, 2517. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Shanmugavel, M.; Kampasi, H.; Singh, R.; Mondhe, D.M.; Rao, J.M.; Qazi, G.N. Chemically standardized isolates from Cedrus deodara stem wood having anticancer activity. Planta Med. 2007, 73, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Monira, K.M.; Munan, S.M. Review on Datura metel: A potential medicinal plant. Glob. J. Res. Med. Plants Indig. Med. 2012, 1, 123. [Google Scholar]
- Moraes, R.M.; Dayan, F.E.; Canel, C. The lignans of Podophyllum. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 26, pp. 149–182. [Google Scholar]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P.V. Therapeutic perspectives of molecules from Urtica dioica extracts for cancer treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef] [PubMed]
- Gueritte, F.; Fahy, J. Anticancer Agents from Natural Products; CRC Press: Boca Raton, FL, USA, 2005; pp. 123–135. [Google Scholar]
- David, M.; Karekalammanavar, G. Spectrographic analysis and in vitro study of antibacterial anticancer activity of aqueous ethanolic fruit extract of Carissa carandas. J. Adv. Sci. Res. 2015, 6, 10–13. [Google Scholar]
- Karunakar, H.; Satyanarayana, D.; Joshi, A.B. Phytochemical investigation of root extract of the plant Carissa spinarum. Rajiv Gandhi Univ. Health Sci. J. Pharm. Sci. 2012, 2, 55–59. [Google Scholar]
- Oberlies, N.H.; Kroll, D.J. Camptothecin and taxol: Historic achievements in natural products research. J. Nat. Prod. 2004, 67, 129–135. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhao, C.; Jing, Q.; Jiang, C.; Lin, B.; Li, D.; Li, B.; Jing, Y.; Yuan, J.; et al. Cephalotaxine-type alkaloids with antiproliferation effects from the branches and leaves of Cephalotaxus fortunei var. alpina. Fitoterapia 2021, 155, 105037. [Google Scholar] [CrossRef]
- Ghani AE, A.; Dora, G.A.; Hassan, W.H.; Abdallah, R.H.; Abd El-Salam, E. New saponins from Albizia lebbeck (L) Benth flowers. Int. J. Pharm. Sci. Res. 2016, 7, 3617. [Google Scholar]
- Al-Snafi, A.E. Chemical constituents and pharmacological effects of Asclepias curassavica—A review. Asian J. Pharm. Res. 2015, 5, 83–87. [Google Scholar]
- Joshi, B.C.; Verma, P.; Juyal, V.; Sah, A.N. A Review of Himalayan Medicinal Plants against Cancer. Curr. Tradit. Med. 2021, 8, 31–47. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentao, P.; Correia-da-Silva, G.; Teixeira, N.; Andrade, P.B. Plant secondary metabolites in cancer chemotherapy: Where are we? Curr. Pharm. Biotechnol. 2012, 13, 632–650. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pathania, R.; Khan, M.; Tonk, R.K.; Kumar, D.; Dash, A.K. Identification and quantification of some natural compounds of Pinus gerardiana leaf extract and its antimicrobial and antioxidant activities. Pharmacologyonline 2021, 2, 333–351. [Google Scholar]
- Banu, K.S.; Cathrine, L. General techniques involved in phytochemical analysis. Int. J. Adv. Res. Chem. Sci. 2015, 2, 25–32. [Google Scholar]
- Poole, C.F. Thin-layer chromatography: Challenges and opportunities. J. Chromatogr. A 2003, 1000, 963–984. [Google Scholar] [CrossRef]
- Mane, N.B.; Khilare, C.J. Phytochemical Analysis and Study of Functional Groups by FTIR Analysis of Withania somnifera L Dunal. J. Sci. Res. 2021, 65, 6. [Google Scholar] [CrossRef]
- Lawania, R.D.; Mishra, A. Anticancer potential of plants and natural products: A review. J. Diagn. Tech. Biomed. Anal. 2013, 1, 104–115. [Google Scholar]
- Bhoomika, R.; Ramesh, K.G.; Anita, A.M. Phyto-pharmacology of Achyranthes aspera: A Review. Pharmacogn. Rev. 2007, 1, 143. [Google Scholar]
- Arora, S.; Tandon, S. Achyranthes aspera root extracts induce human colon cancer cell (COLO-205) death by triggering the mitochondrial apoptosis pathway and S phase cell cycle arrest. Sci. World J. 2014, 2014, 129697. [Google Scholar] [CrossRef]
- Scharfenberg, K.; Wagner, R.; Wagner, K.G. The cytotoxic effect of ajoene, a natural product from garlic, investigated with different cell lines. Cancer Lett. 1990, 53, 103–108. [Google Scholar] [CrossRef]
- Thomson, M.; Ali, M. Garlic [Allium sativum]: A review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targets 2003, 3, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Balasenthil, S.; Ramachandran, C.R.; Nagini, S. Prevention of 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis by garlic. Fitoterapia 2001, 72, 524–531. [Google Scholar] [CrossRef]
- Țigu, A.B.; Moldovan, C.S.; Toma, V.A.; Farcaș, A.D.; Moț, A.C.; Jurj, A.; Pârvu, M. Phytochemical analysis and in vitro effects of Allium fistulosum L. and Allium sativum L. extracts on human normal and tumor cell lines: A comparative study. Molecules 2021, 26, 574. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, G.Y.; Hwang, H.J.; Kim, W.J.; Choi, Y.H. Diallyl trisulfide-induced apoptosis of bladder cancer cells is caspase-dependent and regulated by PI3K/Akt and JNK pathways. Environ. Toxicol. Pharmacol. 2014, 37, 74–83. [Google Scholar] [CrossRef]
- Gao, X.Y.; Geng, X.J.; Zhai, W.L.; Zhang, X.W.; Wei, Y.; Hou, G.J. Effect of combined treatment with cyclophosphamidum and allicin on neuroblastoma–bearing mice. Asian Pac. J. Trop. Med. 2015, 8, 137–141. [Google Scholar] [CrossRef]
- Geethangili, M.; Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in jurkat cells. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 1336–1341. [Google Scholar]
- Rajeshkumar, S.; Nagalingam, M.; Ponnanikajamideen, M.; Vanaja, M.; Malarkodi, C. Anticancer activity of andrographis paniculata leaves extract against neuroblastima (IMR-32) and human colon (HT-29) cancer cell line. World J. Pharm. Pharm. Sci. 2015, 4, 1667–1675. [Google Scholar]
- Muriel, J.M. Herbs or natural products that decrease cancer growth. Oncol. Nurs. Forum 2004, 31, 75. [Google Scholar]
