MRI in the Evaluation of Locally Advanced Vulvar Cancer Treated with Chemoradiotherapy and Vulvar Cancer Recurrence: The 2021 Revision of FIGO Classification and the Need for Multidisciplinary Management
Abstract
Simple Summary
Abstract
1. Introduction
2. MRI Technique
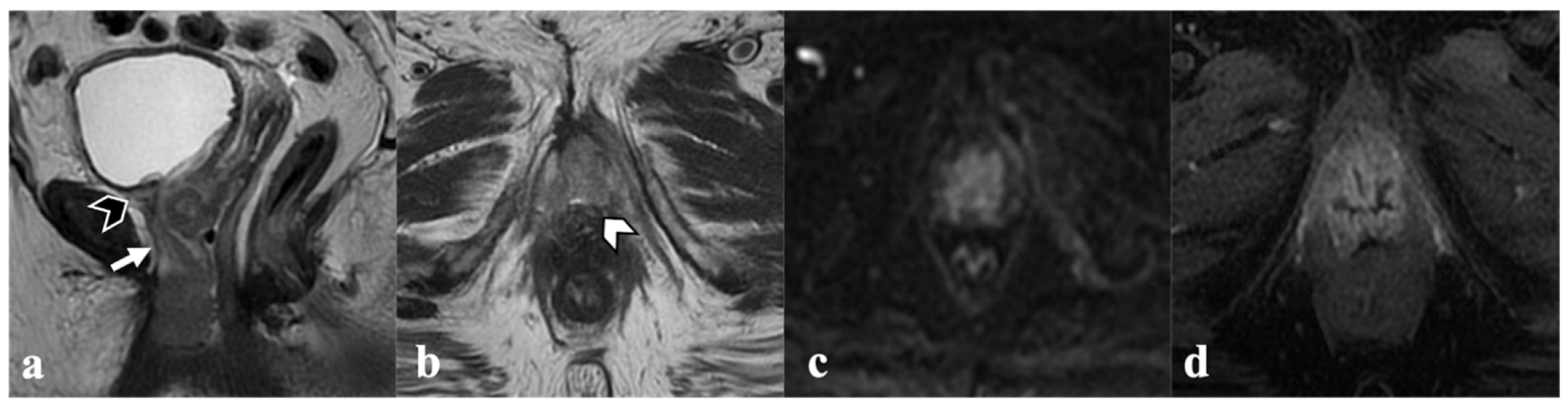
