Calorimetric Markers for Detection and Monitoring of Multiple Myeloma
Abstract
:Simple Summary
Abstract
1. Application of Differential Scanning Calorimetry for Plasma/Serum Proteome Characterization
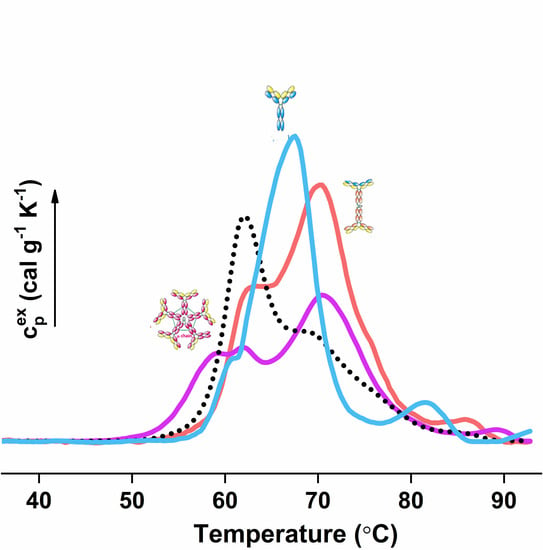
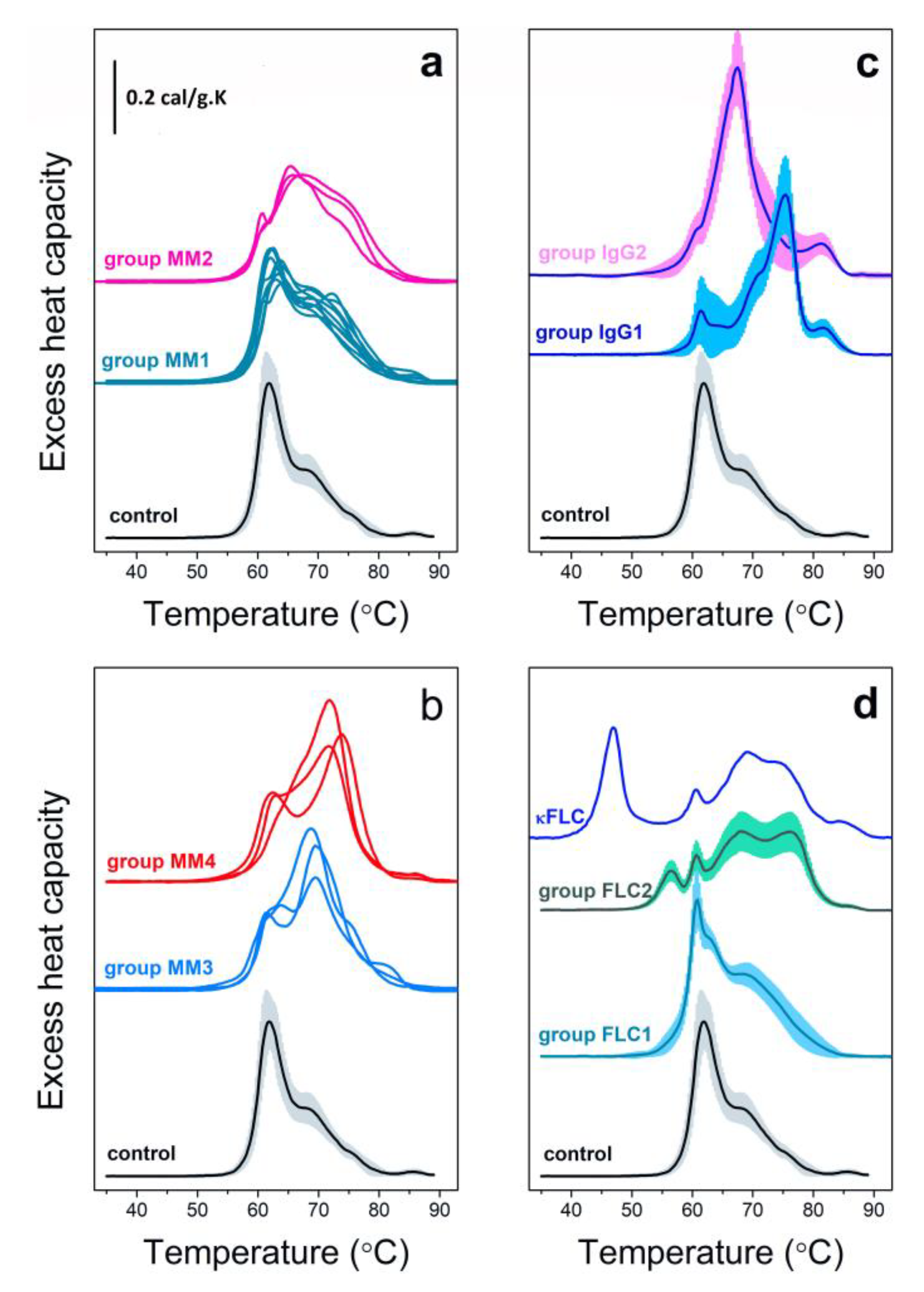
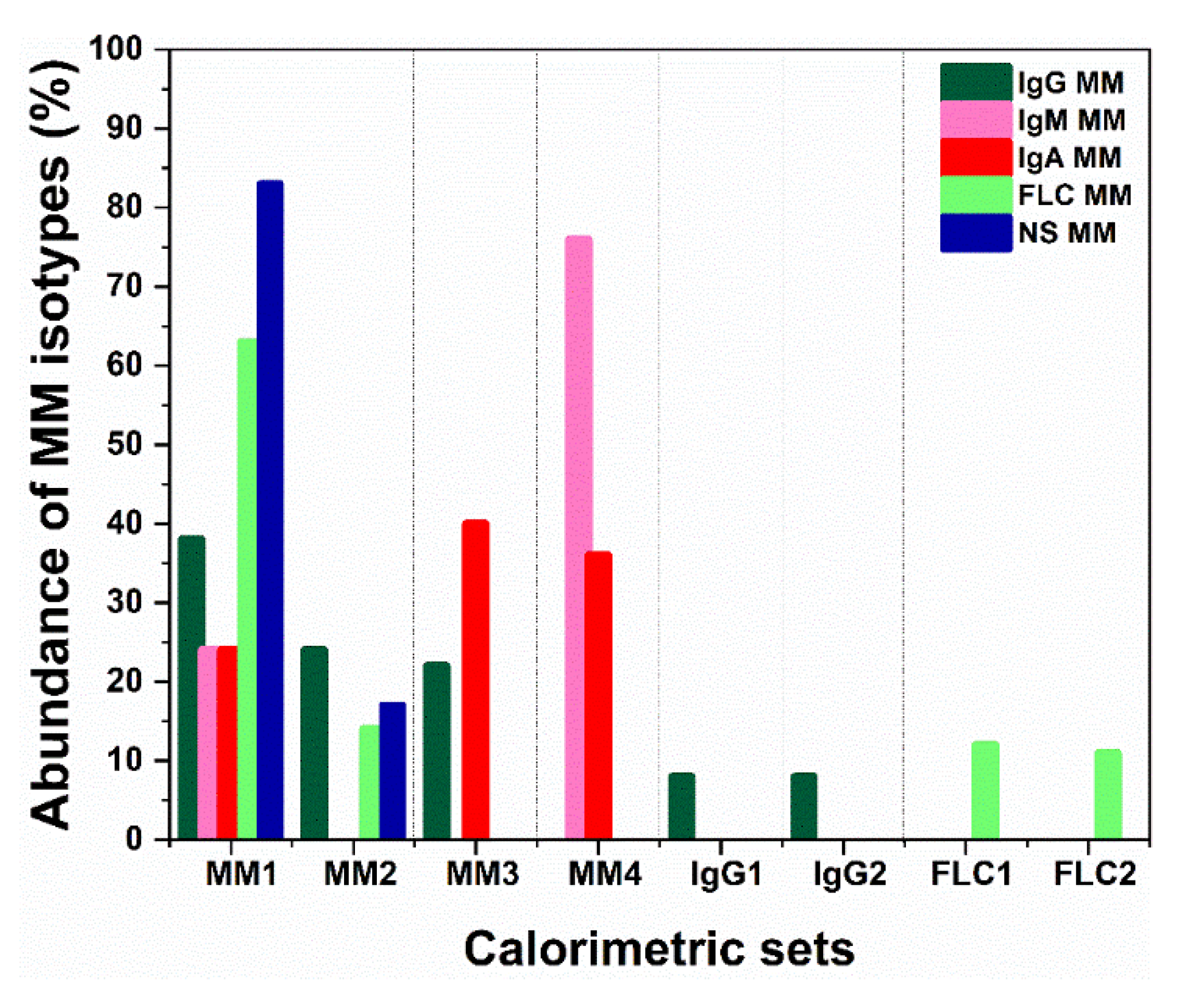
2. Multiple Myeloma Discrimination and Calorimetry-Based Classification
3. Calorimetric Signatures of Secretory and Non-Secretory MM Types
4. Monitoring of MM Patients by DSC
5. DSC Based Discrimination of MM and Other Diseases
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garbett, N.C.; Miller, J.J.; Jenson, A.B.; Miller, D.M.; Chaires, J.B. Interrogation of the plasma proteome with differential scanning calorimetry. Clin. Chem. 2007, 53, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Garbett, N.C.; Miller, J.J.; Jenson, A.B.; Chaires, J.B. Calorimetric analysis of the plasma proteome. Semin. Nephrol. 2007, 27, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Garbett, N.C.; Mekmaysy, C.S.; DeLeeuw, L.; Chaires, J.B. Clinical application of plasma thermograms. Utility, practical approaches and considerations. Methods 2015, 76, 41–50. [Google Scholar] [CrossRef]
- Garbett, N.C.; Mekmaysy, C.S.; Helm, C.W.; Jenson, A.B.; Chaires, J.B. Differential scanning calorimetry of blood plasma for clinical diagnosis and monitoring. Exp. Mol. Pathol. 2009, 86, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Todinova, S.; Krumova, S.; Gartcheva, L.; Robeerst, C.; Taneva, S.G. Microcalorimetry of blood serum proteome: A modified interaction network in the multiple myeloma case. Anal. Chem. 2011, 83, 7992–7998. [Google Scholar] [CrossRef]
- Chagovetz, A.A.; Jensen, R.L.; Recht, L.; Glantz, M.; Chagovetz, A.M. Preliminary use of differential scanning calorimetry of cerebrospinal fluid for the diagnosis of glioblastoma multiforme. J. Neurooncol. 2011, 105, 499–506. [Google Scholar] [CrossRef]
- Antonova, B.; Naydenov, E.; Koynova, R.; Tumangelova-Yuzeir, K.; Tenchov, B. Exothermic transitions in the heat capacity profiles of human cerebrospinal fluid. Eur. Biophys. J. 2020, 49, 231–238. [Google Scholar] [CrossRef]
- Dandé, A.; Kocsis, B.; ·Lőrinczy, D. Thermal analysis of synovial fluids in different stages of osteoarthritis and after bacterial infections. J. Therm. Anal. Calorim. 2020, 142, 797–808. [Google Scholar] [CrossRef]
