Integrative, In Silico and Comparative Analysis of Breast Cancer Secretome Highlights Invasive-Ductal-Carcinoma-Grade Progression Biomarkers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Review
2.2. Data Processing, Visualization, and Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. BC Secretome Dataset Reconstruction and Mining
3.3. BC and Other Adenocarcinoma Secretome Comparative Analysis
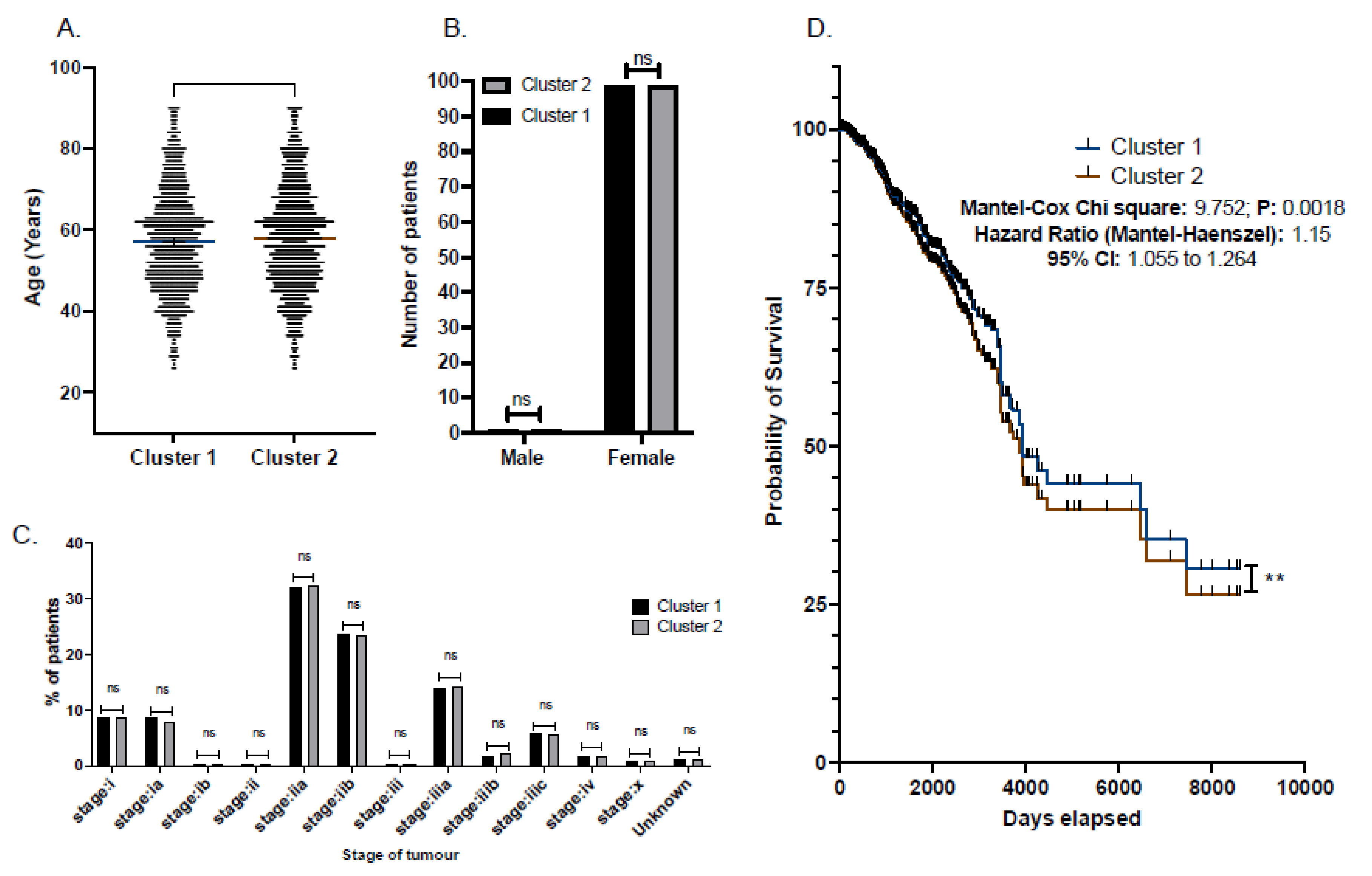
3.4. Central Cluster of BC Secreted Proteins and Patient Survival Correlations
3.5. Pathway Reconstruction
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory. Cancer Today. International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.fr/today (accessed on 28 June 2022).
