Myofibroblastic CAF Density, Not Activated Stroma Index, Indicates Prognosis after Neoadjuvant Therapy of Pancreatic Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
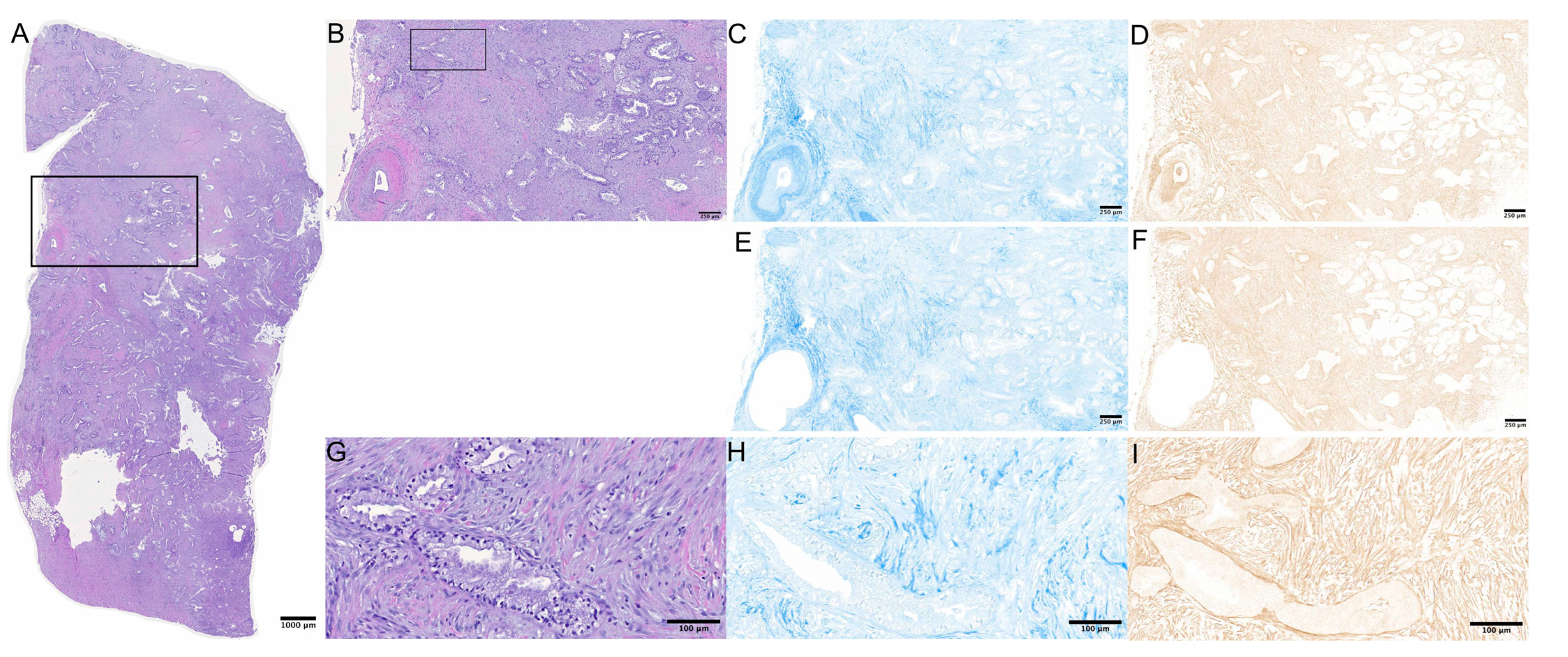
2.2. Stainings and Microscopy
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Tissue Markers
3.3. Survival
3.3.1. ASI and Survival
3.3.2. α-SMA/Aniline Blue and Survival
3.3.3. Fibrogenic, Inert, Dormant and Fibrolytic Stroma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hackert, T.; Buchler, M.W. Pancreatic cancer: Advances in treatment, results and limitations. Dig. Dis. 2013, 31, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The pancreas cancer microenvironment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef] [PubMed]
- Erkan, M.; Reiser-Erkan, C.; Michalski, C.W.; Kong, B.; Esposito, I.; Friess, H.; Kleeff, J. The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Curr. Mol. Med. 2012, 12, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Ohlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Pereira, B.A.; Vennin, C.; Papanicolaou, M.; Chambers, C.R.; Herrmann, D.; Morton, J.P.; Cox, T.R.; Timpson, P. CAF Subpopulations: A New Reservoir of Stromal Targets in Pancreatic Cancer. Trends Cancer 2019, 5, 724–741. [Google Scholar] [CrossRef]
- Phillips, P.A.; McCarroll, J.A.; Park, S.; Wu, M.J.; Pirola, R.; Korsten, M.; Wilson, J.S.; Apte, M.V. Rat pancreatic stellate cells secrete matrix metalloproteinases: Implications for extracellular matrix turnover. Gut 2003, 52, 275–282. [Google Scholar] [CrossRef]
- Apte, M.V.; Wilson, J.S.; Lugea, A.; Pandol, S.J. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 2013, 144, 1210–1219. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
- Yu, Y.; Schuck, K.; Friess, H.; Kong, B. Targeting Aggressive Fibroblasts to Enhance the Treatment of Pancreatic Cancer. Expert Opin. Ther. Targets 2021, 25, 5–13. [Google Scholar] [CrossRef]
- Dalin, S.; Sullivan, M.R.; Lau, A.N.; Grauman-Boss, B.; Mueller, H.S.; Kreidl, E.; Fenoglio, S.; Luengo, A.; Lees, J.A.; Vander Heiden, M.G.; et al. Deoxycytidine Release from Pancreatic Stellate Cells Promotes Gemcitabine Resistance. Cancer Res. 2019, 79, 5723–5733. [Google Scholar] [CrossRef] [PubMed]
- Erkan, M.; Michalski, C.W.; Rieder, S.; Reiser-Erkan, C.; Abiatari, I.; Kolb, A.; Giese, N.A.; Esposito, I.; Friess, H.; Kleeff, J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2008, 6, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Hamberger, F.; Ravichandra, A.; Miller, M.; Nair, A.; Affo, S.; Filliol, A.; Chin, L.; Savage, T.M.; Yin, D.; et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kim, J.; Yang, S.; Wang, H.; Wu, C.J.; Sugimoto, H.; LeBleu, V.S.; Kalluri, R. Type I collagen deletion in alphaSMA(+) myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 2021, 39, 548–565. [Google Scholar] [CrossRef]