- Kamakura, M.; Sakaki, T. A hypopharyngeal gland protein of the worker honeybee Apis mellifera L. enhances proliferation of primary-cultured rat hepatocytes and suppresses apoptosis in the absence of serum. Protein Expr. Purif. 2006, 45, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kang, S.J.; Kim, B.M.; Kim, Y.J.; Woo, H.D.; Chung, H.W. Cytotoxicity of honeybee (Apis mellifera) venom in normal human lymphocytes and HL-60 cells. Chem. Biol. Interact. 2007, 169, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoglu, İ.; Saribeyoglu, K.; Durak, H.; Karahasanoglu, T.; Bayrak, İ.; Altug, T.; Sarıyar, M. Protective covering of surgical wounds with honey impedes tumor implantation. Arch. Surg. 2000, 135, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ito, H.; Shimura, K. Enhancing effect of antitumor polysaccharide from Astragalus or Radix hedysarum on C3 cleavage production of macrophages in mice. Jpn. J. Pharmacol. 1989, 51, 432–434. [Google Scholar] [CrossRef]
- Cho, W.C.S.; Leung, K.N. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J. Ethnopharmacol. 2007, 113, 132–141. [Google Scholar] [CrossRef]
- Nechepurenko, I.V.; Polovinka, M.P.; Komarova, N.I.; Korchagina, D.V.; Salakhutdinov, N.F.; Nechepurenko, S.B. Low-molecular-weight phenolic compounds from Hedysarum theinum roots. Chem. Nat. Compd. 2008, 44, 31–34. [Google Scholar] [CrossRef]
- Yang, L.; Junshan, Y. Studies on chemical constituents isolated from Hedysarum polybotrys. Zhongguo Yao Xue Za Zhi (Zhongguo Yao Xue Hui 1989) 2005, 40, 1215–1216. [Google Scholar]
- Liu, Y.; Zhang, Q.Y.; Zhao, Y.Y.; Wang, B.; Hai, L.Q.; Ying, Y.P.; Chen, H.B. Saponins from the roots of Hedysarum polybotrys. Biochem. Syst. Ecol. 2007, 6, 389–391. [Google Scholar] [CrossRef]
- LI, Y.; Huang, J.; Guo, H.; Ren, B. Chemical constituents from Hedysarum polybotrys and their antitumor activities. Chin. Tradit. Herb. Drugs 1994, 36, 777–780. [Google Scholar]
- Dong, Y.; Tang, D.; Zhang, N.; Li, Y.; Zhang, C.; Li, L.; Li, M. Phytochemicals and biological studies of plants in genus Hedysarum. Chem. Cent. J. 2013, 7, 124. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, Z.; Shi, P.; Wang, G.; Wu, Y.; Li, S.; Yao, H. Anticancer effect of petroleum ether extract from Bidens pilosa L and its constituent’s analysis by GC-MS. J. Ethnopharmacol. 2018, 217, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Kviecinski, M.R.; Benelli, P.; Felipe, K.B.; Correia, J.F.G.; Pich, C.T.; Ferreira, S.R.S.; Pedrosa, R.C. SFE from Bidens pilosa Linné to obtain extracts rich in cytotoxic polyacetylenes with antitumor activity. J. Supercrit. Fluids 2011, 56, 243–248. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Y.; Zhang, X.; Tang, H.F.; Cao, W.D.; Gao, D.K.; Wang, X.L. Tubeimoside V, a new cyclic bisdesmoside from tubers of Bolbostemma paniculatum, functions by inducing apoptosis in human glioblastoma U87MG cells. Bioorganic Med. Chem. Lett. 2006, 16, 4575–4580. [Google Scholar] [CrossRef]
- Yu, L.; Ma, R.; Wang, Y.; Nishino, H. Potent anti-tumor activity and low toxicity of tubeimoside 1 isolated from Bolbostemma paniculatum. Planta Med. 1994, 60, 204–208. [Google Scholar] [CrossRef]
- Dou, J.W.; Shang, R.G.; Lei, X.Q.; Li, K.L.; Guo, Z.Z.; Ye, K.; Huang, Q. Total saponins of Bolbostemma paniculatum (maxim.) Franquet exert antitumor activity against MDA-MB-231 human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. BMC Complementary Altern. Med. 2019, 19, 304. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; del Pulgar, T.G.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef]
- Guzman, M, Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [CrossRef]
- Bala, A.; Mukherjee, P.K.; Braga, F.C.; Matsabisa, M.G. Comparative inhibition of MCF-7 breast cancer cell growth, invasion and angiogenesis by Cannabis sativa L. sourced from sixteen different geographic locations. S. Afr. J. Bot. 2018, 119, 154–162. [Google Scholar] [CrossRef]
- Janatová, A.; Doskočil, I.; Božik, M.; Fraňková, A.; Tlustoš, P.; Klouček, P. The chemical composition of ethanolic extracts from six genotypes of medical cannabis (Cannabis sativa L.) and their selective cytotoxic activity. Chem. Biol. Interact. 2022, 353, 109800. [Google Scholar] [CrossRef]
- Alper, M.; Güneş, H. The anticancer and anti-inflammatory effects of Centaurea solstitialis extract on human cancer cell lines. Turk. J. Pharm. Sci. 2019, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Yang, S.F.; Peng, C.Y.; Chou, M.Y.; Chang, Y.C. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase-plasminogen activator. J. Oral. Pathol. Med. 2007, 36, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Nakajima, T.; Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Okanoue, T. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J. Hepatol. 2006, 44, 1074–1082. [Google Scholar] [CrossRef]
- Spinella, F.; Rosanò, L.; Decandia, S.; Di Castro, V.; Albini, A.; Elia, G.; Bagnato, A. Antitumor effect of green tea polyphenol epigallocatechin-3-gallate in ovarian carcinoma cells: Evidence for the endothelin-1 as a potential target. Exp. Biol. Med. 2006, 231, 1123–1127. [Google Scholar]
- Hwang, J.T.; Ha, J.; Park, I.J.; Lee, S.K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef]
- Peng, G.; Dixon, D.A.; Muga, S.J.; Smith, T.J.; Wargovich, M.J. Green tea polyphenol (−)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2006, 45, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Harshavardhan, S.J.; Chirumarry, S.; Poornachandra, Y.; Jang, K.; Kumar, C.G.; Yoon, Y.-J.; Zhao, B.-X.; Miao, J.-Y.; Shin, D.-S. Design, synthesis in vitro anticancer activity and docking studies of (−)-catechin derivatives. Bull. Kor. Chem. Soc. 2015, 36, 564–570. [Google Scholar]
- Bushman, J.L. Green tea and cancer in humans: A review of the literature. Nutr. Cancer 1998, 31, 151–159. [Google Scholar] [CrossRef]
- Yang, C.S.; Maliakal, P.; Meng, X. Inhibition of carcinogenesis by tea. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 25–54. [Google Scholar] [CrossRef]