- Van Seijen, M.; Lips, E.H.; Thompson, A.M.; Nik-Zainal, S.; Futreal, A.; Hwang, E.S.; Verschuur, E.; Lane, J.; Jonkers, J.; Rea, D.W.; et al. Ductal carcinoma in situ: To treat or not to treat, that is the question. Br. J. Cancer 2019, 121, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C. Tracing the cellular origin of cancer. Nat. Cell Biol. 2013, 15, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Parise, C.A.; Bauer, K.R.; Brown, M.M.; Caggiano, V. Breast Cancer Subtypes as Defined by the Estrogen Receptor (ER), Progesterone Receptor (PR), and the Human Epidermal Growth Factor Receptor 2 (HER2) among Women with Invasive Breast Cancer in California, 1999–2004. Breast J. 2009, 15, 593–602. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Cole, S.R.; Tse, C.-K.; Perou, C.M.; Carey, L.A.; Foulkes, W.D.; Dressler, L.G.; Geradts, J.; Millikan, R.C. Intrinsic Breast Tumor Subtypes, Race, and Long-Term Survival in the Carolina Breast Cancer Study. Clin. Cancer Res. 2010, 16, 6100–6110. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.V.; Jacobson, H.I. Fate of Steroid Estrogens in Target Tissues Biological Activities of Steroids in Relation to Cancer; Pincus, G., Vollmer, E.P., Eds.; National Cancer Institute, National Institutes of Health, US Department of Health, Education and Welfare; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Williams, C.; Lin, C.Y. Oestrogen receptors in breast cancer: Basic mechanisms and clinical implications. Ecancermedicalscience 2013, 7, 370. [Google Scholar] [PubMed]
- National Institute for Health and Care Excellence. New Injectable Immunotherapy Treatment for Rare Form of Triple Negative Breast Cancer Recommended by NICE. 2022. Available online: https://www.nice.org.uk/guidance/indevelopment/gid-ta10417/documents (accessed on 28 June 2022).
- Inwald, E.C.; Klinkhammer-Schalke, M.; Hofstädter, F.; Zeman, F.; Koller, M.; Gerstenhauer, M.; Ortmann, O. Ki-67 is a prognostic parameter in breast cancer patients: Results of a large population-based cohort of a cancer registry. Breast Cancer Res. Treat. 2013, 139, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Luporsi, E.; André, F.; Spyratos, F.; Martin, P.-M.; Jacquemier, J.; Penault-Llorca, F.; Tubiana-Mathieu, N.; Sigal-Zafrani, B.; Arnould, L.; Gompel, A.; et al. Ki-67: Level of evidence and methodological considerations for its role in the clinical management of breast cancer: Analytical and critical review. Breast Cancer Res. Treat. 2012, 132, 895–915. [Google Scholar] [CrossRef]
- De Azambuja, E.; Cardoso, F.; De Castro, G.; Colozza, M.; Mano, M.S.; Durbecq, V.; Sotiriou, C.; Larsimont, D.; Pic-cart-Gebhart, M.; Paesmans, M. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies in-volving 12,155 patients. Br. J. Cancer 2007, 96, 1504–1513. [Google Scholar] [CrossRef]
- Stuart-Harris, R.; Caldas, C.; Pinder, S.; Pharoah, P. Proliferation markers and survival in early breast cancer: A systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 2008, 17, 323–334. [Google Scholar] [CrossRef]
- Petitjean, A.; Achatz, M.I.W.; Borresen-Dale, A.L.; Hainaut, P.; Olivier, M. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene 2007, 26, 2157–2165. [Google Scholar] [CrossRef]
- Bonnefoi, H.; Piccart, M.; Bogaerts, J.; Mauriac, L.; Fumoleau, P.; Brain, E.; Petit, T.; Rouanet, P.; Jassem, J.; Blot, E.; et al. TP53 status for prediction of sensitivity to taxane versus non-taxane neoadjuvant chemotherapy in breast cancer (EORTC 10994/BIG 1-00): A randomised phase 3 trial. Lancet Oncol. 2011, 12, 527–539. [Google Scholar] [CrossRef]
- Olivier, M.; Langerød, D.A.; Carrieri, P.; Bergh, J.; Klaar, S.; Eyfjord, J.; Theillet, C.; Rodriguez, C.; Lidereau, R.; Bièche, I.; et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin. Cancer Res. 2006, 12, 1157–1167. [Google Scholar] [CrossRef]
- Heimann, R.; Ferguson, D.J.; Hellman, S. The relationship between nm23, angiogenesis, and the metastatic proclivity of node-negative breast cancer. Cancer Res. 1998, 58, 2766–2771. [Google Scholar] [CrossRef]
- Heimann, R.; Lan, F.; McBride, R.; Hellman, S. Separating favorable from unfavorable prognostic markers in breast cancer: The role of E-cadherin. Cancer Res. 2000, 60, 298–304. [Google Scholar]