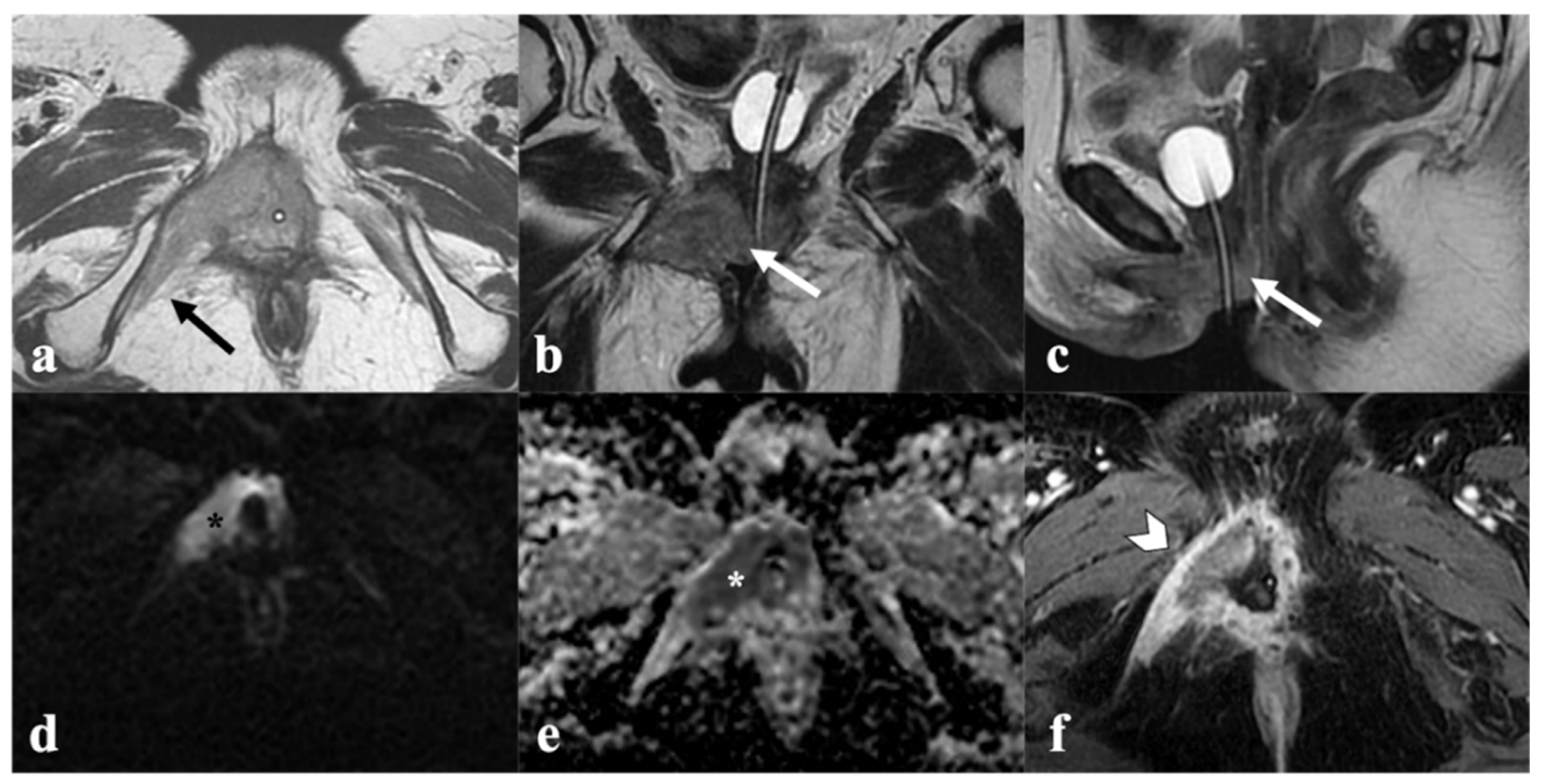
3. MRI Findings of LAVC Prior to CRT
3.1. Vagina
3.2. Urethra
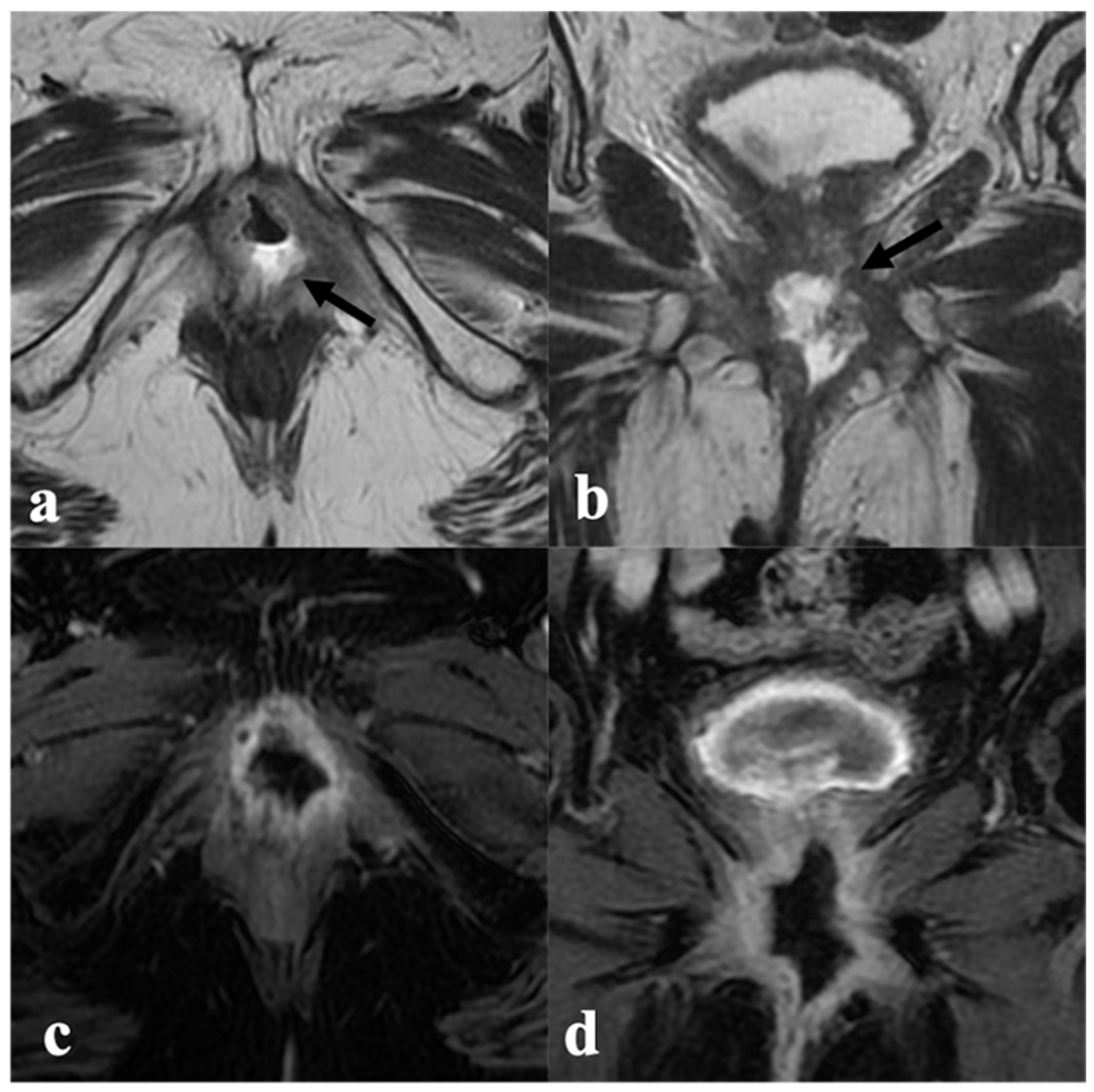
3.3. Anal Sphincter Complex
3.4. Bladder and Rectum
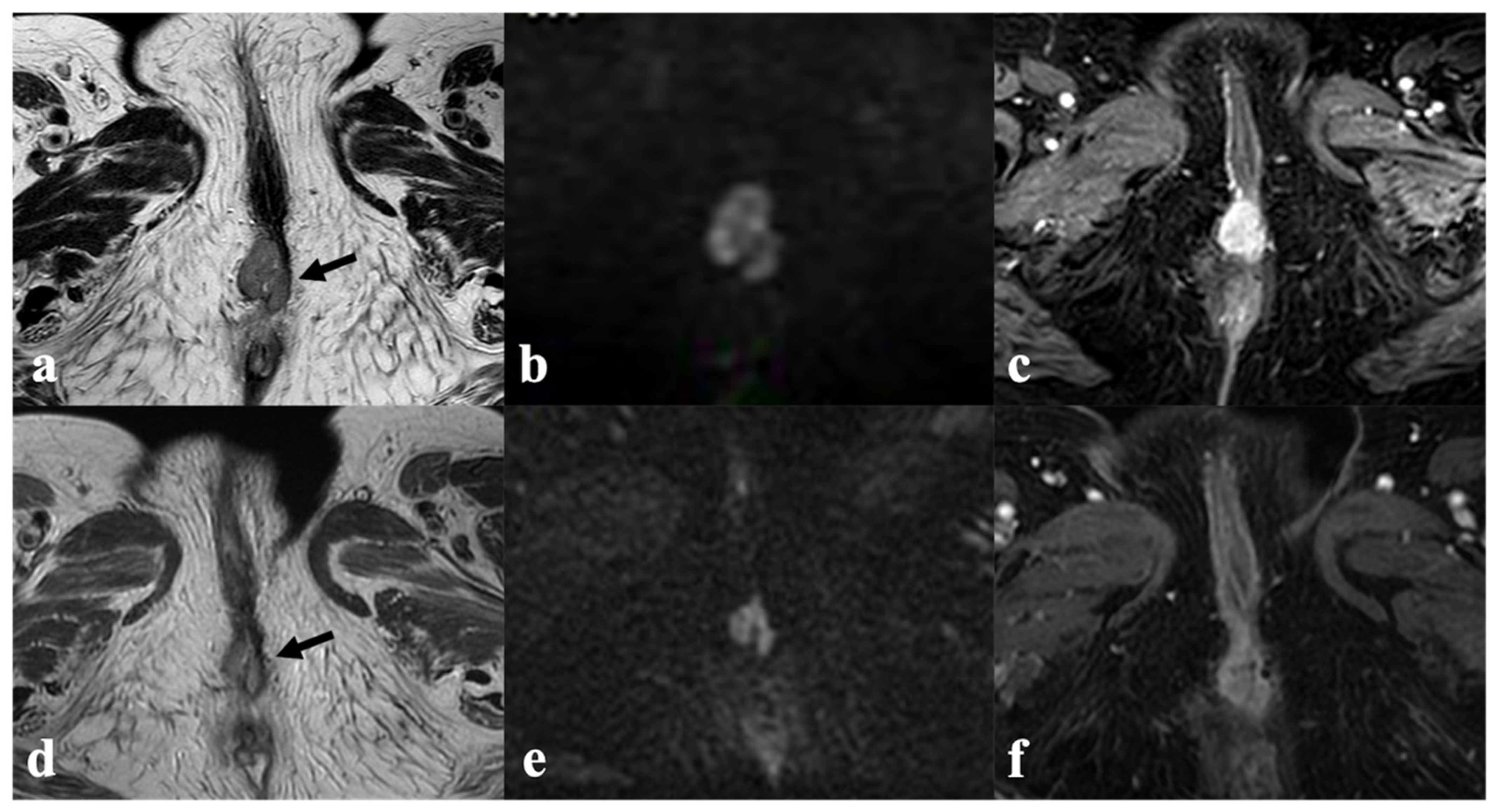
3.5. Inguinal or Pelvic Nodes
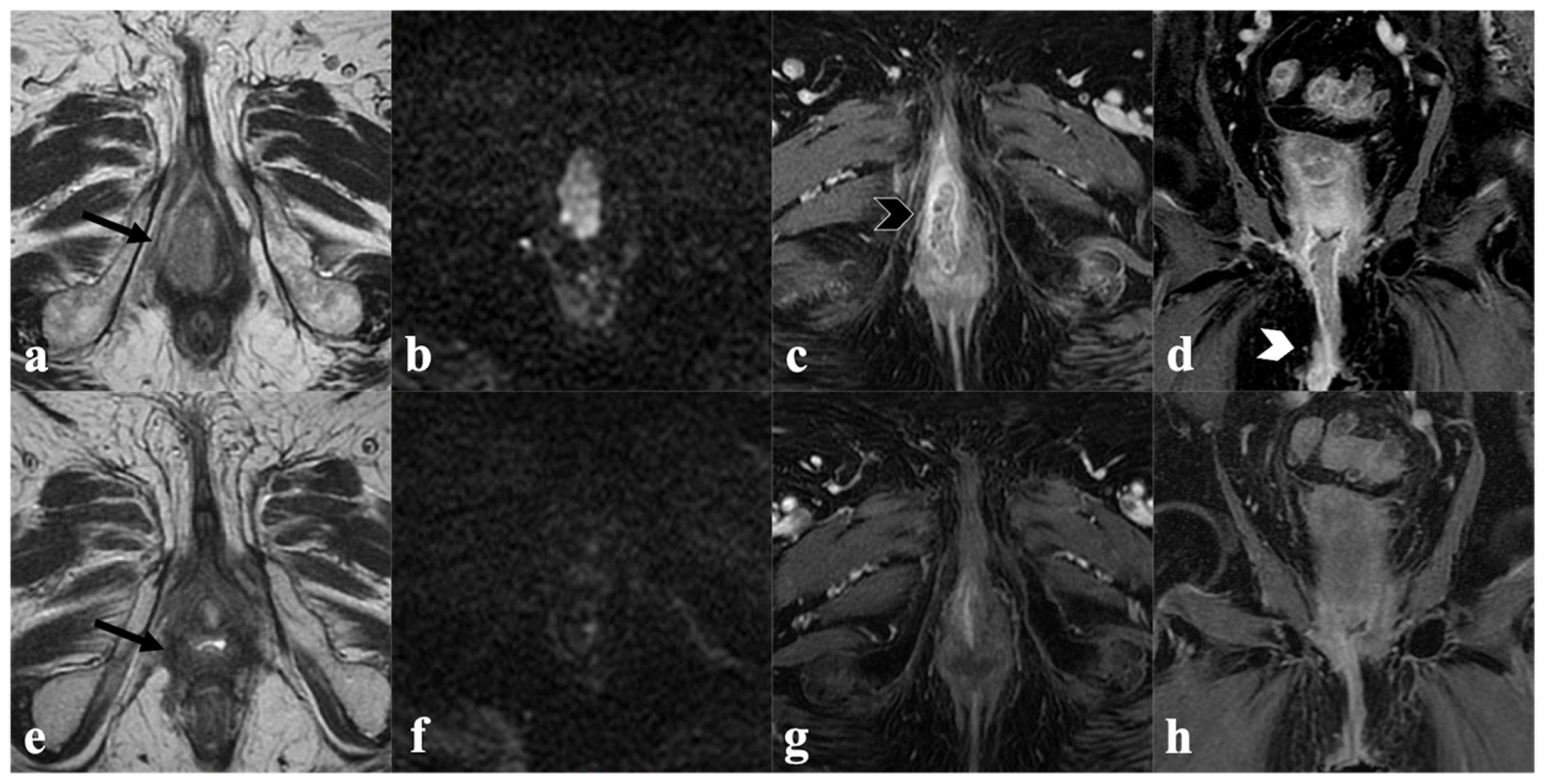