- Garbett, N.C.; Miller, J.J.; Jenson, A.B.; Chaires, J.B. Calorimetry Outside the Box: A New Window into the Plasma Proteome. Biophys. J. 2008, 94, 1377–1383. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell Proteomics 2002, 1, 845–867. [Google Scholar] [CrossRef]
- Garbett, N.C.; Merchant, M.L.; Chaires, J.B.; Klein, J.B. Calorimetric analysis of the plasma proteome: Identification of type 1 diabetes patients with early renal function decline. Biochim. Biophys. Acta 2013, 1830, 4675–4680. [Google Scholar] [CrossRef]
- Garbett, N.C.; Brock, G.N.; Chaires, J.B.; Mekmaysy, C.S.; DeLeeuw, L.; Sivils, K.L.; Harley, J.B.; Rovin, B.H.; Kulasekera, K.B.; Jarjour, W.N. Characterization and classification of lupus patients based on plasma thermograms. PLoS ONE 2017, 12, e0186398. [Google Scholar] [CrossRef]
- Michnik, A.; Drzazga, Z.; Michalik, K.; Barczyk, A.; Santura, I.; Sozańska, E.; Pierzchała, W. Differential scanning calorimetry study of blood serum in chronic obstructive pulmonary disease. J. Therm. Anal. Calorim. 2010, 102, 57–60. [Google Scholar] [CrossRef]
- Moezzi, M.; Fekecs, T.; Zapf, I.; Ferencz, A.; Lörinczy, D. Differential scanning calorimetry (DSC) analysis of human plasma in different psoriasis stages. J. Therm. Anal. Calorim. 2013, 111, 1801–1804. [Google Scholar]
- Krumova, S.; Rukova, B.; Todinova, S.; Gartcheva, L.; Milanova, V.; Toncheva, D.; Taneva, S.G. Calorimetric monitoring of the serum proteome in schizophrenia patients. Thermochim. Acta 2013, 572, 59–64. [Google Scholar] [CrossRef]
- Barceló, F.; Cerdà, J.J.; Gutiérrez, A.; Jimenez-Marco, T.; Durán, M.M.; Novo, A.; Ros, T.; Sampol, A.; Portugal, J. Characterization of monoclonal gammopathy of undetermined significance by calorimetric analysis of blood serum proteome. PLoS ONE 2015, 10, e0120316. [Google Scholar]
- Kendrick, S.K.; Zheng, Q.; Garbett, N.C.; Brock, G.N. Application and interpretation of functional data analysis techniques to differential scanning calorimetry data from lupus patients. PLoS ONE 2017, 12, e0186232. [Google Scholar]