- Yu, H.; Levesque, M.A.; Clark, G.M.; Diamandis, E.P. Enhanced prediction of breast cancer prognosis by evaluating expression of p53 and prostate-specific antigen in combination. Br. J. Cancer 1999, 81, 490–495. [Google Scholar] [CrossRef]
- Remacle, A.; McCarthy, K.; Noel, A.; Maguire, T.; McDermott, E.; O’Higgins, N.; Foidart, J.; Duffy, M. High levels of TIMP-2 correlate with adverse prognosis in breast cancer. Int. J. Cancer 2000, 89, 118–121. [Google Scholar] [CrossRef]
- Yoshida, R.; Kimura, N.; Harada, Y.; Ohuchi, N. The loss of E-cadherin, α-and β-catenin expression is associated with me-tastasis and poor prognosis in invasive breast cancer. Int. J. Oncol. 2001, 18, 513–520. [Google Scholar]
- Ueno, T.; Toi, M.; Koike, M.; Nakamura, S.; Tominaga, T. Tissue factor expression in breast cancer tissues: Its correlation with prognosis and plasma concentration. Br. J. Cancer 2000, 83, 164–170. [Google Scholar] [CrossRef]
- Prat, A.; Chaudhury, A.; Solovieff, N.; Paré, L.; Martinez, D.; Chic, N.; Martínez-Sáez, O.; Brasó-Maristany, F.; Lteif, A.; Taran, T.; et al. Correlative Biomarker Analysis of Intrinsic Subtypes and Efficacy Across the MONALEESA Phase III Studies. J. Clin. Oncol. 2021, 39, 1458–1467. [Google Scholar] [CrossRef]
- Harbeck, N.; Schmitt, M.; Meisner, C.; Friedel, C.; Untch, M.; Schmidt, M.; Sweep, C.G.; Lisboa, B.W.; Lux, M.P.; Beck, T.; et al. Ten-year analysis of the prospective multicentre Chemo-N0 trial validates American Society of Clinical Oncology (AS-CO)-recommended biomarkers uPA and PAI-1 for therapy decision making in node-negative breast cancer patients. Eur. J. Cancer 2013, 49, 1825–1835. [Google Scholar] [CrossRef]
- Tjalsma, H.; Antelmann, H.; Jongbloed, J.D.; Braun, P.G.; Darmon, E.; Dorenbos, R.; Dubois, J.Y.; Westers, H.; Zanen, G.; Quax, W.J.; et al. Proteomics of protein secretion by Bacillus subtilis: Separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 2004, 68, 207–233. [Google Scholar] [CrossRef]
- Wang, C.-I.; Wang, C.-L.; Chen, C.-D.; Wu, C.-C.; Liang, Y.; Tsai, Y.-H.; Chang, Y.-S.; Yu, J.-S.; Yu, C.-J. Importin subunit alpha-2 is identified as a potential biomarker for non-small cell lung cancer by integration of the cancer cell secretome and tissue transcriptome. Int. J. Cancer 2011, 128, 2364–2372. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Wu, C.-C.; Shyr, Y.-M.; Chen, T.-C.; Hwang, T.-L.; Yeh, T.-S.; Chang, K.-P.; Liu, H.-P.; Liu, Y.-L.; Tsai, M.-H.; et al. Secretome-Based Identification of ULBP2 as a Novel Serum Marker for Pancreatic Cancer Detection. PLoS ONE 2011, 6, e20029. [Google Scholar] [CrossRef]
- Grønborg, M.; Kristiansen, T.Z.; Iwahori, A.; Chang, R.; Reddy, R.; Sato, N.; Molina, H.; Jensen, O.N.; Hruban, R.H.; Goggins, M.G.; et al. Biomarker Discovery from Pancreatic Cancer Secretome Using a Differential Proteomic Approach* S. Mol. Cell. Proteom. 2006, 5, 157–171. [Google Scholar] [CrossRef]
- Schaaij-Visser, T.B.; De Wit, M.; Lam, S.W.; Jiménez, C.R. The cancer secretome, current status and opportunities in the lung, breast and colorectal cancer context. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 2242–2258. [Google Scholar] [CrossRef]
- Mannello, F.; Ligi, D. Resolving breast cancer heterogeneity by searching reliable protein cancer biomarkers in the breast fluid secretome. BMC Cancer 2013, 13, 344. [Google Scholar] [CrossRef]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn Diagrams. 2007. Available online: http://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 28 June 2022).
- Venn Diagram Tools—Evolutionary Genomics and Bioinformatics Group. Available online: https://bioinfo2.ugr.es/ceUGR/venn-diagram (accessed on 28 June 2022).
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Morpheus. Available online: https://software.broadinstitute.org/morpheus (accessed on 28 June 2022).
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Pontén, F. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein—Protein association networks with increased coverage, supporting functional discovery in ge-nome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Blache, U.; Horton, E.R.; Xia, T.; Schoof, E.M.; Blicher, L.H.; Schönenberger, A.; Snedeker, J.G.; Martin, I.; Erler, J.T.; Ehrbar, M. Mesenchymal stromal cell activation by breast cancer secretomes in bioengineered 3D microenvironments. Life Sci. Alliance 2019, 2, e201900304. [Google Scholar] [CrossRef] [PubMed]