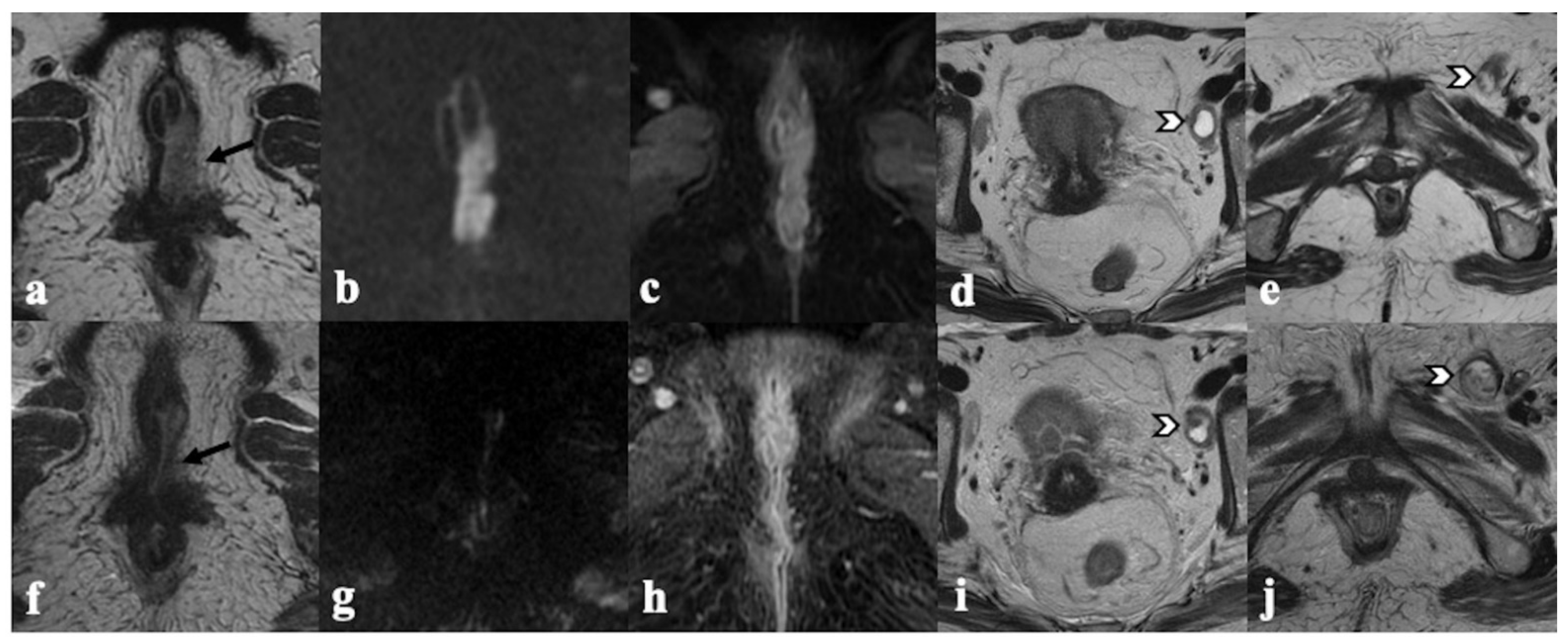
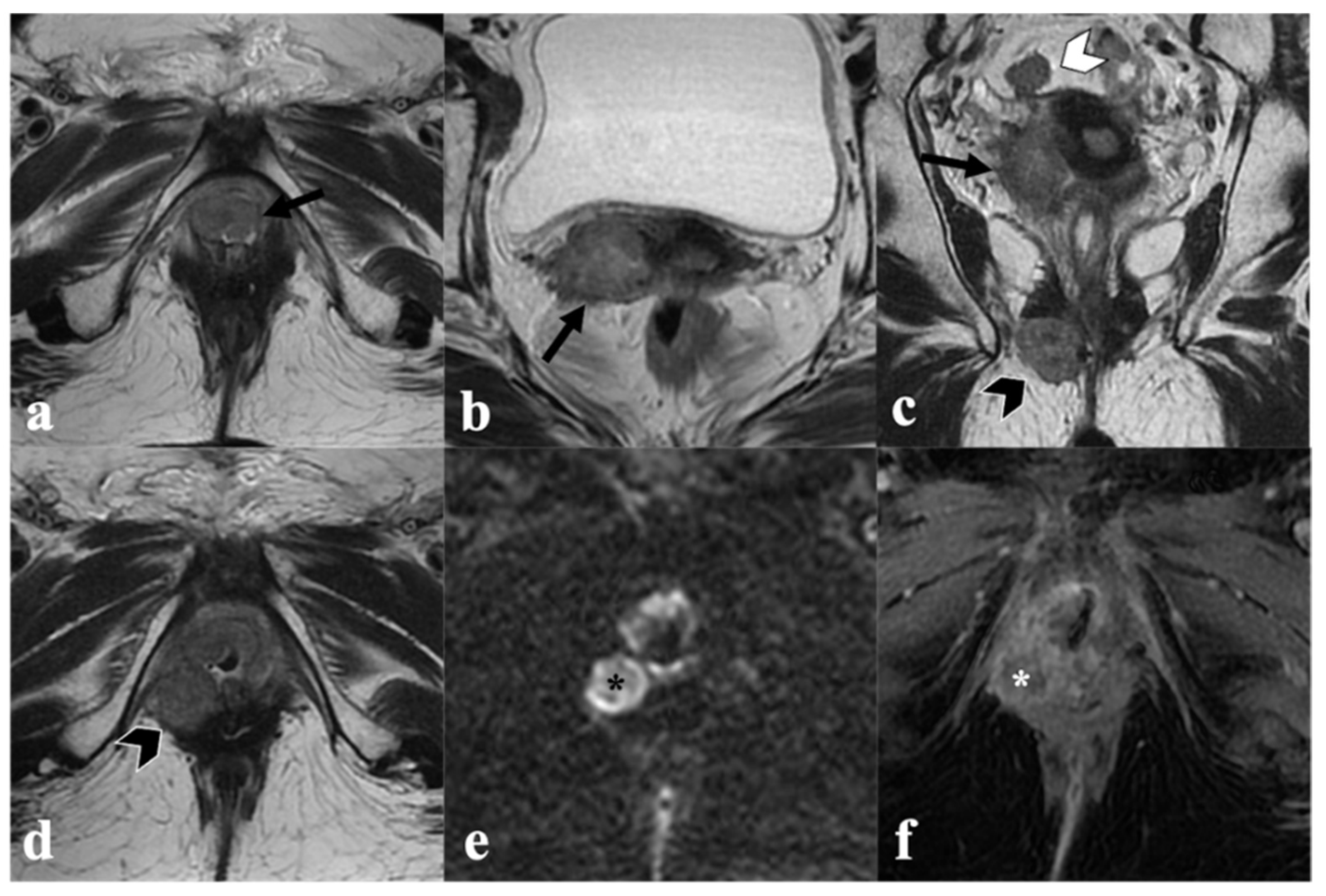
4. Post-CRT MRI Findings
4.1. Local Tumor Status and Residual Invasion of Adjacent Organs
4.2. Lymph Node Status
4.3. Structured MRI Report
- Residual tumor location (right, left, central, bilateral);
- Residual tumor size (three-dimensional measurements);
- Comparative evaluation of tumor T2 signal intensity, restricted diffusion, and degree of enhancement;
- Residual invasion to adjacent organs (urethra, vagina, anus, rectum, pelvic sidewall);
- Presence of post-treatment fibrosis;
- Lymph node assessment;
- Description of the remaining pelvic organs.