- Todinova, S.; Krumova, S.; Kurtev, P.; Dimitrov, V.; Djongov, L.; Dudunkov, Z.; Taneva, S.G. Calorimetry-based profiling of blood plasma from colorectal cancer patients. Biochim. Biophys. Acta 2012, 1820, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Annesi, F.; Hermoso-Durán, S.; Rizzuti, B.; Bruno, R.; Pirritano, D.; Petrone, A.; Del Giudice, F.; Ojeda, J.; Vega, S.; Sanchez-Gracia, O.; et al. Thermal Liquid Biopsy (TLB) of blood plasma as a potential tool to help in the early diagnosis of multiple sclerosis. J. Pers. Med. 2021, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Zapf, I.; Fekecs, T.; Ferencz, A.; Tizedes, G.; Pavlovics, G.; Kálmánd, E.; Lőrinczy, D. DSC analysis of human plasma in breast cancer patients. Thermochim. Acta 2011, 524, 88–91. [Google Scholar] [CrossRef]
- Zapf, I.; Moezzi, M.; Fekecs, T.; Nedvig, K.; Lőrinczy, D.; Ferencz, A. Influence of oxidative injury and monitoring of blood plasma by DSC on breast cancer patients. J. Therm. Anal. Calorim. 2016, 123, 2029–2035. [Google Scholar] [CrossRef]
- Kikalishvili, L.; Ramishvili, M.; Nemsadze, G.; Lezhava, T.; Khorava, P.; Gorgoshidze, M.; Kiladze, M.; Monaselidze, J. Thermal stability of blood plasma proteins of breast cancer patients, DSC study. J. Therm. Anal. Calorim. 2015, 120, 501–505. [Google Scholar] [CrossRef]
- Faroongsarng, D.; Sunpaweravong, S.; Raksawong, A. Thermally induced denaturing energetics of human blood plasma albumin by differential scanning calorimetry (DSC) as an indicator for breast cancer diagnosis in female patients. AAPS Pharm. Sci. Tech. 2019, 20, 146. [Google Scholar] [CrossRef] [PubMed]
- Hermoso-Durán, S.; García-Rayado, G.; Ceballos-Laita, L.; Sostres, C.; Vega, S.; Millastre, J.; Sánchez-Gracia, O.; Ojeda, J.L.; Lanas, A.; Velázquez-Campoy, A.; et al. Thermal Liquid Biopsy (TLB) focused on benign and premalignant pancreatic cyst diagnosis. J. Pers. Med. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Garbett, N.C.; Merchant, M.L.; Helm, C.W.; Jenson, A.B.; Klein, J.B.; Chaires, J.B. Detection of cervical cancer biomarker patterns in blood plasma and urine by differential scanning calorimetry and mass spectrometry. PLoS ONE 2014, 9, e84710. [Google Scholar] [CrossRef]
- Rai, S.N.; Pan, J.; Cambon, A.; Chaires, J.B.; Garbett, N.C. Group classification based on high-dimensional data: Application to differential scanning calorimetry plasma thermogram analysis of cervical cancer and control samples. Open Access Med. Stat. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Rai, S.N.; Srivastava, S.; Pan, J.; Wu, X.; Rai, S.P.; Mekmaysy, C.S.; DeLeeuw, L.; Chaires, J.B.; Garbett, N.C. Multi-group diagnostic classification of high-dimensional data using differential scanning calorimetry plasma thermograms. PLoS ONE 2019, 14, e0220765. [Google Scholar]