- Helms, E.; Onate, M.K.; Sherman, M.H. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov. 2020, 10, 648–656. [Google Scholar] [CrossRef]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Chang, P.M.; Cheng, C.T.; Wu, R.C.; Chung, Y.H.; Chiang, K.C.; Yeh, T.S.; Liu, C.Y.; Chen, M.H.; Chen, M.H.; Yeh, C.N. Nab-paclitaxel is effective against intrahepatic cholangiocarcinoma via disruption of desmoplastic stroma. Oncol. Lett. 2018, 16, 566–572. [Google Scholar] [CrossRef]
- Alvarez, R.; Musteanu, M.; Garcia-Garcia, E.; Lopez-Casas, P.P.; Megias, D.; Guerra, C.; Munoz, M.; Quijano, Y.; Cubillo, A.; Rodriguez-Pascual, J.; et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br. J. Cancer 2013, 109, 926–933. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ramanathan, R.K.; Borad, M.J.; Laheru, D.A.; Smith, L.S.; Wood, T.E.; Korn, R.L.; Desai, N.; Trieu, V.; Iglesias, J.L.; et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 4548–4554. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Sachsenmaier, M.; Hinz, U.; Schneider, L.; Michalski, C.W.; Springfeld, C.; Strobel, O.; Jager, D.; Ulrich, A.; Buchler, M.W. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann. Surg. 2016, 264, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Seufferlein, T.; Van Laethem, J.L.; Laurent-Puig, P.; Smolenschi, C.; Malka, D.; Boige, V.; Hollebecque, A.; Conroy, T. Systemic treatment of pancreatic cancer revisited. Semin Oncol. 2019, 46, 28–38. [Google Scholar] [CrossRef]
- Xie, L.; Xia, L.; Klaiber, U.; Sachsenmaier, M.; Hinz, U.; Bergmann, F.; Strobel, O.; Buchler, M.W.; Neoptolemos, J.P.; Fortunato, F.; et al. Effects of neoadjuvant FOLFIRONOX and gemcitabine-based chemotherapy on cancer cell survival and death in patients with pancreatic ductal adenocarcinoma. Oncotarget 2019, 10, 7276–7287. [Google Scholar] [CrossRef]
- Mota Reyes, C.; Teller, S.; Muckenhuber, A.; Konukiewitz, B.; Safak, O.; Weichert, W.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Neoadjuvant Therapy Remodels the Pancreatic Cancer Microenvironment via Depletion of Protumorigenic Immune Cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 220–231. [Google Scholar] [CrossRef]
- Diener, M.K.; Mihaljevic, A.L.; Strobel, O.; Loos, M.; Schmidt, T.; Schneider, M.; Berchtold, C.; Mehrabi, A.; Muller-Stich, B.P.; Jiang, K.; et al. Periarterial divestment in pancreatic cancer surgery. Surgery 2021, 169, 1019–1025. [Google Scholar] [CrossRef]