- Chen, C.; Shen, G.; Hebbar, V.; Hu, R.; Owuor, E.D.; Kong, A.N.T. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1369–1378. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Sanusi, J.; Rajarajeswaran, J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015, 169, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Baxter, R.L. Mezerein: Antileukemic principle isolated from Daphne mezereum L. Science 1975, 187, 652–653. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Peruzzi, L.; Efferth, T.; Daphne, S.T.; Mezereum, L. A study of anti-proliferative activity towards human cancer cells and antioxidant properties. Nat. Prod. Res. 2019, 33, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Coyle, T.; Levante, S.; Shetler, M.; Winfield, J. In vitro and in vivo cytotoxicity of gossypol against central nervous system tumor cell lines. J. Neuro-Oncol. 1994, 19, 25–35. [Google Scholar] [CrossRef]
- Gilbert, N.E.; O’Reilly, J.E.; Chang, C.G.; Lin, Y.C.; Brueggemeier, R.W. Antiproliferative activity of gossypol and gossypolone on human breast cancer cells. Life Sci. 1995, 57, 61–67. [Google Scholar] [CrossRef]
- Liang, X.S.; Rogers, A.J.; Webber, C.L.; Ormsby, T.J.; Tiritan, M.E.; Matlin, S.A.; Benz, C.C. Developing gossypol derivatives with enhanced antitumor activity. Investig. New Drugs 1995, 13, 181–186. [Google Scholar] [CrossRef]
- Wu, D. An overview of the clinical pharmacology and therapeutic potential of gossypol as a male contraceptive agent and in gynaecological disease. Drugs 1989, 38, 333–341. [Google Scholar] [CrossRef]
- Patil, C.D.; Borase, H.P.; Salunkhe, R.B.; Suryawanshi, R.K.; Narkhade, C.P.; Salunke, B.K.; Patil, S.V. Mosquito larvicidal potential of Gossypium hirsutum (Bt cotton) leaves extracts against Aedes aegypti and Anopheles stephensi larvae. J. Arthropod-Borne Dis. 2014, 8, 91. [Google Scholar]
- Han, M.L.; Wang, Y.F.; Tang, M.Y.; Ge, Q.S.; Zhou, L.F.; Sun, Y.T. Gossypol in the treatment of endometriosis and uterine myoma. In Endometriosis; Karger Publishers: Basel, Switzerland, 1987; Volume 16, pp. 268–270. [Google Scholar]
- Gruenwald, J.; Brendler, T.; Jaenicke, C. PDR for Herbal Medicines; Thomson, Reuters: Toronto, ON, Canada, 2007. [Google Scholar]
- Newall, C.A.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines. A Guide for Health-Care Professionals; The Pharmaceutical Press: London, UK, 1996. [Google Scholar]
- Pittella, F.; Dutra, R.C.; Junior, D.D.; Lopes, M.T.; Barbosa, N.R. Antioxidant and cytotoxic activities of Centella asiatica (L) Urb. Int. J. Mol. Sci. 2009, 10, 3713–3721. [Google Scholar] [CrossRef]
- Babu, T.D.; Kuttan, G.; Padikkala, J. Cytotoxic and anti-tumour properties of certain taxa of Umbelliferae with special reference to Centella asiatica (L.) Urban. J. Ethnopharmacol. 1995, 48, 53–57. [Google Scholar] [CrossRef]
- Planas-Silva, M.D.; Weinberg, R.A. The restriction point and control of cell proliferation. Curr. Opin. Cell Biol. 1997, 9, 768–772. [Google Scholar] [CrossRef]
- Alali, F.Q.; Liu, X.X.; McLaughlin, J.L. Annonaceous acetogenins: Recent progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar] [CrossRef]
- Wada, A.; Sakaeda, T.; Takara, K.; Hirai, M.; Kimura, T.; Ohmoto, N.; Okumura, K. Effects of St John’s wort and hypericin on cytotoxicity of anticancer drugs. Drug Metab. Pharmacokinet. 2002, 17, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Martarelli, D.; Martarelli, B.; Pediconi, D.; Nabissi, M.I.; Perfumi, M.; Pompei, P. Hypericum perforatum methanolic extract inhibits growth of human prostatic carcinoma cell line orthotopically implanted in nude mice. Cancer Lett. 2004, 210, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Girgin, S.N. A unique phenolic extraction method from olive oil macerate of Hypericum perforatum using DMSO: Assessment of in vitro anticancer activity, LC-MS/MS profile, total phenolic content and antioxidant capacity. S. Afr. J. Bot. 2021, 139, 6–11. [Google Scholar] [CrossRef]
- Gönenç, T.M.; Ozturk, M.; Türkseven, S.G.; Kirmizibayrak, P.B.; Günal, S.; Yilmaz, S. Hypericum perforatum L. An overview of the anticancer potencies of the specimens collected from different ecological environments. Pak. J. Bot. 2020, 52, 1003–1010. [Google Scholar] [CrossRef]
- Garrido-Garrido, G.; Martínez-Sánchez, G.; Pardo-Andreu, G.; García-Rivera, D.; Hernández-Casaña, P.; Rodeiro-Guerra, I.; Núñez-Sellés, A.J. Recent advances in the research & development of an aqueous stem bark extract obtained from Mangifera indica L. Recent Dev. Med. Plant Res. 2007, 1, 169–192. [Google Scholar]
- Tamayo, D.; Mari, E.; González, S.; Guevara, M.; Garrido, G.; Delgado, R.; Núñez, A.J. Vimang as natural antioxidant supplementation in patients with malignant tumors. Minerva Med. 2001, 92 (Suppl. 1), 95–97. [Google Scholar]
- Núñez Sellés, A.J.; Vélez Castro, H.T.; Agüero-Agüero, J.; González-González, J.; Naddeo, F.; De Simone, F.; Rastrelli, L. Isolation and quantitative analysis of phenolic antioxidants, free sugars, and polyols from mango (Mangifera indica L.) stem bark aqueous decoction used in Cuba as a nutritional supplement. J. Agric. Food Chem. 2002, 50, 762–766. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Yang, B.; Xiang, T.; Yin, X.; Peng, W.; Ren, G. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicol. Appl. Pharmacol. 2013, 272, 180–190. [Google Scholar] [CrossRef]
- Li, M.; Ma, H.; Yang, L.; Li, P. Mangiferin inhibition of proliferation and induction of apoptosis in human prostate cancer cells is correlated with downregulation of B-cell lymphoma-2 and upregulation of microRNA-182. Oncol. Lett. 2016, 11, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.S.; Zhou, Y.Y.; Yuan, Y.F.; Zhong, Z.G.; Liang, C.Y.; Qiu, Q. Study on anticancer effect in vivo of active fraction from Nervilia fordii. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2007, 30, 1095–1098. [Google Scholar]