- Kedra-Krolik, K.; Chmielewska, I.; Michnik, A.; Zarzycki, P. Blood serum calorimetry indicates the chemotherapeutic efficacy in lung cancer treatment. Sci. Rep. 2017, 7, 16796. [Google Scholar] [CrossRef]
- Koynova, R.; Antonova, B.; Sezanova, B.; Tenchov, B. Beneficial effect of sequential chemotherapy treatments of lung cancer patients revealed by calorimetric monitoring of blood plasma proteome denaturation. Thermochim. Acta 2018, 659, 1–7. [Google Scholar] [CrossRef]
- Schneider, G.; Kaliappan, A.; Nguyen, T.Q.; Buscaglia, R.; Brock, G.N.; Hall, M.B.; DeSpirito, C.; Wilkey, D.W.; Merchant, M.L.; Klein, J.B.; et al. The utility of differential scanning calorimetry curves of blood plasma for diagnosis, subtype differentiation and predicted survival in lung cancer. Cancers 2021, 13, 5326. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, F.; Devred, F. Plasmatic signature of disease by differential scanning calorimetry (DSC). In Microcalorimetry of Biological Molecules. Methods in Molecular Biology; Ennifar, E., Ed.; Humana Press: Totowa, NJ, USA, 2019; Volume 1964, pp. 45–57. [Google Scholar]
- Rodrigo, A.; Ojeda, J.L.; Vega, S.; Sanchez-Gracia, O.; Lanas, A.; Isla, D.; Velazquez-Campoy, A.; Abian, O. Thermal Liquid Biopsy (TLB): A predictive score derived from serum thermograms as a clinical tool for screening lung cancer patients. Cancers (Basel) 2019, 11, 1012. [Google Scholar] [CrossRef]
- Tsvetkov, P.O.; Tabouret, E.; Roman, A.Y.; Romain, S.; Bequet, C.; Ishimbaeva, O.; Honoré, S.; Figarella- Branger, D.; Chinot, O.; Devred, F. Differential scanning calorimetry of plasma in glioblastoma: Toward a new prognostic/monitoring tool. Oncotarget 2018, 9, 9391–9399. [Google Scholar] [CrossRef]
- Vega, S.; Garcia-Gonzalez, M.A.; Lanas, A.; Velazquez-Campoy, A.; Abian, O. Deconvolution analysis for classifying gastric adenocarcinoma patients based on differential scanning calorimetry serum thermograms. Sci. Rep. 2015, 5, 7988. [Google Scholar] [CrossRef]
- Ferencz, A.; Fekecs, T.; Lorinczy, D. Differential scanning calorimetry, as a new method to monitor human plasma in melanoma patients with regional lymph node or distal metastases. In Skin Cancer Overview; Xi, Y., Ed.; InTech: London, UK, 2011; pp. 141–151. [Google Scholar]