- Mahajan, U.M.; Langhoff, E.; Goni, E.; Costello, E.; Greenhalf, W.; Halloran, C.; Ormanns, S.; Kruger, S.; Boeck, S.; Ribback, S.; et al. Immune Cell and Stromal Signature Associated With Progression-Free Survival of Patients With Resected Pancreatic Ductal Adenocarcinoma. Gastroenterology 2018, 155, 1625–1639. [Google Scholar] [CrossRef]
- Nakajima, K.; Ino, Y.; Naito, C.; Nara, S.; Shimasaki, M.; Ishimoto, U.; Iwasaki, T.; Doi, N.; Esaki, M.; Kishi, Y.; et al. Neoadjuvant therapy alters the collagen architecture of pancreatic cancer tissue via Ephrin-A5. Br. J. Cancer 2022, 126, 628–639. [Google Scholar] [CrossRef]
- Miyashita, T.; Tajima, H.; Makino, I.; Okazaki, M.; Yamaguchi, T.; Ohbatake, Y.; Nakanuma, S.; Hayashi, H.; Takamura, H.; Ninomiya, I.; et al. Neoadjuvant Chemotherapy with Gemcitabine Plus Nab-paclitaxel Reduces the Number of Cancer-associated Fibroblasts Through Depletion of Pancreatic Stroma. Anticancer Res. 2018, 38, 337–343. [Google Scholar] [CrossRef]
- Laklai, H.; Miroshnikova, Y.A.; Pickup, M.W.; Collisson, E.A.; Kim, G.E.; Barrett, A.S.; Hill, R.C.; Lakins, J.N.; Schlaepfer, D.D.; Mouw, J.K.; et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 2016, 22, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Ohlund, D.; Franklin, O.; Lundberg, E.; Lundin, C.; Sund, M. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer 2013, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhong, D.W.; Wang, Q.W.; Miao, X.Y.; Xu, X.D. Paclitaxel ameliorates fibrosis in hepatic stellate cells via inhibition of TGF-beta/Smad activity. World J. Gastroenterol. 2010, 16, 3330–3334. [Google Scholar] [CrossRef]
- Tsukada, T.; Fushida, S.; Harada, S.; Terai, S.; Yagi, Y.; Kinoshita, J.; Oyama, K.; Tajima, H.; Ninomiya, I.; Fujimura, T.; et al. Low-dose paclitaxel modulates tumour fibrosis in gastric cancer. Int. J. Oncol. 2013, 42, 1167–1174. [Google Scholar] [CrossRef]
- Erstad, D.J.; Sojoodi, M.; Taylor, M.S.; Jordan, V.C.; Farrar, C.T.; Axtell, A.L.; Rotile, N.J.; Jones, C.; Graham-O'Regan, K.A.; Ferreira, D.S.; et al. Fibrotic Response to Neoadjuvant Therapy Predicts Survival in Pancreatic Cancer and Is Measurable with Collagen-Targeted Molecular MRI. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 5007–5018. [Google Scholar] [CrossRef]
- Han, C.; Liu, T.; Yin, R. Biomarkers for cancer-associated fibroblasts. Biomark Res. 2020, 8, 64. [Google Scholar] [CrossRef]