- Kamkaen, N.; Wilkinson, J.M.; Cavanagh, H.M. Cytotoxic effect of four Thai edible plants on mammalian cell proliferation. Thai Pharma Health Sci. J. 2006, 1, 189–195. [Google Scholar]
- Roy, M.K.; Nakahara, K.; Thalang, V.N.; Trakoontivakorn, G.; Takenaka, M.; Isobe, S.; Tsushida, T. Baicalein, a flavonoid extracted from a methanolic extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. Pharmazie 2007, 62, 149–153. [Google Scholar]
- Nakahara, K.; Onishi-Kameyama, M.; Ono, H.; Yoshida, M.; Trakoontivakorn, G. Antimutagenic Acitivity against Trp-P-1 of the Edible Thai Plant, Oroxylum indicum Vent. Biosci. Biotechnol. Biochem. 2001, 65, 2358–2360. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, T.R.; Akerele, O. (Eds.) Medicinal Plants: Their Role in Health and Biodiversity; University of Pennsylvania Press: Philadelphia, PA, USA, 2015. [Google Scholar]
- Costa-Lotufo, L.V.; Khan, M.T.H.; Ather, A.; Wilke, D.V.; Jimenez, P.C.; Pessoa, C.; de Moraes, M.O. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J. Ethnopharmacol. 2005, 99, 21–30. [Google Scholar] [CrossRef]
- Kumar, S.; Bansal, P.; Gupta, V.; Sannd, R.; Rao, M. The clinical effect of Albizia lebbeck stem bark decoction on bronchial asthma. Int. J. Pharm. Sci. Drug Res. 2010, 2, 48–50. [Google Scholar]
- Kupchan, S.M.; Knox, J.R.; Kelsey, J.E.; Renauld, J.S. Calotropin, a cytotoxic principle isolated from Asclepias curassavica L. Science 1964, 146, 1685–1686. [Google Scholar] [CrossRef]
- Morita, H.; Yamamiya, T.; Takeya, K.; Itokawa, H. New antitumor bicyclic hexapeptides, RA-XI,-XII,-XIII and-XIV from Rubia cordifolia. Chem. Pharm. Bull. 1992, 40, 1352–1354. [Google Scholar] [CrossRef]
- Morita, H.; Yamamiya, T.; Takeya, K.; Itokawa, H.; Sakuma, C.; Yamada, J.; Suga, T. Conformational recognition of RA-XII by 80S Ribosomes: A differential line broadening study in 1H NMR spectroscopy. Chem. Pharm. Bull. 1993, 41, 781–783. [Google Scholar] [CrossRef]
- Gupta, P.P.; Srimal, R.C.; Verma, N.; Tandon, J.S. Biological activity of Rubia cordifolia and isolation of an active principle. Pharm. Biol. 1999, 37, 46–49. [Google Scholar] [CrossRef]
- Son, J.K.; Jung, J.H.; Lee, C.S.; Moon, D.C.; Choi, S.W.; Min, B.S.; Woo, M.H. DNA Topoisomerases I and II inhibition and cytotoxicity of constituents from the roots of Rubia cordifolia. Bull. Korean Chem. Soc. 2006, 27, 1231–1234. [Google Scholar] [CrossRef]
- Parajuli, P.; Joshee, N.; Rimando, A.M.; Mittal, S.; Yadav, A.K. In vitro antitumor mechanisms of various Scutellaria extracts and constituent flavonoids. Planta Med. 2009, 75, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Y.; Yuan, S.; Liu, G.; Lu, Y.; Zhang, J.; Wang, W. Potential anticancer activity of tanshinone IIA against human breast cancer. Int. J. Cancer 2005, 116, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Tayarani-Najaran, Z.; Emami, S.A.; Asili, J.; Mirzaei, A.; Mousavi, S.H. Analyzing cytotoxic and apoptogenic properties of Scutellaria litwinowii root extract on cancer cell lines. Evid. Based Complementary Altern. Med. 2011, 2011, 160682. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Korman, N.J.; Mukhtar, H.; Agarwal, R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997, 89, 556–565. [Google Scholar] [CrossRef]
- Shukla, M.K.; Thakur, A.; Verma, R.; Lalhlenmawia, H.; Bhattacharyya, S.; Bisht, D.; Singh, A.; Parcha, V.; Kumar, D. Unravelling the therapeutic potential of orchid plant against cancer. S. Afr. J. Bot. 2022, 150, 69–79. [Google Scholar] [CrossRef]
- Cancer (who.int). Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 24 July 2022).
- Wang, H.; Oo Khor, T.; Shu, L.; Su, Z.Y.; Fuentes, F.; Lee, J.H.; Tony Kong, A.N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Sharma, A.; Nagraik, R.; Venkidasamy, B.; Khan, A.; Dulta, K.; Chauhan, P.K.; Kumar, D.; Shin, D.S. In-vitro antidiabetic, antioxidant, antimicrobial and cytotoxic activity of Murraya koenigii leaf extract Intercedes ZnO nanoparticles. Luminescence 2022, 1. Early View. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, K.R.B.; Ghate, M.D.; Lalhlenmawia, H.; Kumar, D.; Singh, J. Bioinspired quantum dots for cancer therapy: A mini-review. Mater. Lett. 2022, 313, 131742. [Google Scholar] [CrossRef]
- Madhuri, S.; Pandey, G. Some dietary agricultural plants with anticancer properties. Plant Arch. 2008, 8, 13–16. [Google Scholar]
- Sivalokanathan, S.; Ilayaraja, M.; Balasubramanian, M.P. Efficacy of Terminalia Arjuna (Roxb.) on N-Nitrosodiethylamine Induced Hepatocellular Carcinoma in Rats; NISCAIR-CSIR: New Delhi, India, 2005. [Google Scholar]
- Park, W.C.; Jordan, V.C. Selective estrogen receptor modulators (SERMS) and their roles in breast cancer prevention. Trends Mol. Med. 2002, 8, 82–88. [Google Scholar] [CrossRef]
- Cragg, G.M.; Kingston, D.G.; Newman, D.J. (Eds.) Anticancer Agents from Natural Products; Taylor & Francis: Boca Raton, FL, USA, 2005; Volume 23, pp. 676–686. [Google Scholar]
- Rivera, G.; Bowman, W.P.; Murphy, S.B.; Dahl, G.V.; Aur, R.J.; Kalwinsky, D.K.; Avery, T.L. Vm-26 with prednisone and vincristine for treatment of refractory acute lymphocytic leukemia. Med. Pediatric Oncol. 1982, 10, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bosl, G.J.; Geller, N.L.; Bajorin, D.; Leitner, S.P.; Yagoda, A.; Golbey, R.B.; Carey, R. A randomized trial of etoposide + cisplatin versus vinblastine+ bleomycin+ cisplatin+ cyclophosphamide+ dactinomycin in patients with good-prognosis germ cell tumors. J. Clin. Oncol. 1988, 6, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H. Management of small cell lung cancer: Current state of the art. Chest 1999, 116, 525S–530S. [Google Scholar] [CrossRef]