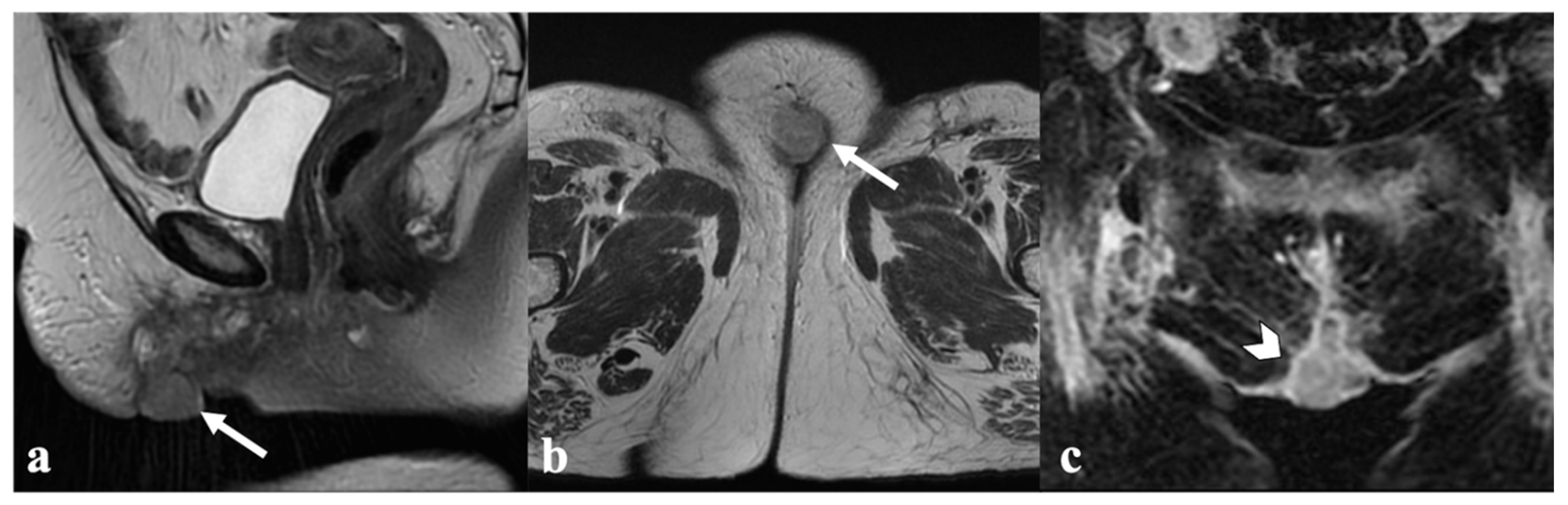
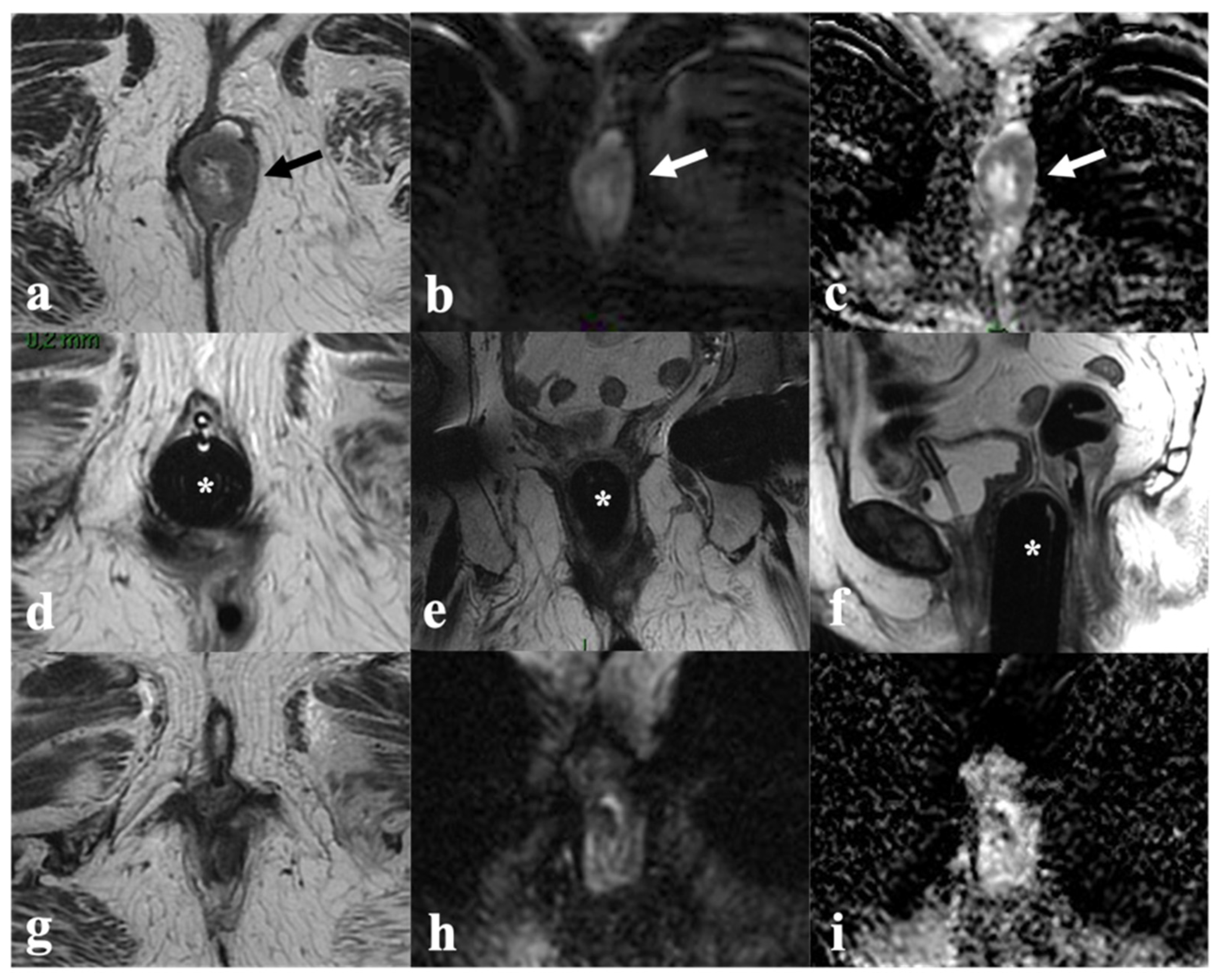
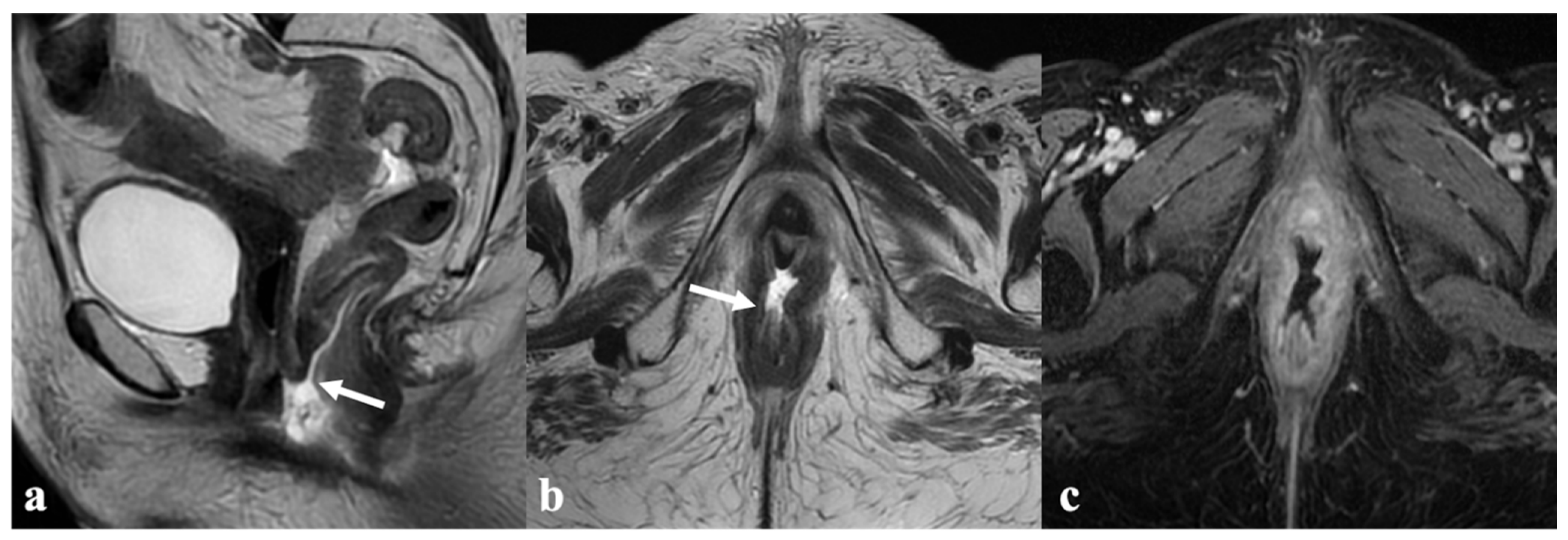
5. Vulvar Cancer Recurrence
6. Post-Therapy Complications
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koh, W.J.; Greer, B.E.; Abu-Rustum, N.R.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Scavone, J. Vulvar Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 92–120. [Google Scholar] [CrossRef]
- Daily, L.J.; Kaplan, A.L.; Kaufman, R.H. Exenteration for advanced carcinoma of the vulva. Obstet. Gynecol. 1970, 36, 845–849. [Google Scholar] [PubMed]
- National Cancer Institute—NIH Cancer of the Vulva-Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/vulva.html (accessed on 5 May 2021).