- Darvishi, B.; Eisavand, M.R.; Majidzadeh, A.K.; Farahmand, L. Matrix stiffening and acquired resistance to chemotherapy: Concepts and clinical significance. Br. J. Cancer 2022, 126, 1253–1263. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef]
- Michelakos, T.; Cai, L.; Villani, V.; Sabbatino, F.; Kontos, F.; Fernandez-Del Castillo, C.; Yamada, T.; Neyaz, A.; Taylor, M.S.; Deshpande, V.; et al. Tumor Microenvironment Immune Response in Pancreatic Ductal Adenocarcinoma Patients Treated With Neoadjuvant Therapy. J. Natl. Cancer Inst. 2021, 113, 182–191. [Google Scholar] [CrossRef]
- Peng, H.; James, C.A.; Cullinan, D.R.; Hogg, G.D.; Mudd, J.L.; Zuo, C.; Takchi, R.; Caldwell, K.E.; Liu, J.; DeNardo, D.G.; et al. Neoadjuvant FOLFIRINOX Therapy Is Associated with Increased Effector T Cells and Reduced Suppressor Cells in Patients with Pancreatic Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 6761–6771. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020, 10, 1330–1351. [Google Scholar] [CrossRef] [PubMed]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856. [Google Scholar] [CrossRef]





| Type of Neoadjuvant Therapy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RES | FOL | COMB | GEM | Total | |||||||
| n | % | n | % | n | % | n | % | n | % | p-Value | |
| Overall | 69 | 59.0 | 31 | 26.5 | 10 | 8.5 | 7 | 6.0 | 117 | NA | |
| Age (median (IQR)) | 64.7 (12.5) | 59.8 (12.9) | 59.2 (14.8) a | 69.6 (14.8) a | 62.4 (13.4) | 0.039 a | |||||
| Sex | |||||||||||
| male | 35 | 50.7 | 17 | 54.8 | 8 | 80.0 | 3 | 42.9 | 63 | 53.8 | ns |
| female | 34 | 49.3 | 14 | 45.2 | 2 | 20.0 | 4 | 57.1 | 54 | 46.2 | |
| ASA score | |||||||||||
| 1 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | ns |
| 2 | 28 b | 40.6 | 22 b | 71.0 | 5 | 50.0 | 4 | 57.1 | 59 | 50.4 | 0.030 b |
| 3 | 40 c | 58.0 | 9 c | 29.0 | 5 | 50.0 | 3 | 42.9.9 | 57 | 48.7 | 0.045 c |
| Neoadj. RCTx | |||||||||||
| no | 6 | 60.0 | 113 | 96.6 | NA | ||||||
| yes | 4 | 40.0 | 4 | 3.4 | |||||||
| Type of surgery | |||||||||||
| DP | 16 | 23.2 | 7 | 22.6 | 0 | 0.0 | 2 | 28.6 | 25 | 21.4 | ns |
| TP | 28 | 40.6 | 13 | 41.9.9 | 3 | 30.0 | 3 | 42.9.9 | 47 | 40.2 | ns |
| PD | 25 | 36.2 | 11 | 35.5 | 7 | 70.0 | 2 | 28.6 | 45 | 38.5 | ns |
| Extended resection | |||||||||||
| no | 39 d | 56.5 | 9 d | 29.0 | 2 | 20.0 | 2 | 28.6 | 52 | 44.4 | 0.017 d |
| yes | 30 d | 43.5 | 22 d | 71.0 | 8 | 80.0 | 5 | 71.4 | 65 | 55.6 | |
| R-status | |||||||||||
| R0 | 15 | 21.7 | 6 | 19.4 | 2 | 20.0 | 3 | 42.9 | 26 | 22.2 | ns |
| R1 | 54 | 78.3 | 24 | 77.4 | 7 | 70.0 | 4 | 57.1 | 89 | 76.1 | ns |
| R2 | 0 | 0.0 | 1 | 3.2 | 1 | 10.0 | 0 | 0.0 | 2 | 1.7 | ns |
| T-stage | |||||||||||