- Malik, S.; Biba, O.; Grúz, J.; Arroo, R.R.J.; Strnad, M. Biotechnological approaches for producing aryltetralin lignans from Linum species. Phytochem. Rev. 2014, 13, 893–913. [Google Scholar] [CrossRef]
- Utsugi, T.; Shibata, J.; Sugimoto, Y.; Aoyagi, K.; Wierzba, K.; Kobunai, T.; Yamada, Y. Antitumor activity of a novel podophyllotoxin derivative (TOP-53) against lung cancer and lung metastatic cancer. Cancer Res. 1996, 56, 2809–2814. [Google Scholar]
- You, Y. Podophyllotoxin derivatives: Current synthetic approaches for new anticancer agents. Curr. Pharm. Des. 2005, 11, 1695–1717. [Google Scholar] [CrossRef]
- Nisar, M.; Khan, I.; Simjee, S.U.; Gilani, A.H.; Perveen, H. Anticonvulsant, analgesic and antipyretic activities of Taxus wallichiana Zucc. J. Ethnopharmacol. 2008, 116, 490–494. [Google Scholar] [CrossRef]
- Khan, I.; Nisar, M.; Shah, M.R.; Shah, H.; Gilani, S.N.; Gul, F.; Ullah, M. Anti-inflammatory activities of Taxusabietane A isolated from Taxus wallichiana Zucc. Fitoterapia 2011, 82, 1003–1007. [Google Scholar] [CrossRef]
- Chattopadhyay, S.K.; Pal, A.; Maulik, P.R.; Kaur, T.; Garg, A.; Khanuja, S.P.S. Taxoid from the needles of the Himalayan yew Taxus wallichiana with cytotoxic and immunomodulatory activities. Bioorganic Med. Chem. Lett. 2006, 16, 2446–2449. [Google Scholar] [CrossRef] [PubMed]
- Dexheimer, T.S.; Antony, S.; Marchand, C.; Pommier, Y. Tyrosyl-DNA phosphodiesterase as a target for anticancer therapy. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem -Anti-Cancer Agents) 2008, 8, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, F.C.; El Khamisy, S.F.; Zuma, M.C.; Osborn, K.; Caldecott, K.W. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 2009, 461, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; Lea, W.A.; Jadhav, A.; Dexheimer, T.S.; Austin, C.P.; Inglese, J.; Simeonov, A. Identification of phosphotyrosine mimetic inhibitors of human tyrosyl-DNA phosphodiesterase I by a novel AlphaScreen high-throughput assay. Mol. Cancer Ther. 2009, 8, 240–248. [Google Scholar] [CrossRef]
- Dancey, J.; Eisenhauer, E.A. Current perspectives on camptothecins in cancer treatment. Br. J. Cancer 1996, 74, 327–338. [Google Scholar] [CrossRef]
- Fujita, K.I.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234. [Google Scholar] [CrossRef]
- Jakobsen, A.K.; Lauridsen, K.L.; Samuel, E.B.; Proszek, J.; Knudsen, B.R.; Hager, H.; Stougaard, M. Correlation between topoisomerase I and tyrosyl-DNA phosphodiesterase 1 activities in non-small cell lung cancer tissue. Exp. Mol. Pathol. 2015, 99, 56–64. [Google Scholar] [CrossRef]
- Zakharenko, A.; Luzina, O.; Koval, O.; Nilov, D.; Gushchina, I.; Dyrkheeva, N.; Lavrik, O. Tyrosyl-DNA phosphodiesterase 1 inhibitors: Usnic acid enamines enhance the cytotoxic effect of camptothecin. J. Nat. Prod. 2016, 79, 2961–2967. [Google Scholar] [CrossRef]
- Itokawa, H.; Ibraheim, Z.Z.; Qiao, Y.F.; Takeya, K. Anthraquinones, naphthohydroquinones and naphthohydroquinone dimers from Rubia cordifolia and their cytotoxic activity. Chem. Pharm. Bull. 1993, 41, 1869–1872. [Google Scholar] [CrossRef]
- Na, W.; Ma, B.; Shi, S.; Chen, Y.; Zhang, H.; Zhan, Y.; An, H. Procyanidin B1, a novel and specific inhibitor of Kv10. 1 channel, suppresses the evolution of hepatoma. Biochem. Pharmacol. 2020, 178, 114089. [Google Scholar] [CrossRef]
- Guo, S.; Bai, X.; Shi, S.; Deng, Y.; Kang, X.; An, H. TMEM16A, a Homoharringtonine Receptor, as a Potential Endogenic Target for Lung Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 10930. [Google Scholar] [CrossRef] [PubMed]
- Gürel, G.; Blaha, G.; Moore, P.B.; Steitz, T.A. U2504 Determines the Species Specificity of the A-Site Cleft Antibiotics: The Structures of Tiamulin, Homoharringtonine, and Bruceantin Bound to the Ribosome. J. Mol. Biol. 2009, 389, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Ahmed, M. The analgesic and anti-inflammatory activities of the extract of Albizia lebbeck in animal model. Pak. J. Pharm. Sci. 2009, 22, 74–77. [Google Scholar] [PubMed]
- Bartek, J.; Lukas, C.; Lukas, J. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 2004, 5, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Kokila, K.; Priyadharshini, S.D.; Sujatha, V. Phytopharmacological properties of Albizia species: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 70–73. [Google Scholar]
- Faisal, M.; Singh, P.P.; Irchhaiya, R. Review on Albizia lebbeck a potent herbal drug. Int. Res. J. Pharm. 2012, 3, 63–68. [Google Scholar]
- Sharma, G.K.; Dubey, N. Review of Shirish (Albizia lebbeck) therapeutic properties. Int. J. Ayurvedic Herb. Med. 2015, 5, 1683–1688. [Google Scholar]
- Bobby, M.N.; Wesely, E.G.; Johnson, M. High performance thin layer chromatography profile studies on the alkaloids of Albizia lebbeck. Asian Pac. J. Trop. Biomed. 2012, 2, S1–S6. [Google Scholar] [CrossRef]
- Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa being an Account of their Medicinal and other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal. In The Medicinal and Poisonous Plants of Southern and Eastern Africa Being an Account of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal; E. & S. Livingstone Ltd.: Edinburgh, UK, 1962. [Google Scholar]
- Abd El-Ghany, A.E.S.; Dora, G.; Abdallah, R.H.; Hassan, W.; El-Salam, E.A. Phytochemical and biological study of Albizia lebbeck stem bark. J. Chem. Pharma. Res. 2015, 7, 29–43. [Google Scholar]
- Xu, F.; Wang, P.; Zhang, X.; Hou, T.; Qu, L.; Wang, C.; Liang, X. Identification and target-pathway deconvolution of FFA4 agonists with anti-diabetic activity from Arnebia euchroma (Royle) Johnst. Pharmacol. Res. 2021, 163, 105173. [Google Scholar] [CrossRef]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Kooti, W.; Ali-Akbari, S.; Asadi-Samani, M.; Ghadery, H.; Ashtary-Larky, D. A review on medicinal plant of Apium graveolens. Adv. Herb. Med. 2015, 1, 48–59. [Google Scholar]
- Watt, G. Periodic Expert. Dict. Econ. Prod. India 1972, 1, 260. [Google Scholar]