- Gadducci, A.; Aletti, G.D. Locally advanced squamous cell carcinoma of the vulva: A challenging question for gynecologic oncologists. Gynecol. Oncol. 2020, 158, 208–217. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Cuello, M.A.; Rogers, L.J. Cancer of the vulva: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 7–18. [Google Scholar] [CrossRef]
- Fischerova, D.; Garganese, G.; Reina, H.; Fragomeni, S.M.; Cibula, D.; Nanka, O.; Valentin, L. Terms, definitions and measurements to describe sonographic features of lymph nodes: Consensus opinion from the Vulvar International Tumor Analysis (VITA) group. Ultrasound Obs. Gynecol. 2021, 57, 861–879. [Google Scholar] [CrossRef]
- Verri, D.; Moro, F.; Fragomeni, S.M.; Zaçe, D.; Bove, S.; Pozzati, F.; Garganese, G. The Role of Ultrasound in the Evaluation of Inguinal Lymph Nodes in Patients with Vulvar Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3082. [Google Scholar] [CrossRef]
- Gui, B.; Persiani, S.; Miccò, M.; Pignatelli, V.; Rodolfino, E.; Avesani, G.; Manfredi, R. MRI Staging in Locally Advanced Vulvar Cancer: From Anatomy to Clinico-Radiological Findings. A Multidisciplinary VulCan Team Point of View. J. Pers. Med. 2021, 11, 1219. [Google Scholar] [CrossRef]
- Nikolić, O.; Cunha, T.M.; Nikolić, M.B.; Otero-García, M.M.; Gui, B.; Nougaret, S.; Leonhardt, H. Vulvar cancer staging: Guidelines of the European Society of Urogenital Radiology (ESUR). Insights Imaging 2021, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Serrado, M.A.; Horta, M.; Cunha, T.M. State of the art in vulvar cancer imaging. Radiol Bras 2019, 52, 316–324. [Google Scholar] [CrossRef]
- Shetty, A.S.; Menias, C.O. MR Imaging of Vulvar and Vaginal Cancer. Mag. Reson. Imaging Clin. N. Am. 2017, 25, 481–502. [Google Scholar] [CrossRef]
- Viswanathan, C.; Kirschner, K.; Truong, M.; Balachandran, A.; Devine, C.; Bhosale, P. Multimodality imaging of vulvar cancer: Staging, therapeutic response, and complications. AJR Am. J. Roentgenol. 2013, 200, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Management of Advanced Squamous Cell Carcinoma of the Vulva. Cancers 2021, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, L.; Garganese, G.; D’Aviero, A.; Lancellotta, V.; Fragomeni, S.M.; Fionda, B.; Macchia, G. Multidisciplinary personalized approach in the management of vulvar cancer—The Vul.Can Team experience. Int. J. Gynecol. Cancer 2020, 30, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Gentileschi, S.; Servillo, M.; Garganese, G.; Fragomeni, S.; De Bonis, F.; Scambia, G.; Salgarello, M. Surgical therapy of vulvar cancer: How to choose the correct reconstruction? J. Gynecol. Oncol. 2016, 27, e60. [Google Scholar] [CrossRef]
- Corrado, G.; Cutillo, G.; Fragomeni, S.M.; Bruno, V.; Tagliaferri, L.; Mancini, E.; Garganese, G. Palliative electrochemotherapy in primary or recurrent vulvar cancer. Int. J. Gynecol. Cancer 2020, 30, 927–931. [Google Scholar] [CrossRef]
- Ferrandina, G.; Lucidi, A.; Paglia, A.; Corrado, G.; Macchia, G.; Tagliaferri, L.; Scambia, G. Role of comorbidities in locally advanced cervical cancer patients administered preoperative chemoradiation: Impact on outcome and treatment-related complications. Eur. J. Surg. Oncol. 2012, 38, 238–244. [Google Scholar] [CrossRef]
- Lancellotta, V.; Kovács, G.; Tagliaferri, L.; Perrucci, E.; Colloca, G.; Valentini, V.; Aristei, C. Age Is Not a Limiting Factor in Interventional Radiotherapy (Brachytherapy) for Patients with Localized Cancer. Biomed. Res. Int. 2018, 2018, 2178469. [Google Scholar] [CrossRef]
- Di Capua, B.; Bellieni, A.; Fusco, D.; Gambacorta, M.A.; Tagliaferri, L.; Villani, E.R.; Colloca, G.F. Perspectives and limits of cancer treatment in an oldest old population. Aging Clin. Exp. Res. 2021, 33, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Gentileschi, S.; Servillo, M.; Garganese, G.; Fragomeni, S.; De Bonis, F.; Cina, A.; Salgarello, M. The lymphatic superficial circumflex iliac vessels deep branch perforator flap: A new preventive approach to lower limb lymphedema after groin dissection-preliminary evidence. Microsurgery 2017, 37, 564–573. [Google Scholar] [CrossRef]
- Gentileschi, S.; Caretto, A.A.; Servillo, M.; Stefanizzi, G.; Alberti, C.; Garganese, G.; Scambia, G. Feasibility, indications and complications of SCIP flap for reconstruction after extirpative surgery for vulvar cancer. J. Plast. Reconstr. Aesthet. Surg. 2021, 75, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, L.; Lancellotta, V.; Casà, C.; Fragomeni, S.M.; Ferioli, M.; Gentileschi, S.; Macchia, G. The Radiotherapy Role in the Multidisciplinary Management of Locally Advanced Vulvar Cancer: A Multidisciplinary VulCan Team Review. Cancers 2021, 13, 5747. [Google Scholar] [CrossRef]
- Kunos, C.; Simpkins, F.; Gibbons, H.; Tian, C.; Homesley, H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: A randomized controlled trial. Obstet. Gynecol. 2009, 114, 537–546. [Google Scholar] [CrossRef]
- Skliarenko, J.; Barnes, E.A. Palliative pelvic radiotherapy for gynaecologic cancer. J. Radiat. Oncol. 2012, 1, 239–244. [Google Scholar] [CrossRef]
- Lancellotta, V.; Macchia, G.; Garganese, G.; Fionda, B.; Fragomeni, S.M.; D’Aviero, A.; Tagliaferri, L. The role of brachytherapy (interventional radiotherapy) for primary and/or recurrent vulvar cancer: A Gemelli Vul.Can multidisciplinary team systematic review. Clin. Transl. Oncol. 2021, 23, 1611–1619. [Google Scholar] [CrossRef]
- Gaffney, D.K.; King, B.; Viswanathan, A.N.; Barkati, M.; Beriwal, S.; Eifel, P.; Bosch, W. Consensus Recommendations for Radiation Therapy Contouring and Treatment of Vulvar Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1191–1200. [Google Scholar] [CrossRef]