- Fekecs, T.; Zapf, I.; Ferencz, A.; Lőrinczy, D. Differential scanning calorimetry (DSC) analysis of human plasma in melanoma patients with or without regional lymph node metastases. J. Therm. Anal. Calorim. 2012, 108, 149–152. [Google Scholar] [CrossRef]
- Velazquez-Campoy, A.; Vega, S.; Sanchez-Gracia, O.; Lanas, A.; Rodrigo, A.; Kaliappan, A.; Hall, M.B.; Nguyen, T.Q.; Brock, G.N.; Chesney, J.A.; et al. Thermal liquid biopsy for monitoring melanoma patients under surveillance during treatment: A pilot study. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Todinova, S.; Krumova, S.; Radoeva, R.; Gartcheva, L.; Taneva, S.G. Calorimetric markers of Bence Jones and nonsecretory multiple myeloma serum proteome. Anal. Chem. 2014, 86, 12355–12361. [Google Scholar] [CrossRef] [PubMed]
- Todinova, S.; Krumova, S.; Andreeva, T.; Dimitrova, K.; Gartcheva, L.; Taneva, L.G. Unusual thermal transition in the serum calorimetric profile of a patient diagnosed with multiple myeloma with secretion of monoclonal κ free light chains: A case report. Cancer Res. Front. 2016, 2, 416–426. [Google Scholar] [CrossRef]
- Todinova, S.; Krumova, S.; Danailova, A.; Petkova, V.; Guenova, M.; Mihaylov, G.; Gartcheva, L.; Taneva, S.G. Calorimetric markers for monitoring of multiple myeloma and Waldenström’s macroglobulinemia patients. Eur. Biophys. J. 2018, 47, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Krumova, S.; Todinova, S.; Danailova, A.; Petkova, V.; Dimitrova, K.; Gartcheva, L.; Taneva, S.G. Calorimetric features of IgM gammopathies. Implication for patient’s diagnosis and monitoring. Thermochim. Acta 2015, 615, 23–29. [Google Scholar] [CrossRef]
- Todinova, S.; Krumova, S.; Gartcheva, L.; Dimitrova, K.; Petkova, V.; Taneva, S.G. Calorimetric manifestation of IgA monoclonal immunoglobulins in multiple myeloma sera. Thermochim. Acta 2018, 666, 208–211. [Google Scholar] [CrossRef]
- Danailova, A.; Todinova, S.; Dimitrova, K.; Petkova, K.; Guenova, M.; Mihaylov, G.; Gartcheva, L.; Krumova, S.; Taneva, S. Effect of autologous stem-cells transplantation of patients with multiple myeloma on the calorimetric markers of the serum proteome. Correlation with the immunological markers. Thermochim. Acta 2017, 655, 351–357. [Google Scholar] [CrossRef]