- Asolkar, L.V.; Kakkar, K.K.; Chakre, O.J.; Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Publications & Information Directorate: New Delhi, India, 1992. [Google Scholar]
- Oberlies, N.H.; Chang, C.J.; McLaughlin, J.L. Structure–Activity relationships of diverse annonaceous acetogenins against multidrug resistant human mammary adenocarcinoma (MCF-7/Adr) Cells. J. Med. Chem. 1997, 40, 2102–2106. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Eguchi, T.; Kakinuma, K.; Ikekawa, N.; Sahai, M.; Gupta, Y.K. Squamocin, a new cytotoxic bis-tetrahydrofuran containing acetogenin from Annona squamosa. Chem. Pharm. Bull. 1988, 36, 4802–4806. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Murasaki, C.; Shimada, H.; Nishioka, S.; Kakinuma, K.; Singh, S.; Sahai, M. Annonaceous acetogenins from the seeds of Annona squamosa. Non-adjacent bis-tetrahydrofuranic acetogenins. Chem. Pharm. Bull. 1994, 42, 1175–1184. [Google Scholar] [CrossRef]
- Saha, R. Pharmacognosy and pharmacology of Annona squamosa. Int. J. Pharm. Life Sci. 2011, 2, 1183–1189. [Google Scholar]
- Kadali, V.N.; Pola, S.R.; Sandeep, B.V. Anti cancer properties of plants present in west Godavari district of Andhra Pradesh, India-a mini review. Indian J. Tradit. Knowl. 2010, 3, 211–217. [Google Scholar]
- Shirwaikar, A.; Rajendran, K.; Kumar, C.D.; Bodla, R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. J. Ethnopharmacol. 2004, 91, 171–175. [Google Scholar] [CrossRef]
- Niu, N.; Schaid, D.J.; Abo, R.P.; Kalari, K.; Fridley, B.L.; Feng, Q.; Wang, L. Genetic association with overall survival of taxane-treated lung cancer patients-a genome-wide association study in human lymphoblastoid cell lines followed by a clinical association study. BMC Cancer 2012, 12, 422. [Google Scholar] [CrossRef]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Antioxidant activities of various solvent extracts of custard apple (Annona squamosa L.) fruit pulp. Nutrafoods 2012, 11, 137–144. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kesari, A.N.; Murthy, P.S.; Chandra, R.; Tandon, V.; Watal, G. Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. in experimental animals. J. Ethnopharmacol. 2005, 99, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.K.; Pathak, M.; Yadav, D.K.; Maurya, R.; Sahai, M.; Jain, S.K.; Misra-Bhattacharya, S. Immunomodulatory constituents from Annona squamosa twigs provoke differential immune response in BALB/c mice. Curr. Sci. 2013, 104, 1224–1230. [Google Scholar]
- Pandey, N.; Barve, D. Phytochemical and pharmacological review on Annona squamosa Linn. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1404–1412. [Google Scholar]
- Damasceno, D.C.; Volpato, G.T.; Sartori, T.C.F.; Rodrigues, P.F.; Perin, E.A.; Calderon, I.D.M.P.; Rudge, M.V.C. Effects of Annona squamosa extract on early pregnancy in rats. Phytomedicine 2002, 9, 667–672. [Google Scholar] [CrossRef]
- Jain, S.C.; Singh, B.; Jain, R. Arnebins and antimicrobial activities of Arnebia hispidissima DC. cell cultures. Phytomedicine 2000, 6, 474–476. [Google Scholar] [CrossRef]
- Hosseini, A.; Mirzaee, F.; Davoodi, A.; Jouybari, H.B.; Azadbakht, M. The traditional medicine aspects, biological activity and phytochemistry of Arnebia spp. Med. Glas. 2018, 15, 1–9. [Google Scholar]
- Lin, Z.B.; Chai, B.L.; Wang, P.; Guo, Q.X.; Lu, F.S.; Xiang, G.Q. Studies on the anti-inflammatory effect of chemical principle of Zi-cao (Arnebia euchroma). Pei Ching I Hsueh Yuan Hsueh Pao 1980, 12, 101–106. [Google Scholar]
- Tanaka, S.; Tajima, M.; Tsukada, M.; Tabata, M. A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J. Nat. Prod. 1986, 49, 466–469. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nishizawa, M.; Yamagishi, T.; Tanaka, T.; Nonaka, G.I.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents, 18. Sodium and potassium salts of caffeic acid tetramers from Arnebia euchroma as anti-HIV agents. J. Nat. Prod. 1995, 58, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Sankawa, U.; Ebizuka, Y.; Miyazaki, T.; Isomura, Y.; Otsuka, H.; Shibata, S.; Fukuoka, F. Antitumor activity of shikonin and its derivatives. Chem. Pharm. Bull. 1977, 25, 2392–2395. [Google Scholar] [CrossRef] [PubMed]
- Searle, J.; Lawson, T.A.; Abbott, P.J.; Harmon, B.; Kerr, J.F. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J. Pathol. 1975, 116, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.L.; Lin, C.H.; Li, C.H.; Tsai, C.H.; Ho, J.D.; Chiou, G.C.; Cheng, Y.W. Anti-inflammatory properties of shikonin contribute to improved early-stage diabetic retinopathy. Sci. Rep. 2017, 7, 44985. [Google Scholar] [CrossRef]
- Ganie, S.A.; Jan, A.; Muzaffar, S.; Zargar, B.A.; Hamid, R.; Zargar, M.A. Radical scavenging and antibacterial activity of Arnebia benthamii methanol extract. Asian Pac. J. Trop. Med. 2012, 5, 766–772. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Antioxidant and antimicrobial activities of Arnebia hispidissima. Am. J. Adv. Drug Deliv. 2014, 2, 224–237. [Google Scholar]
- Shameem, N.; Kamili, A.N.; Parray, J.A.; Hamid, R.; Bandh, S.A. Antimicrobial and antioxidant activity of methanol extracts of Arnebia benthamii (Wall ex. G. Don) Johnston—A critically endangered medicinal plant of North western Himalaya. J. Anal. Sci. Technol. 2015, 6, 36. [Google Scholar] [CrossRef]
- Parray, J.A.; Hamid, R.; Kamili, A.N.; Shameem, N.; Jan, S.; Ganai, B.A. Biological efficacy and radical scavenging potential of shikonin in Arnebia benthamii (Wall ex. G Don) Johnston. Ind. Crops Prod. 2015, 74, 434–439. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, W.; Liu, D.; Hou, M.; Liu, S.; He, W.; Shao, M. Identification, in vitro evaluation and modeling studies of the constituents from the roots of Arnebia euchroma for antitumor activity and STAT3 inhibition. Bioorganic Chem. 2020, 96, 103655. [Google Scholar] [CrossRef]