| T1 | 6 | 8.7 | 3 | 9.7 | 0 | 0.0 | 1 | 14.3 | 10 | 8.5 | ns |
| T2 | 44 | 63.8 | 17 | 54.8 | 6 | 60.0 | 4 | 57.1 | 71 | 60.7 | ns |
| T3 | 19 | 27.5 | 7 | 22.6 | 3 | 30.0 | 2 | 28.6 | 31 | 26.5 | ns |
| T4 | 0 | 0.0 | 4 | 12.9 | 1 | 10.0 | 0 | 0.0 | 5 | 4.3 | ns |
| N-stage | |||||||||||
| N0 | 29 | 42.0 | 11 | 35.5 | 5 | 50.0 | 3 | 42.9 | 48 | 41.0 | ns |
| N1 | 24 | 34.8 | 11 | 35.5 | 4 | 40.0 | 2 | 28.6 | 41 | 35.0 | ns |
| N2 | 16 | 23.2 | 9 | 29.0 | 1 | 10.0 | 2 | 28.6 | 28 | 23.9 | ns |
| Grading | |||||||||||
| G1 | 1 | 1.4 | NA | ||||||||
| G2 | 30 | 43.5 | |||||||||
| G3 | 38 | 55.1 | |||||||||
| Path. regression | |||||||||||
| minor | 15 | 78.9 | 3 | 37.5 | 1 | 33.3 | ns | ||||
| major | 4 | 21.1 | 5 | 62.5 | 2 | 66.7 | ns | ||||
| M-stage | |||||||||||
| M0 | 58 | 84.1 | 24 | 77.4 | 7 | 70.0 | 5 | 71.4 | 94 | 80.3 | ns |
| M1 | 11 | 15.9 | 7 | 22.6 | 3 | 30.0 | 2 | 28.6 | 23 | 19.7 | ns |
| Tumor stage (UICC 8th) | |||||||||||
| I | 22 | 31.9 | 7 | 18.9 | 1 | 10.0 | 1 | 12.5 | 22 | 31.9 | ns |
| II | 22 | 31.9 | 8 | 21.6 | 4 | 40.0 | 2 | 25.0 | 22 | 31.9 | ns |
| III | 15 | 21.7 | 12 | 32.4 | 2 | 20.0 | 3 | 37.5 | 15 | 21.7 | ns |
| IV | 10 | 14.5 | 10 | 27.0 | 3 | 30.0 | 2 | 25.0 | 10 | 14.5 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heger, U.; Martens, A.; Schillings, L.; Walter, B.; Hartmann, D.; Hinz, U.; Pausch, T.; Giese, N.; Michalski, C.W.; Hackert, T. Myofibroblastic CAF Density, Not Activated Stroma Index, Indicates Prognosis after Neoadjuvant Therapy of Pancreatic Carcinoma. Cancers 2022, 14, 3881. https://doi.org/10.3390/cancers14163881
Heger U, Martens A, Schillings L, Walter B, Hartmann D, Hinz U, Pausch T, Giese N, Michalski CW, Hackert T. Myofibroblastic CAF Density, Not Activated Stroma Index, Indicates Prognosis after Neoadjuvant Therapy of Pancreatic Carcinoma. Cancers. 2022; 14(16):3881. https://doi.org/10.3390/cancers14163881
Chicago/Turabian StyleHeger, Ulrike, Anna Martens, Lisa Schillings, Britta Walter, Domenic Hartmann, Ulf Hinz, Thomas Pausch, Nathalia Giese, Christoph W. Michalski, and Thilo Hackert. 2022. "Myofibroblastic CAF Density, Not Activated Stroma Index, Indicates Prognosis after Neoadjuvant Therapy of Pancreatic Carcinoma" Cancers 14, no. 16: 3881. https://doi.org/10.3390/cancers14163881
APA StyleHeger, U., Martens, A., Schillings, L., Walter, B., Hartmann, D., Hinz, U., Pausch, T., Giese, N., Michalski, C. W., & Hackert, T. (2022). Myofibroblastic CAF Density, Not Activated Stroma Index, Indicates Prognosis after Neoadjuvant Therapy of Pancreatic Carcinoma. Cancers, 14(16), 3881. https://doi.org/10.3390/cancers14163881