- Brudar, S.; Černigoj, U.; Podgornik, H.; Kržan, M.; Prislan, I. Use of differential scanning calorimetry and immunoaffinity chromatography to identify disease induced changes in human blood plasma proteome. Acta Chim. Slov. 2017, 64, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Fish, D.J.; Brewood, G.P.; Kim, J.S.; Garbett, N.C.; Chaires, J.B.; Benight, A.S. Statistical analysis of plasma thermograms measured by differential scanning calorimetry. Biophys. Chem. 2010, 152, 184–190. [Google Scholar] [CrossRef]
- Garbett, N.C.; Brock, G.N. Differential scanning calorimetry as a complementary diagnostic tool for the evaluation of biological samples. Biochim. Biophys. Acta 2016, 1860, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Atanassov, K. Intuitionistic fuzzy sets. Fuzzy Sets Syst. 1986, 20, 87–96. [Google Scholar] [CrossRef]
- Todinova, S.; Mavrov, D.; Krumova, S.; Marinov, P.; Atanassova, V.; Atanassov, K.; Taneva, S.G. Blood plasma thermograms dataset analysis by means of intercriteria and correlation analyses for the case of colorectal cancer. Int. J. Bioautomation 2016, 20, 115–124. [Google Scholar]
- Krumova, S.; Todinova, S.; Mavrov, D.; Marinov, P.; Atanassova, V.; Atanassov, K.; Taneva, S.G. Intercriteria analysis of calorimetric data of blood serum proteome. Biochim. Biophys. Acta 2017, 1861, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Sikkink, L.A.; Ramirez-Alvarado, M. Biochemical and aggregation analysis of Bence Jones proteins from different light chain diseases. Amyloid 2008, 15, 29–39. [Google Scholar] [CrossRef]
- Blancas-Mejía, L.M.; Tischer, A.; Thompson, J.R.; Tai, J.; Wang, L.; Auton, M.; Ramirez-Alvarado, M. Kinetic control in protein folding for light chain amyloidosis and the differential effects of somatic mutations. J. Mol. Biol. 2014, 426, 347–361. [Google Scholar] [CrossRef]
- Poshusta, T.L.; Katoh, N.; Gertz, M.A.; Dispenzieri, A.; Ramirez-Alvarado, M. Thermal stability threshold for amyloid formation in light chain amyloidosis. Int. J. Mol. Sci. 2013, 14, 22604–22617. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, T.X.; Horita, T.J.; Kasravi, B. Understanding and interpreting serum protein electrophoresis. Am. Fam. Physician 2005, 71, 105–112. [Google Scholar] [PubMed]
- Dispenzieri, A.; Kyle, R.; Merlini, G.; Miguel, J.S.; Ludwig, H.; Hajek, R.; Palumbo, A.; Jagannath, S.; Blade, J.; Lonial, S.; et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia 2009, 23, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.S.; Kumar, S.K.; Dispenzieri, A.; Greenberg, A.J.; Larson, D.R.; Kyle, R.A.; Lacy, M.Q.; Gertz, M.A.; Rajkumar, S.V. Clinical course and prognosis of non-secretory multiple myeloma. Eur. J. Haematol. 2015, 95, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef] [PubMed]