- Buck, K.T. The bisbenzylisoquinoline alkaloids. In The Alkaloids: Chemistry and Pharmacology; Academic Press: Cambridge, MA, USA, 1999; Volume 30, pp. 1–222. [Google Scholar]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K.C. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 1999, 38, 270–301. [Google Scholar] [CrossRef]
- Nakoti, S.S.; Juyal, D.; Josh, A.K. A review on pharmacognostic and phytochemical study of a plant Nardostachys Jatamansi. Pharma Innov. 2017, 6, 936. [Google Scholar]
- Hegde, K.; Joshi, A.B. Phytochemical investigation of root extract of the plant Carissa carandas Linn. Res. J. Pharm. Technol. 2010, 3, 217–220. [Google Scholar]
- Afzal, M.; Al-Oriquat, G. Shikonin derivatives, Part VI. Chemical investigations of Arnebia decumbens. Agric. Biol. Chem. 1986, 50, 1651–1652. [Google Scholar] [CrossRef]
- El-Desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in-vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef]
- Kim, J.; Park, E. Cytotoxic anticancer candidates from natural resources. Curr. Med. Chem.-Anti-Cancer Agents 2002, 2, 485–537. [Google Scholar] [CrossRef]
- Rumjuankiat, K.; Sonhom, N.; Showpanish, K.; Somsri, A.; Pilasombut, K. In vitro antioxidant activities and volatile compounds from Karanda (Carissa carandas L) fruit wine. Int. J. Agric. Res. 2018, 14, 1843–1860. [Google Scholar]
- Hayes, P.Y.; Jahidin, A.H.; Lehmann, R.; Penman, K.; Kitching, W.; De Voss, J.J. Steroidal saponins from the roots of Asparagus racemosus. Phytochemistry 2008, 69, 796–804. [Google Scholar] [CrossRef]
- Rao, A.R. Inhibitory action of Asparagus racemosus on DMBA-induced mammary carcinogenesis in rats. Int. J. Cancer 1981, 28, 607–610. [Google Scholar] [CrossRef]
- Diwanay, S.; Chitre, D.; Patwardhan, B. Immunoprotection by botanical drugs in cancer chemotherapy. J. Ethnopharmacol. 2004, 90, 49–55. [Google Scholar] [CrossRef]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; M/S Bishen Singh Mahendra Pal Singh: Dehradun, India, 1994; Volume 3, pp. 2186–2188. [Google Scholar]
- Ahmed, B.; Rahman, A. Bacosterol, a New 13, 14-Seco-Steroid and Bacosine, a New Triterpene from Bacopa monniera; NISCAIR-CSIR: New Delhi, India, 2000. [Google Scholar]
- Bhandari, P.; Kumar, N.; Singh, B.; Kaul, V.K. Bacosterol glycoside, a new 13, 14-seco-steroid glycoside from Bacopa monnieri. Chem. Pharm. Bull. 2006, 54, 240–241. [Google Scholar] [CrossRef]
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C. Phenolic profile and bioactive properties of Carissa macrocarpa (Eckl) A DC: An in-vitro comparative study between leaves stems and flowers. Molecules 2019, 24, 1696. [Google Scholar] [CrossRef] [PubMed]
- Hogland, H.C. Hematological complications of cancer chemotherapy. Semin. Oncol. 1982, 9, 95–102. [Google Scholar]
- Gupta, M.; Mazumder, U.K.; Kumar, R.S.; Gomathi, P.; Rajeshwar, Y.; Kakoti, B.B.; Selven, V.T. Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. J. Ethnopharmacol. 2005, 98, 267–273. [Google Scholar] [CrossRef] [PubMed]

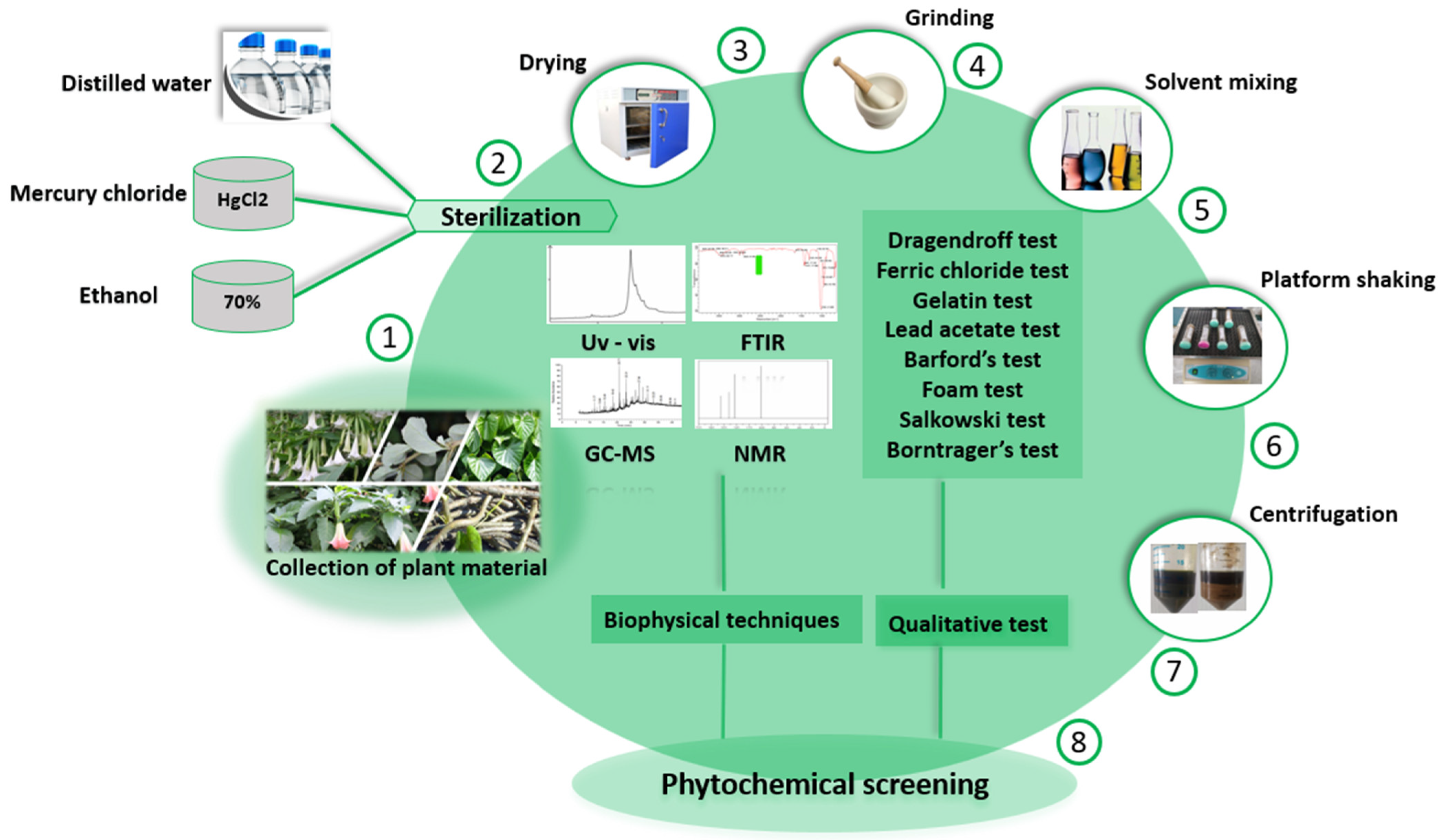
| Name of Medicinal Plants | Local Name | Family Name | Parts Used | Type of Anticancer Activity | Habitat | Reference |
|---|---|---|---|---|---|---|
| Semecarpus anacardium | Marking nut tree, phobi nut tree, and varnish tree, Ballataka | Anacardiaceae | Mature fruit | Anticancer | Sub-Himalayan regions | [89] |
| Annona squamosa | Seetapalam | Annonaceae | Aerial part | Colon and breast cancer | Lower regions of the Himalayas | [90] |
| Centella asiatica | Pegaga | Apiaceae | Leaves | Lung cancer | India | [91] |
| Asclepiascura ssavica | Kakatundi | Apocynaceae | Plant | Anti-nasopharynx human carcinoma | India | [92] |
| Catharanthus roseus | Nayantara, Sada Sawagan | Apocynaceae | Root | Breast cancer | India | [93] |
| Calotropis gigantea | Dryand, Erukku | Asclepiadaceae | flowers (flower petals) | Anti-Ehrlich ascites carcinoma cells | India | [94] |
| Bidens pilosa | Kateeli | Asteraceae | Leaves | Oral, liver, colon, and breast cancer | Garhwal, Uttaranchal, India | [37] |
| Blumealance olaria (Roxb.) | Buarze | Asteraceae | Leaves | Anticancer | Uttara Kannada district, Karnataka, India | [95] |
| Inula racemosa | Pushkarmula | Compositae | Roots | Colon, prostate, CNS, ovary, leukemia, and lung cancer | Jammu and Kashmir (India) | [61] |
| Mikania micrantha Kunth, Maotamgui | Maotamgui | Asteraceae | Leaves | Anti-esophageal adenocarcinoma | India | [96] |
| Cyanthillium cinereum | Little ironweed | Asteraceae | Plant | Anti-matrix metallopeptidases | India | [97] |
| Vernonia cinerea | Mookkuthi Poondu | Asteraceae | Aerial part | Anti-oral squamous cell carcinoma and lung carcinoma | India | [98] |
| Xanthium strumarium | Banokra, lanetsuru | Asteraceae | aerial parts (stems and leaves) | Anti-proliferative against HepG2 cancer cells | India, Kashmir | [99] |
| Begonia malabarica | Rathasurai, Kakidziihe | Begoniaceae | Plant | Colon, lung, and stomach cancer | India | [100] |
| Arnebia euchroma | Johnst, Ratan jot. | Boraginaceae | Roots/leaves | Anti-tumor | India | [33] |
| Croton tiglium | Koni-bih | Euphorbiaceae | Seeds | Anti-tumor S-180 and Ehrlich | India | [101] |
| Euphorbia hirta | Jar dudli | Euphorbiaceae | Whole plant | Anti-myeloid leukemia cancer cell line | Kashmir (India) | [102] |
| Mallotus philippensis | Rohini | Euphorbiaceae | Fruit Root, hairs | Anti-promyelocytic leukemia cells | India | [103] |
| Albizia lebbeck | Siris tree | Fabaceae | Bark | Breast cancer | India | [104] |
| Butea monosperma | Taub., Pala | Fabaceae | Flowers | Breast cancer | Kashmir (India) | [105] |
| Vitex negundo | Nira gundi | Verbenaceae | Leaves | Anti-Dalton’s ascitic lymphoma | India | [106] |
| Woodfordia fruticosa | Dhai | Lythraceae | Whole plant/flowers | Inhibit the proliferation of CML K562 cells | Kashmir (India) | [107] |