- Brunoro, G.V.F.; Carvalho, P.C.; Barbosa, V.C.; Pagnoncelli, D.; De Moura Gallo, C.V.; Perales, J.; Zahedi, R.P.; Valente, R.H.; De Costa Neves-Ferreira, A.G. Differential proteomic comparison of breast cancer secretome using a quantitative paired analysis workflow. BMC Cancer 2019, 19, 365. [Google Scholar] [CrossRef] [PubMed]
- Ankney, J.A.; Xie, L.; Wrobel, J.A.; Wang, L.; Chen, X. Novel secretome-to-transcriptome integrated or secre-to-transcriptomic approach to reveal liquid biopsy biomarkers for predicting individualized prognosis of breast cancer patients. BMC Med. Genom. 2019, 12, 78. [Google Scholar] [CrossRef]
- Cox, T.R.; Schoof, E.M.; Gartland, A.; Erler, J.T.; Linding, R. Dataset for the proteomic inventory and quantitative analysis of the breast cancer hypoxic secretome associated with osteotropism. Data Brief 2015, 5, 621–625. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, H.; Li, X.; Li, X.; Cong, M.; Peng, F.; Yu, J.; Zhang, X.; Yang, Q.; Hu, G. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat. Cell Biol. 2017, 19, 1274–1285. [Google Scholar] [CrossRef]
- Boersema, P.J.; Geiger, T.; Wiśniewski, J.R.; Mann, M. Quantification of the N-glycosylated Secretome by Super-SILAC During Breast Cancer Progression and in Human Blood Samples. Mol. Cell. Proteom. 2013, 12, 158–171. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Li, H.; Yao, L.; Fu, D.; Yao, X.; Xu, L.X.; Hu, X.; Hu, G. Differential secretome analysis reveals CST6 as a suppressor of breast cancer bone metastasis. Cell Res. 2012, 22, 1356–1373. [Google Scholar] [CrossRef]
- Shaashua, L.; Eckerling, A.; Israeli, B.; Yanovich, G.; Rosenne, E.; Fichman-Horn, S.; Ben Zvi, I.; Sorski, L.; Haldar, R.; Satchi-Fainaro, R.; et al. Spontaneous regression of micro-metastases following primary tumor excision: A critical role for primary tumor secretome. BMC Biol. 2020, 18, 163. [Google Scholar] [CrossRef]
- Kwon, Y.; Park, S.-J.; Nguyen, B.T.; Kim, M.J.; Oh, S.; Lee, H.; Park, N.; Kim, H.S.; Kang, M.-J.; Min, B.S.; et al. Multi-layered proteogenomic analysis unravels cancer metastasis directed by MMP-2 and focal adhesion kinase signaling. Sci. Rep. 2021, 11, 17130. [Google Scholar] [CrossRef]
- Barderas, R.; Mendes, M.; Torres, S.; Bartolome, R.A.; López-Lucendo, M.; Villar-Vázquez, R.; Peláez-García, A.; Fuente, E.; Bonilla, F.; Casal, J.I. In-depth Characterization of the Secretome of Colorectal Cancer Metastatic Cells Identifies Key Proteins in Cell Adhesion, Migration, and Invasion. Mol. Cell. Proteom. 2013, 12, 1602–1620. [Google Scholar] [CrossRef]
- Yan, G.-R.; Ding, W.; Xu, S.-H.; Xu, Z.; Xiao, C.-L.; Yin, X.-F.; He, Q.-Y. Characterization of Phosphoproteins in Gastric Cancer Secretome. OMICS A J. Integr. Biol. 2011, 15, 83–90. [Google Scholar] [CrossRef]
- Wang, J.; Gao, F.; Mo, F.; Hong, X.; Wang, H.; Zheng, S.; Lin, B. Identification of CHI3L1 and MASP2 as a biomarker pair for liver cancer through integrative secretome and transcriptome analysis. Proteom. Clin. Appl. 2009, 3, 541–551. [Google Scholar] [CrossRef]
- Pich, C.; Meylan, P.; Mastelic-Gavillet, B.; Nguyen, T.N.; Loyon, R.; Trang, B.K.; Moser, H.; Moret, C.; Goepfert, C.; Hafner, J.; et al. Induction of Paracrine Signaling in Metastatic Melanoma Cells by PPARγ Agonist Rosiglitazone Activates Stromal Cells and Enhances Tumor GrowthRGZ Activates Paracrine Signaling, Enhancing Melanoma Growth. Cancer Res. 2018, 78, 6447–6461. [Google Scholar] [CrossRef]
- Liberato, T.; Pessotti, D.S.; Fukushima, I.; Kitano, E.S.; Serrano, S.M.; Zelanis, A. Signatures of protein expression revealed by secretome analyses of cancer associated fibroblasts and melanoma cell lines. J. Proteom. 2018, 174, 1–8. [Google Scholar] [CrossRef]
- Granado-Martínez, P.; Garcia-Ortega, S.; González-Sánchez, E.; McGrail, K.; Selgas, R.; Grueso, J.; Gil, R.; Naldaiz-Gastesi, N.; Rhodes, A.C.; Hernandez-Losa, J.; et al. STK11 (LKB1) missense somatic mutant isoforms promote tumor growth, motility and inflammation. Commun. Biol. 2020, 3, 366. [Google Scholar] [CrossRef]
- Böttger, F.; Schaaij-Visser, T.B.; de Reus, I.; Piersma, S.R.; Pham, T.V.; Nagel, R.; Brakenhoff, R.H.; Thunnissen, E.; Smit, E.F.; Jimenez, C.R. Proteome analysis of non-small cell lung cancer cell line secretomes and patient sputum reveals bio-fluid biomarker candidates for cisplatin response prediction. J. Proteom. 2019, 196, 106–119. [Google Scholar] [CrossRef]