- Garbett, N.C.; Miller, J.J.; Jenson, A.B.; Chaires, J.B. Ligand binding alters the calorimetric thermogram of albumin. J. Clin. Ligand Assay 2006, 29, 194–197. [Google Scholar]
- Bruschi, M.; Musante, L.; Ghiggeri, G.M.; Rialdi, G.; Candiano, G. Comparative study of thermal stability of healthy and focal segmental glomerulosclerosis plasma albumin. J. Therm. Anal. Calorim. 2007, 87, 27–31. [Google Scholar] [CrossRef]
- Sharma, A.; Tripathi, M.; Satyam, A.; Kumar, L. Study of antioxidant levels in patients with multiple myeloma. Leuk. Lymphoma 2009, 50, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Allegra, A.; Alonci, A.; Cristani, M.; Russo, S.; Speciale, A.; Penna, G.; Spatari, G.; Cannavò, A.; Bellomo, G.; et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012, 61, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, W.; Zainulabdeen, J.; Mehde, A. Investigation of the antioxidant status in multiple myeloma patients: Effects of therapy. Asian Pac. J. Cancer Prev. 2013, 14, 3663–3667. [Google Scholar] [CrossRef]
- Anna Michnik, A.; Drzazga, Z. Thermal denaturation of mixtures of human serum proteins. DSC study. J. Therm. Anal. Calorim. 2010, 101, 513–518. [Google Scholar] [CrossRef]
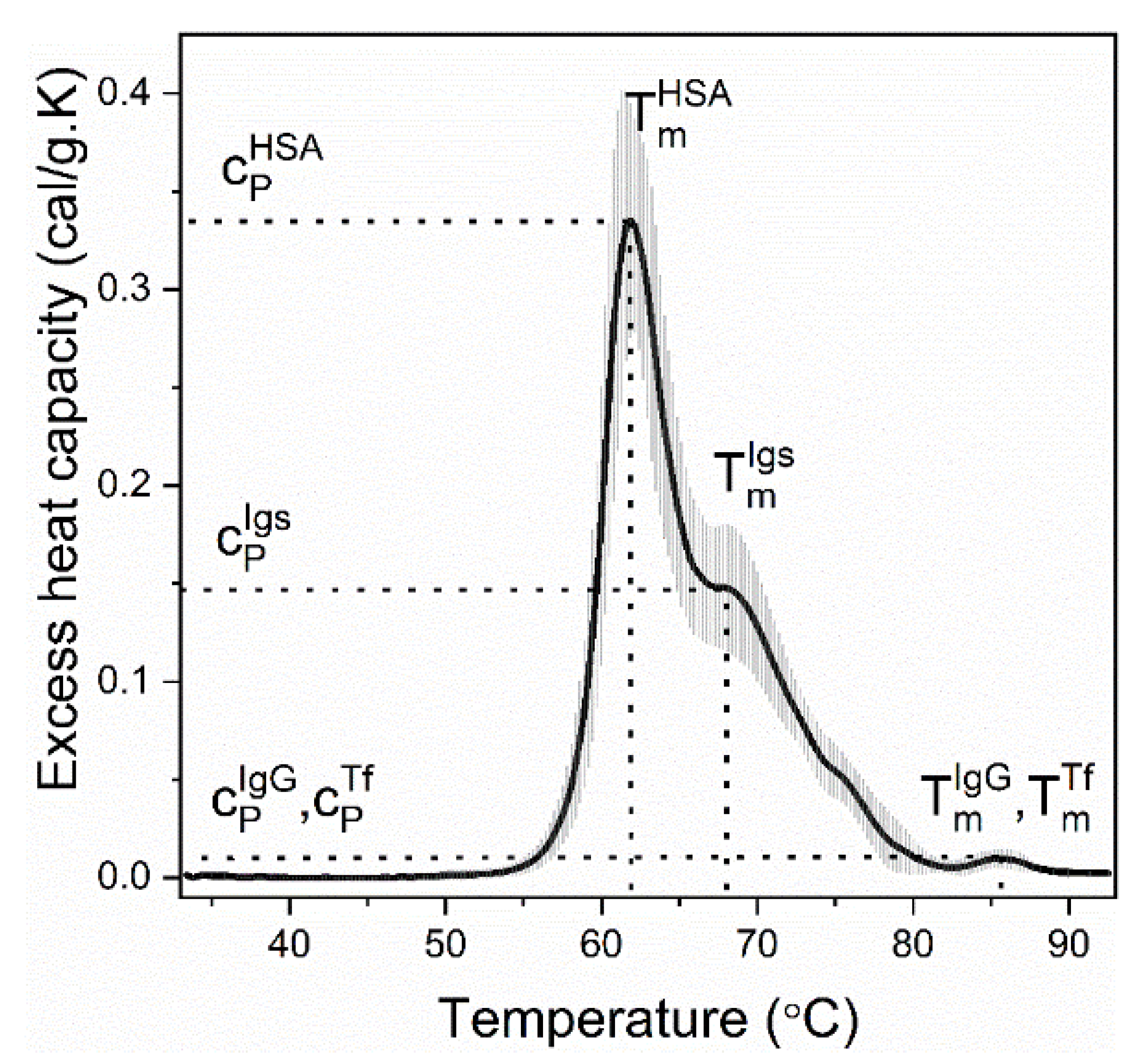
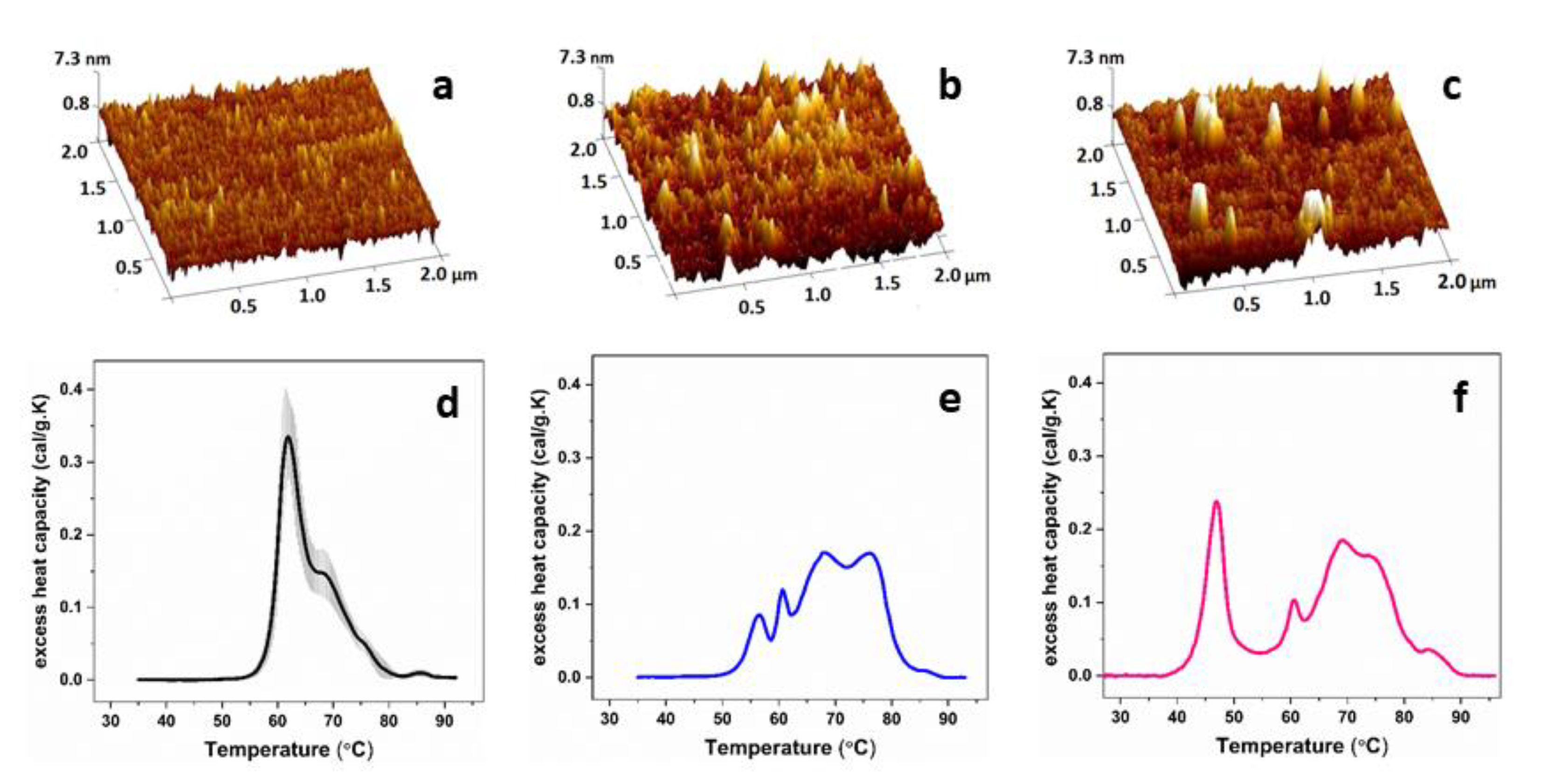

| Calorimetric Parameters | |
| Tmi | transition temperature, i is either albumin (HSA) or immunoglobulins (Igs) |
| cPi | excess heat capacity, i is either albumin (HSA), or immunoglobulins (Igs) |
| cPHSA/cPIgs | ratio of the excess heat capacities of albumin and immunoglobulins transitions |
| ΔHcal = ∫cP. dT | enthalpy of the thermogram (integrated area of the thermogram) |
where T1 is the initial and T2 is the final temperature point of the thermogram | weighted average center of the thermogram |
| Statistical Measures | |
| r | Pearson’s correlation coefficient |
| P | spatial distance metric |
| similarity metric | |
| where 0 ≤ w ≤ 2 | |
| InterCriteria Analysis | |
| μ(ci,cj) | degree of similarity between the pair of criteria (ci, cj) determined for sets of MM thermograms by InterCriteria Analysis |