- Lakhman, Y.; Nougaret, S.; Miccò, M.; Scelzo, C.; Vargas, H.A.; Sosa, R.E.; Sala, E. Role of MR Imaging and FDG PET/CT in Selection and Follow-up of Patients Treated with Pelvic Exenteration for Gynecologic Malignancies. Radiographics 2015, 35, 1295–1313. [Google Scholar] [CrossRef] [PubMed]
- Virarkar, M.; Vulasala, S.S.; Daoud, T.; Javadi, S.; Lall, C.; Bhosale, P. Vulvar Cancer: 2021 Revised FIGO Staging System and the Role of Imaging. Cancers 2022, 14, 2264. [Google Scholar] [CrossRef]
- Chow, L.; Tsui, B.Q.; Bahrami, S.; Masamed, R.; Memarzadeh, S.; Raman, S.S.; Patel, M.K. Gynecologic tumor board: A radiologist’s guide to vulvar and vaginal malignancies. Abdom. Radiol. 2021, 46, 5669–5686. [Google Scholar] [CrossRef]
- Rockall, A.G.; Ghosh, S.; Alexander-Sefre, F.; Babar, S.; Younis MT, S.; Naz, S.; Reznek, R.H. Can MRI rule out bladder and rectal invasion in cervical cancer to help select patients for limited EUA? Gynecol. Oncol. 2006, 101, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Montana, G.S.; Thomas, G.M.; Moore, D.H.; Saxer, A.; Mangan, C.E.; Lentz, S.S.; Averette, H.E. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: A gynecologic oncology group study. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1007–1013. [Google Scholar] [CrossRef]
- Moore, D.H.; Thomas, G.M.; Montana, G.S.; Saxer, A.; Gallup, D.G.; Olt, G. Preoperative chemoradiation for advanced vulvar cancer: A phase II study of the Gynecologic Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 79–85. [Google Scholar] [CrossRef]
- Shylasree, T.S.; Bryant, A.; Howells, R.E. Chemoradiation for advanced primary vulval cancer. Cochrane Database Syst. Rev. 2011, CD003752. [Google Scholar] [CrossRef]
- Ju, F.J. Evaluation of the efficacy of chemoradiotherapy in cervical cancer using diffusion-weighted imaging and apparent diffusion coefficient. Onco Targets Ther. 2016, 9, 7555–7561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oonk, M.H.; Planchamp, F.; Baldwin, P.; Bidzinski, M.; Brännström, M.; Landoni, F.; Van Der Zee, A.G. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer. Int. J. Gynecol. Cancer 2017, 27, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Nooij, L.S.; Brand, F.A.; Gaarenstroom, K.N.; Creutzberg, C.L.; de Hullu, J.A.; van Poelgeest, M.I. Risk factors and treatment for recurrent vulvar squamous cell carcinoma. Crit. Rev. Oncol. Hematol. 2016, 106, 1–13. [Google Scholar] [CrossRef]
- Rose, P.G. Skin bridge recurrences in vulvar cancer: Frequency and management. Int. J. Gynecol. Cancer 1999, 9, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Salom, E.M.; Penalver, M. Recurrent vulvar cancer. Curr. Treat. Options Oncol. 2002, 3, 143–153. [Google Scholar] [CrossRef] [PubMed]
| Stage | Definition |
|---|---|
| I | Tumor confined to the vulva. |
| IA | Tumor size ≤ 2 cm and stromal invasion ≤ 1 mm |
| IB | Tumor size > 2 cm or stromal invasion > 1 mm |
| II | Tumor of any size with extension to lower one-third of the urethra, lower one-third of the vagina, lower one-third of the anus with negative nodes |
| III | Tumor of any size with extension to upper part of adjacent perineal structures, or with any number of nonfixed, nonulcerated lymph node |
| IIIA | Tumor of any size with disease extension to upper two-thirds of the urethra, upper two-thirds of the vagina, bladder mucosa, rectal mucosa, or regional lymph node metastases ≤ 5 mm |
| IIIB | Regional lymph node metastases > 5 mm |
| IIIC | Regional lymph node metastases with extracapsular spread |
| IV | Tumor of any size fixed to bone, or fixed, ulcerated lymph node metastases, or distant metastases |
| IVA | Disease fixed to pelvic bone, or fixed or ulcerated regional lymph node metastases |
| IVB | Distant metastasis |
| CE-DW-MRI |
|---|
| Imaging Protocol |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miccò, M.; Russo, L.; Persiani, S.; Dolciami, M.; Manganaro, L.; Cunha, T.M.; Janicas, C.; Rizzo, S.; Nicolic, O.; Garganese, G.; et al. MRI in the Evaluation of Locally Advanced Vulvar Cancer Treated with Chemoradiotherapy and Vulvar Cancer Recurrence: The 2021 Revision of FIGO Classification and the Need for Multidisciplinary Management. Cancers 2022, 14, 3852. https://doi.org/10.3390/cancers14163852
Miccò M, Russo L, Persiani S, Dolciami M, Manganaro L, Cunha TM, Janicas C, Rizzo S, Nicolic O, Garganese G, et al. MRI in the Evaluation of Locally Advanced Vulvar Cancer Treated with Chemoradiotherapy and Vulvar Cancer Recurrence: The 2021 Revision of FIGO Classification and the Need for Multidisciplinary Management. Cancers. 2022; 14(16):3852. https://doi.org/10.3390/cancers14163852
Chicago/Turabian StyleMiccò, Maura, Luca Russo, Salvatore Persiani, Miriam Dolciami, Lucia Manganaro, Teresa Margarida Cunha, Catarina Janicas, Stefania Rizzo, Olivera Nicolic, Giorgia Garganese, and et al. 2022. "MRI in the Evaluation of Locally Advanced Vulvar Cancer Treated with Chemoradiotherapy and Vulvar Cancer Recurrence: The 2021 Revision of FIGO Classification and the Need for Multidisciplinary Management" Cancers 14, no. 16: 3852. https://doi.org/10.3390/cancers14163852
APA StyleMiccò, M., Russo, L., Persiani, S., Dolciami, M., Manganaro, L., Cunha, T. M., Janicas, C., Rizzo, S., Nicolic, O., Garganese, G., Tagliaferri, L., Lancellotta, V., Scambia, G., Manfredi, R., & Gui, B. (2022). MRI in the Evaluation of Locally Advanced Vulvar Cancer Treated with Chemoradiotherapy and Vulvar Cancer Recurrence: The 2021 Revision of FIGO Classification and the Need for Multidisciplinary Management. Cancers, 14(16), 3852. https://doi.org/10.3390/cancers14163852