- Carbotti, G.; Petretto, A.; Naschberger, E.; Stürzl, M.; Martini, S.; Mingari, M.C.; Filaci, G.; Ferrini, S.; Fabbi, M. Cyto-kine-induced guanylate binding protein 1 (GBP1) release from human ovarian cancer cells. Cancers 2020, 12, 488. [Google Scholar] [CrossRef]
- Worzfeld, T.; Finkernagel, F.; Reinartz, S.; Konzer, A.; Adhikary, T.; Nist, A.; Stiewe, T.; Wagner, U.; Looso, M.; Graumann, J.; et al. Proteotranscriptomics Reveal Signaling Networks in the Ovarian Cancer Microenvironment. Mol. Cell. Proteom. 2018, 17, 270–289. [Google Scholar] [CrossRef]
- Lanfredi, G.P.; Thomé, C.H.; Ferreira, G.A.; Silvestrini, V.C.; Masson, A.P.; Vargas, A.P.; Grassi, M.L.; Poersch, A.; dos Reis, F.J.C.; Faça, V.M. Analysis of ovarian cancer cell secretome during epithelial to mesenchymal transition reveals a protein signature associated with advanced stages of ovarian tumors. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140623. [Google Scholar] [CrossRef]
- Silva, L.M.; Kryza, T.; Stoll, T.; Hoogland, C.; Dong, Y.; Stephens, C.R.; Hastie, M.; Magdolen, V.; Kleifeld, O.; Gorman, J.J.; et al. Integration of Two In-depth Quantitative Proteomics Approaches Determines the Kallikrein-related Peptidase 7 (KLK7) Degradome in Ovarian Cancer Cell Secretome. Mol. Cell. Proteom. 2019, 18, 818–836. [Google Scholar] [CrossRef]
- Zubair, H.; Patel, G.K.; Khan, M.A.; Azim, S.; Zubair, A.; Singh, S.; Srivastava, S.K.; Singh, A.P. Proteomic Analysis of MYB-Regulated Secretome Identifies Functional Pathways and Biomarkers: Potential Pathobiological and Clinical Implications. J. Proteome Res. 2020, 19, 794–804. [Google Scholar] [CrossRef]
- Dalla Brandi, J.; Pozza, E.D.; Dando, I.; Biondani, G.; Robotti, E.; Jenkins, R.; Elliott, V.; Park, K.; Marengo, E.; Costello, E.; et al. Secretome protein signature of human pancreatic cancer stem-like cells. J. Proteom. 2016, 136, 1–12. [Google Scholar] [CrossRef]
- Adamczyk, K.A.; Klein-Scory, S.; Tehrani, M.M.; Warnken, U.; Schmiegel, W.; Schnoelzer, M.; Schwarte-Waldhoff, I. Char-acterization of soluble and exosomal forms of the EGFR released from pancreatic cancer cells. Life Sci. 2011, 89, 304–312. [Google Scholar] [CrossRef]
- Sung, E.; Kwon, O.K.; Lee, J.-M.; Lee, S. Proteomics approach to identify novel metastatic bone markers from the secretome of PC-3 prostate cancer cells. Electrophoresis 2017, 38, 2638–2645. [Google Scholar] [CrossRef]
- Mbeunkui, F.; Metge, B.J.; Shevde, L.A.; Pannell, L.K. Identification of Differentially Secreted Biomarkers Using LC-MS/MS in Isogenic Cell Lines Representing a Progression of Breast Cancer. J. Proteome Res. 2007, 6, 2993–3002. [Google Scholar] [CrossRef]
- Miller, F.R.; Santner, S.J.; Tait, L.; Dawson, P.J. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. JNCI J. Natl. Cancer Inst. 2000, 92, 1185–1186. [Google Scholar] [CrossRef]
- Treeck, O.; Schüler-Toprak, S.; Ortmann, O. Estrogen Actions in Triple-Negative Breast Cancer. Cells 2020, 9, 2358. [Google Scholar] [CrossRef]
- Fang, L.; Che, Y.; Zhang, C.; Huang, J.; Lei, Y.; Lu, Z.; Sun, N.; He, J. LAMC1 upregulation via TGFβ induces inflammatory cancer-associated fibroblasts in esophageal squamous cell carcinoma via NF-κB-CXCL1-STAT3. Mol. Oncol. 2021, 15, 3125–3146. [Google Scholar] [CrossRef]
- Hung, C.F.; Rohani, M.G.; Lee, S.-S.; Chen, P.; Schnapp, L.M. Role of IGF-1 pathway in lung fibroblast activation. Respir. Res. 2013, 14, 102. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, Z.; Lin, P.; Fu, X.; Li, F.; Jiang, J.; Zhu, P. ACPAs promote IL-1β production in rheumatoid arthritis by acti-vating the NLRP3 inflammasome. Cell. Mol. Immunol. 2020, 17, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Shi, Q.; Li, W.; Wu, W.; Zha, Z. ITGB1 promotes the chondrogenic differentiation of human adipose-derived mesen-chymal stem cells by activating the ERK signaling. J. Mol. Histol. 2020, 51, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Zhang, M.; Jin, M.; Bai, C.; Wang, X. Potential mechanism of interleukin-8 production from lung cancer cells: An involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J. Cell. Physiol. 2012, 227, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W.; Zuo, L.; Xu, M.; Wu, Y.; Huang, J.; Zhang, X.; Li, Y.; Wang, J.; Chen, J.; et al. The Fibrillin-1/VEGFR2/STAT2 signaling axis promotes chemoresistance via modulating glycolysis and angiogenesis in ovarian cancer or-ganoids and cells. Cancer Commun. 2022, 42, 245–265. [Google Scholar] [CrossRef]