| Azadirachta indica | Dhrek | Meliaceae | aerial parts | Anti-MCF cancer Cell | Kashmir (India) | [108] |
| Moringa oleifera | Sajina | Moringaceae | Leaves | Lung cancer | India | [109] |
| Syzygium cumini | Jamun | Myrtaceae | Fruits | Anticancer cell lines ovarian adenocarcinoma, prostate carcinoma, non-small cell lung carcinoma | India, m Keshav Shristi, Bhayandar | [110] |
| Phyllanthus emblica | Sohlhu, aomla | Phyllanthaceae | Fruits | Prevented N-nitrosodiethylamine induced hepatocellular carcinoma | India | [111] |
| Plantago major | Jangremriza, Akaba | Plantaginaceae | Leaves | Against Ehrlich ascites carcinoma | India | [112] |
| Potentilla fulgens | Bajradanti | Rosaceae | Root | Breast cancer | India | [33] |
| Citrus limon | Kanji nemu | Rutaceae | Fresh fruits | Colon cancer | India | [113] |
| Taxus wallichiana | Lauthsalla, barmi, banya | Taxaceae | Needles, bark, root, seed, heartwood | Anti-liver, colon, ovarian, and breast cancer cell | India, Kashmir (India) | [114] |
| Curcuma longa | Haldi | Zingiberaceae | Rhizomes | Dalton’s lymphoma cells | India, U.P. Himalaya | [115] |
| Leguminase | Siris, Shiris in Hindi; Lebbeck tree in English | Albizzia lebbeck (Lin.) Benth | Bark | Anti-human breast cancer cells | India | [104] |
| Anona squamosa | Gandagatra | Annonaceae V. N. Sitaphala | Plant | The inhibited proliferation of HL-60 cells | India, U.P. Himalaya | [116] |
| Asparagus racemosus | Wild Shatavari, Ekalkanto | Liliaceae V. N. Satavari | Roots | Anti-breast cancer, colon, adeno carcinoma, kidney carcinoma, and EAC tumor cells | India, U.P. Himalaya | [117] |
| Bacopa monniera | Water hyssop, water hyssop, brahmi | Scrophulariaceae V. N. Brahmi | Plant | Ehrlich ascites carcinoma | India, U.P. Himalaya | [118] |
| Bauhinia racemosa | Asundro Bidi Leaf tree | Caesalpiniaceae V. N. Kandi | Bark | Against a HeLa cancer cell | India, U.P. Himalaya | [119] |
| Berberis asisatica | Daruharidra, Darbi (Sans) | Berberidaceae V. N. Kingori | Roots | Dalton’s lymphoma ascites tumor cells | India, U.P. Himalaya | [120] |
| Berberis aristate, | Daruharidra | Berberidaceae V. N. Daruharidra | Bark | Anticancer | India, U.P. Himalaya | [121] |
| Berberis lyceum | Ishkeen (Kashmal and Darbald) | V. N. Kingore | Root | Anticancer | India, U.P. Himalaya | [122] |
| Bergenia ligulata | Pakhanbheda | Saxifragaceae V. N. Silparo | Roots | SARCOMA-WM1256 IM | India, U.P. Himalaya | [123] |
| Calotropis gigantea | Milkweed, Madara, Akavan, Aak | Asclepiadaceae V. N. Akha, Arka | Root, leaves & Latex | Prevents oral, prostrate, and colon cancer, anti-hepatocellular carcinoma | India, U.P. Himalaya, India, U.P. | [124,125] |
| Cedrus deodara | Dabadru, Devadru, Devadrus, Devdar (Bengali) | Pinaceae V. N. Devadara | Heartwood | Anticancer | India, U.P. Himalaya | [126] |
| Datura metel | Indian Thornapple, Hindu Datura | Solanaceae V. N. Dhatura | Aerial parts | Anti-colorectal carcinoma | India, U.P. Himalaya | [127] |
| Podophyllus emodi | Himalayan/Indian may apple | Berberidaceae V. N. Baryakarkatee | Rhizomes | Anticancer | India, U.P. Himalaya | [128] |
| Urtica dioica | Nettle leaf, or just a nettle or stinger | Urticaceae V. N. Bichubooti, Brishakali, Kandali | leaves and stems | Repress prostate-cell metabolism and proliferation | India, U.P. Himalaya | [129] |
| Species Name | Phytochemicals | Chemical Structure | Types of Cancers | Reference |
|---|---|---|---|---|
| Catharanthus roseus | Vinca alkaloids | 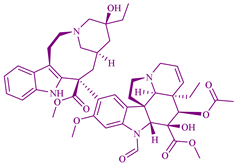 | Kaposi’s sarcoma, breast, leukemia, testicular and lung cancers, and lymphomas | [130] |
| Podophyllum peltatum | Podophyllotoxin | 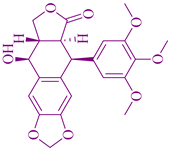 | Testicular, bronchial, and lymphomas cancer | [131] |
| Taxus brevifolia | Taxanes | 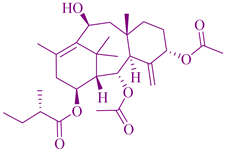 | Kaposi sarcoma, lung cancer, ovarian and breast cancer | [132] |
| Camptotheca acuminata | Camptothecin |  | Colorectal, lung, ovarian cancer | [133] |
| Cephalotaxus harringtonia var. drupacea | Homoharringtonine |  | Chronic myelogenous leukemia and myelogenous leukemia | [134] |
| Albizia lebbeck | Saponin | 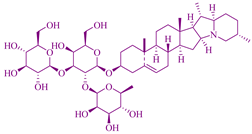 | Cervical, larynx, hepatocarcinoma, breast, and colon carcinoma | [135] |
| Annona squamosa | Isoquinoline alkaloid |  | Liver, breast, and colon cancer | [90] |
| Arnebia euchroma | Shikonin | 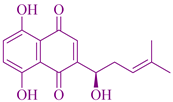 | Prevents cancer-causing and malfunction compounds | [33] |
| Asclepias curassavica | Calotropin |  | Nasopharynx carcinoma | [136] |
| Asparagus racemosus | Shatavarin IV |  | Ehrlich ascites carcinoma (EAC) tumor | [137] |
| Bacopa monnieri | Stigmasterol |  | Inhibits EAC (Ehrlich ascites carcinoma) and protects the liver of the EAC patient | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regassa, H.; Sourirajan, A.; Kumar, V.; Pandey, S.; Kumar, D.; Dev, K. A Review of Medicinal Plants of the Himalayas with Anti-Proliferative Activity for the Treatment of Various Cancers. Cancers 2022, 14, 3898. https://doi.org/10.3390/cancers14163898
Regassa H, Sourirajan A, Kumar V, Pandey S, Kumar D, Dev K. A Review of Medicinal Plants of the Himalayas with Anti-Proliferative Activity for the Treatment of Various Cancers. Cancers. 2022; 14(16):3898. https://doi.org/10.3390/cancers14163898
Chicago/Turabian StyleRegassa, Hailemeleak, Anuradha Sourirajan, Vikas Kumar, Sadanand Pandey, Deepak Kumar, and Kamal Dev. 2022. "A Review of Medicinal Plants of the Himalayas with Anti-Proliferative Activity for the Treatment of Various Cancers" Cancers 14, no. 16: 3898. https://doi.org/10.3390/cancers14163898
APA StyleRegassa, H., Sourirajan, A., Kumar, V., Pandey, S., Kumar, D., & Dev, K. (2022). A Review of Medicinal Plants of the Himalayas with Anti-Proliferative Activity for the Treatment of Various Cancers. Cancers, 14(16), 3898. https://doi.org/10.3390/cancers14163898