| ν(ci,cj) | degree of differentiation between the pair of criteria (ci, cj) determined for sets of MM thermograms by InterCriteria Analysis |
| Calorimetric Group | TmHSA (°C) | TmIgs (°C) | cPHSA/cPIgs | TFM (°C) | ΔHcal (cal/g) | r | P | ρ |
|---|---|---|---|---|---|---|---|---|
| control | 61.9 ± 0.7 | 68.4 ± 0.3 | 2.45 ± 0.72 | 64.0 ± 1.1 | 3.1 ± 0.3 | 0.97 ± 0.20 | 0.76 ± 0.05 | 0.80 ± 0.10 |
| MM1 | 63.1 ± 0.7 | 68.8 ± 1.4 | 1.41 ± 0.23 | 67.0 ± 1.1 | 3.4 ± 0.2 | 0.89 ± 0.80 | 0.71 ± 0.09 | 0.75 ± 0.09 |
| MM2 | 66.2 ± 0.9 | 72.1 ± 0.4 | 1.38 ± 0.29 | 68.5 ± 0.9 | 3.5 ± 0.3 | 0.66 ± 0.08 | 0.63 ± 0.08 | 0.64 ± 0.08 |
| MM3 | 62.6 ± 1.1 | 69.3 ± 0.4 | 0.60 ± 0.13 | 68.8 ± 0.4 | 3.8 ± 0.5 | 0.64 ± 0.08 | 0.59 ± 0.10 | 0.61 ± 0.10 |
| MM4 | 63.0 ± 0.7 | 72.8 ± 1.4 | 0.63 ± 0.02 | 70.1 ± 0.9 | 4.0 ± 0.1 | 0.49 ± 0.10 | 0.59 ± 0.07 | 0.56 ± 0.08 |
| IgG1 | 60.0 ± 0.7 | 75.3 ± 0.4 | 0.39 ± 0.05 | 73.3 ± 1.2 | 3.21 ± 0.9 | 0.29 ± 0.10 | 0.42 ± 0.06 | 0.38 ± 0.06 |
| IgG2 | 60.8 ± 0.6 | 67.4 ± 0.2 | 0.25 ± 0.05 | 67.3 ± 0.6 | 4.25 ± 1.1 | 0.29 ± 0.10 | 0.56 ± 0.05 | 0.51 ± 0.07 |
| FLC1 | 62.9 ± 0.6 | 69.5 ± 1.1 | 1.47 ± 0.50 | 65.7 ± 1.3 | 3.80 ± 0.50 | 0.94 ± 0.02 | 0.72 ± 0.09 | 0.74 ± 0.02 |
| FLC2 | 68.2 ± 0.9 | 77.1 ± 1.3 | 0.99 ± 0.26 | 67.8 ± 1.4 | 3.60 ± 0.60 | 0.38 ± 0.09 | 0.55 ± 0.08 | 0.49 ± 0.07 |
| κFLC-case | 60.5 | 69.1 | 0.55 | 67.2 | 4.43 | 0.47 | 0.57 | 0.54 |
| ci,cj | μ(ci,cj) | ν(ci,cj) |
|---|---|---|
| cPIgs, [M] | 0.790 | 0.163 |
| cPIgs, cPHSA | 0.239 | 0.666 |
| cPHSA, [HSA] | 0.560 | 0.391 |
| cPIgs, TFM | 0.713 | 0.239 |
| cPIgs, ρ | 0.204 | 0.725 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumova, S.; Todinova, S.; Taneva, S.G. Calorimetric Markers for Detection and Monitoring of Multiple Myeloma. Cancers 2022, 14, 3884. https://doi.org/10.3390/cancers14163884
Krumova S, Todinova S, Taneva SG. Calorimetric Markers for Detection and Monitoring of Multiple Myeloma. Cancers. 2022; 14(16):3884. https://doi.org/10.3390/cancers14163884
Chicago/Turabian StyleKrumova, Sashka, Svetla Todinova, and Stefka G. Taneva. 2022. "Calorimetric Markers for Detection and Monitoring of Multiple Myeloma" Cancers 14, no. 16: 3884. https://doi.org/10.3390/cancers14163884
APA StyleKrumova, S., Todinova, S., & Taneva, S. G. (2022). Calorimetric Markers for Detection and Monitoring of Multiple Myeloma. Cancers, 14(16), 3884. https://doi.org/10.3390/cancers14163884