- Hegarty, S.V.; O’Keeffe, G.W.; Sullivan, A.M. BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog. Neurobiol. 2013, 109, 28–41. [Google Scholar] [CrossRef]
- Cowan, K.J.; Storey, K. Mitogen-activated protein kinases: New signaling pathways functioning in cellular responses to environmental stress. J. Exp. Biol. 2003, 206, 1107–1115. [Google Scholar] [CrossRef]
- Vallet, S.D.; Berthollier, C.; Salza, R.; Muller, L.; Ricard-Blum, S. The Interactome of Cancer-Related Lysyl Oxidase and Lysyl Oxidase-like Proteins. Cancers 2021, 13, 71. [Google Scholar] [CrossRef]
- Nisar, M.; Paracha, R.Z.; Arshad, I.; Adil, S.; Zeb, S.; Hanif, R.; Rafiq, M.; Hussain, Z. Integrated Analysis of Microarray and RNA-Seq Data for the Identification of Hub Genes and Networks Involved in the Pancreatic Cancer. Front. Genet. 2021, 12, 663787. [Google Scholar] [CrossRef]
- Pitsidianaki, I.; Morgan, J.; Adams, J.; Campbell, K. Mesenchymal-to-epithelial transitions require tissue-specific interactions with distinct laminins. J. Cell Biol. 2021, 220, e202010154. [Google Scholar] [CrossRef]
- Dudha, N.; Rana, J.; Rajasekharan, S.; Gabrani, R.; Gupta, A.; Chaudhary, V.K.; Gupta, S. Host-pathogen interactome analysis of Chikungunya virus envelope proteins E1 and E2. Virus Genes 2015, 50, 200–209. [Google Scholar] [CrossRef]
- Zhan, X.H.; Jiao, J.W.; Zhang, H.F.; Li, C.Q.; Zhao, J.M.; Liao, L.D.; Wu, J.Y.; Wu, B.L.; Wu, Z.Y.; Wang, S.H.; et al. A three-gene signature from protein—protein interaction network of LOXL 2-and actin-related proteins for esophageal squamous cell car-cinoma prognosis. Cancer Med. 2017, 6, 1707–1719. [Google Scholar] [CrossRef]
- Jerhammar, F.; Ceder, R.; Garvin, S.; Grénman, R.; Grafström, R.C.; Roberg, K. Fibronectin 1 is a potential biomarker for ra-dioresistance in head and neck squamous cell carcinoma. Cancer Biol. Ther. 2010, 10, 1244–1251. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; von Maltzahn, J.; Soleimani, V.D.; Yin, H.; Rudnicki, M.A. Fibronectin Regulates Wnt7a Signaling and Satellite Cell Expansion. Cell Stem Cell 2013, 12, 75–87. [Google Scholar] [CrossRef]
- Astudillo, P.; Larrain, J. Wnt signaling and cell-matrix adhesion. Curr. Mol. Med. 2014, 14, 209–220. [Google Scholar] [CrossRef]
- Mahboobnia, K.; Pirro, M.; Marini, E.; Grignani, F.; Bezsonov, E.E.; Jamialahmadi, T.; Sahebkar, A. PCSK9 and cancer: Re-thinking the link. Biomed. Pharmacother. 2021, 140, 111758. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, S.; Li, T.; Yu, L.; Zhang, Y.; Zeng, H.; Qian, X.; Bi, J.; Lin, Y. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J. Exp. Clin. Cancer Res. 2019, 38, 173. [Google Scholar] [CrossRef]
- Kaartinen, V.; Warburton, D. Fibrillin controls TGF-β activation. Nat. Genet. 2003, 33, 331–332. [Google Scholar] [CrossRef]
- Kuemmerle, J.F.; Murthy, K.S.; Bowers, J.G. IGFBP-3 activates TGF-β receptors and directly inhibits growth in human intestinal smooth muscle cells. Am. J. Physiol. Liver Physiol. 2004, 287, G795–G802. [Google Scholar] [CrossRef]
- Ju, L.; Zhou, C. Association of integrin beta1 and c-MET in mediating EGFR TKI gefitinib resistance in non-small cell lung cancer. Cancer Cell Int. 2013, 13, 15. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef]
- Miyazono, K. Transforming growth factor-β signaling in epithelial-mesenchymal transition and progression of cancer. Pro-c. Jpn. Acad. B 2009, 85, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Dihlmann, S.; von Knebel Doeberitz, M. Wnt/β-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int. J. Cancer 2005, 113, 515–524. [Google Scholar] [CrossRef]
- Shin, J.; Song, S.-Y.; Ahn, H.-S.; An, B.C.; Choi, Y.-D.; Yang, E.G.; Na, K.-J.; Lee, S.-T.; Park, J.-I.; Kim, S.-Y.; et al. Integrative analysis for the discovery of lung cancer serological markers and validation by MRM-MS. PLoS ONE 2017, 12, e0183896. [Google Scholar] [CrossRef]
- De Oliveira, G.; Paccielli Freire, P.; Santiloni Cury, S.; de Moraes, D.; Santos Oliveira, J.; Dal-Pai-Silva, M.; Reis PPFrancisco Carvalho, R. An integrated meta-analysis of secretome and proteome identify potential biomarkers of pancreatic ductal ade-nocarcinoma. Cancers 2020, 12, 716. [Google Scholar] [CrossRef]
- Schiarea, S.; Solinas, G.; Allavena, P.; Scigliuolo, G.M.; Bagnati, R.; Fanelli, R.; Chiabrando, C. Secretome Analysis of Multiple Pancreatic Cancer Cell Lines Reveals Perturbations of Key Functional Networks. J. Proteome Res. 2010, 9, 4376–4392. [Google Scholar] [CrossRef]
- Evans, M.F.; Vacek, P.M.; Sprague, B.L.; Stein, G.S.; Stein, J.L.; Weaver, D.L. Microarray and RNA in situ hybridization assay for recurrence risk markers of breast carcinoma and ductal carcinoma in situ: Evidence supporting the use of diverse pathways panels. J. Cell. Biochem. 2020, 121, 1736–1746. [Google Scholar] [CrossRef]
- Jonasson, E.; Ghannoum, S.; Persson, E.; Karlsson, J.; Kroneis, T.; Larsson, E.; Landberg, G.; Ståhlberg, A. Identification of Breast Cancer Stem Cell Related Genes Using Functional Cellular Assays Combined With Single-Cell RNA Sequencing in MDA-MB-231 Cells. Front. Genet. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.C.; Patel, S.; Tyson, K.; Adam, P.J.; Schenker, M.; Loader, J.A.; Daviet, L.; Legrain, P.; Parekh, R.; Harris, A.L.; et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4. 4a and dystroglycan. Br. J. Cancer 2003, 88, 579–585. [Google Scholar] [CrossRef]
- Damaghi, M.; Mori, H.; Byrne, S.; Xu, L.; Chen, T.; Johnson, J.; Gallant, N.D.; Marusyk, A.; Borowsky, A.D.; Gillies, R.J. Collagen production and niche engineering: A novel strategy for cancer cells to survive acidosis in DCIS and evolve. Evol. Appl. 2020, 13, 2689–2703. [Google Scholar] [CrossRef]
- Kalscheuer, S.; Khanna, V.; Kim, H.; Li, S.; Sachdev, D.; Decarlo, A.; Yang, D.; Panyam, J. Discovery of HSPG2 (Perlecan) as a Therapeutic Target in Triple Negative Breast Cancer. Sci. Rep. 2019, 9, 12492. [Google Scholar] [CrossRef]
- Matsumoto, K.; Umitsu, M.; De Silva, D.M.; Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, W.; Gnad, T.; Wex, T.; Higashiyama, S.; Wolke, C.; Naumann, M.; Lendeckel, U. Ectodomain shedding of E-cadherin and c-Met is induced by Helicobacter pylori infection. Exp. Cell Res. 2009, 315, 3500–3508. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Kimura, N.; Miyazaki, K.; Yabuta, T.; Kumamoto, K.; Takenoshita, S.; Chen, J.; Kobayashi, M.; Hosokawa, M.; Taniguchi, A.; et al. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc. Natl. Acad. Sci. USA 2004, 101, 8132–8137. [Google Scholar] [CrossRef]
- Lefèvre, M.; Felmlee, D.; Parnot, M.; Baumert, T.F.; Schuster, C. Syndecan 4 Is Involved in Mediating HCV Entry through Interaction with Lipoviral Particle-Associated Apolipoprotein E. PLoS ONE 2014, 9, e95550. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, Y.K.; Yoon, C.-H.; Kim, K.; Kim, K.-C. Meta-analysis of gene expression profiles in long-term non-progressors infected with HIV-1. BMC Med. Genom. 2019, 12, 3. [Google Scholar] [CrossRef]
- Xie, S.; Shen, C.; Tan, M.; Li, M.; Song, X.; Wang, C. Systematic analysis of gene expression alterations and clinical outcomes of adenylate cyclase-associated protein in cancer. Oncotarget 2017, 8, 27216–27239. [Google Scholar] [CrossRef][Green Version]
- Liu, D.; Song, J.; Ji, X.; Liu, Z.; Cong, M.; Hu, B. Association of Genetic Polymorphisms on VEGFA and VEGFR2 with Risk of Coronary Heart Disease. Medicine 2016, 95, e3413. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Bridges, A.W.; Burns, K.L.; Tate, C.C.; Babensee, J.E.; LaPlaca, M.C.; García, A.J. Role of plasma fibronectin in the foreign body response to biomaterials. Biomaterials 2007, 28, 3626–3631. [Google Scholar] [CrossRef]
- Ensey, J.; Li, S.; Kashon, M.L.; Hollander, M.S.; Cutlip, R.G.; Baker, B.A. Age-dependent differential gene expression in expo-sureresponse of contraction-induced muscle injury. FASEB J. 2013, 27 (Suppl. 1), 1212–1219. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Lin, F.; Sun, H.; Lin, Y.; Wang, Z.; Wang, X. The lnc-CTSLP8 upregulates CTSL1 as a competitive endogenous RNA and promotes ovarian cancer metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 151. [Google Scholar] [CrossRef]
- Bartolini, A.; Cardaci, S.; Lamba, S.; Oddo, D.; Marchiò, C.; Cassoni, P.; Amoreo, C.A.; Corti, G.; Testori, A.; Bussolino, F.; et al. BCAM and LAMA5 Mediate the Recognition between Tumor Cells and the Endothelium in the Metastatic Spreading of KRAS-Mutant Colorectal Cancer. Clin. Cancer Res. 2016, 22, 4923–4933. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, S.; Cui, X.; Lv, X.; Jiao, Y.; Yu, F.; Yao, H.; Song, E.; Chen, Y.; Wang, M.; et al. The Overexpression of Hypomethylated miR-663 Induces Chemotherapy Resistance in Human Breast Cancer Cells by Targeting Heparin Sulfate Proteoglycan 2 (HSPG2). J. Biol. Chem. 2013, 288, 10973–10985. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Li, J.; Yan, M.-X.; Liu, L.; Jia, D.-S.; Geng, Q.; Lin, H.-C.; He, X.; Li, J.-J.; Yao, M. Integrative Analyses Identify Osteopontin, LAMB3 and ITGB1 as Critical Pro-Metastatic Genes for Lung Cancer. PLoS ONE 2013, 8, e55714. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-X.; Sha, R.-L.; Bao, J.-Q.; Luan, W.; Su, R.-L.; Sun, S.-R. Expression of long non-coding RNA linc-ITGB1 in breast cancer and its influence on prognosis and survival. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3397–3401. [Google Scholar]
- Kerslake, R.; Hall, M.; Vagnarelli, P.; Jeyaneethi, J.; Randeva, H.S.; Pados, G.; Kyrou, I.; Karteris, E. A pancancer overview of FBN1, asprosin and its cognate receptor OR4M1 with detailed expression profiling in ovarian cancer. Oncol. Lett. 2021, 22, 1–14. [Google Scholar] [CrossRef]
- Mo, D.; He, F.; Zheng, J.; Chen, H.; Tang, L.; Yan, F. tRNA-derived fragment tRF-17-79MP9PP attenuates cell invasion and migration via THBS1/TGF-β1/Smad3 axis in breast cancer. Front. Oncol. 2021, 11, 1116. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.; Li, Y.; Qiu, H. Upregulation of THBS1 is Related to Immunity and Chemotherapy Resistance in Gastric Cancer. Int. J. Gen. Med. 2021, 14, 4945–4957. [Google Scholar] [CrossRef]
- Weng, T.-Y.; Wang, C.-Y.; Hung, Y.-H.; Chen, W.-C.; Chen, Y.-L.; Lai, M.-D. Differential Expression Pattern of THBS1 and THBS2 in Lung Cancer: Clinical Outcome and a Systematic-Analysis of Microarray Databases. PLoS ONE 2016, 11, e0161007. [Google Scholar] [CrossRef]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- D’Amico, P.; Corvaja, C.; Gerratana, L.; Reduzzi, C.; Curigliano, G.; Cristofanilli, M. The use of liquid biopsy in early breast cancer: Clinical evidence and future perspectives. J. Cancer Metastasis Treat. 2021, 2021, 3. [Google Scholar] [CrossRef]
- Zhang, X.; Ju, S.; Wang, X.; Cong, H. Advances in liquid biopsy using circulating tumor cells and circulating cell-free tumor DNA for detection and monitoring of breast cancer. Clin. Exp. Med. 2019, 19, 271–279. [Google Scholar] [CrossRef]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef]
- Ben-David, U.; Siranosian, B.; Ha, G.; Tang, H.; Oren, Y.; Hinohara, K.; Strathdee, C.A.; Dempster, J.; Lyons, N.J.; Burns, R.; et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature 2018, 560, 325–330. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, Y.; Mueller, T.; Kreibich, S.; Williams, E.G.; Van Drogen, A.; Aebersold, R. Multi-omic measurements of heteroge-neity in HeLa cells across laboratories. Nat. Biotechnol. 2019, 37, 314–322. [Google Scholar] [CrossRef]
- Jarnuczak, A.F.; Najgebauer, H.; Barzine, M.; Kundu, D.J.; Ghavidel, F.; Perez-Riverol, Y.; Papatheodorou, I.; Brazma, A.; Vizcaíno, J.A. An integrated landscape of protein expression in human cancer. Sci. Data 2021, 8, 115. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastora, S.L.; Kounidas, G.; Speirs, V.; Masannat, Y.A. Integrative, In Silico and Comparative Analysis of Breast Cancer Secretome Highlights Invasive-Ductal-Carcinoma-Grade Progression Biomarkers. Cancers 2022, 14, 3854. https://doi.org/10.3390/cancers14163854
Kastora SL, Kounidas G, Speirs V, Masannat YA. Integrative, In Silico and Comparative Analysis of Breast Cancer Secretome Highlights Invasive-Ductal-Carcinoma-Grade Progression Biomarkers. Cancers. 2022; 14(16):3854. https://doi.org/10.3390/cancers14163854
Chicago/Turabian StyleKastora, Stavroula L., Georgios Kounidas, Valerie Speirs, and Yazan A. Masannat. 2022. "Integrative, In Silico and Comparative Analysis of Breast Cancer Secretome Highlights Invasive-Ductal-Carcinoma-Grade Progression Biomarkers" Cancers 14, no. 16: 3854. https://doi.org/10.3390/cancers14163854
APA StyleKastora, S. L., Kounidas, G., Speirs, V., & Masannat, Y. A. (2022). Integrative, In Silico and Comparative Analysis of Breast Cancer Secretome Highlights Invasive-Ductal-Carcinoma-Grade Progression Biomarkers. Cancers, 14(16), 3854. https://doi.org/10.3390/cancers14163854





