The Galaninergic System: A Target for Cancer Treatment
Abstract
Simple Summary
Abstract
1. Introduction
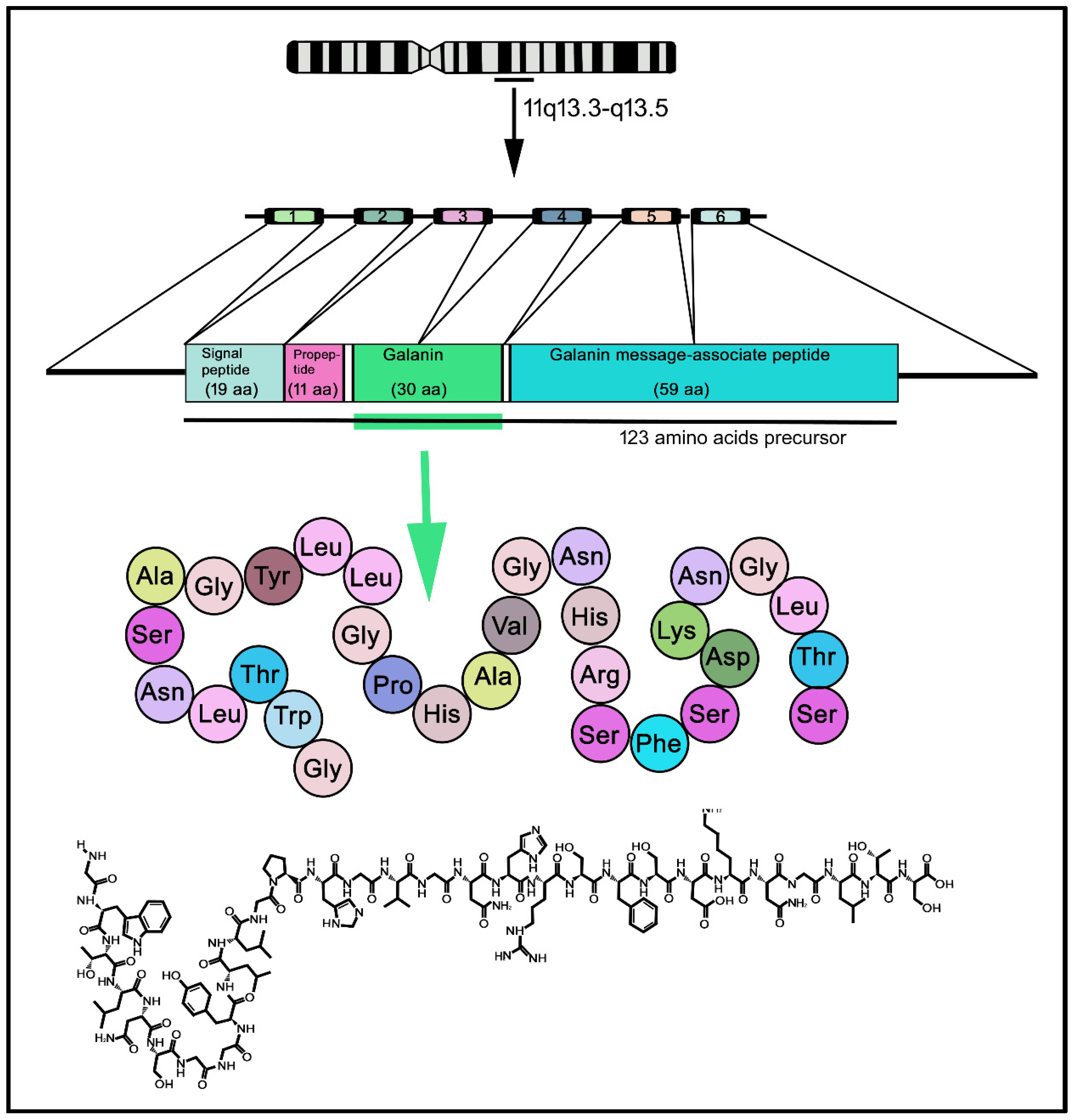
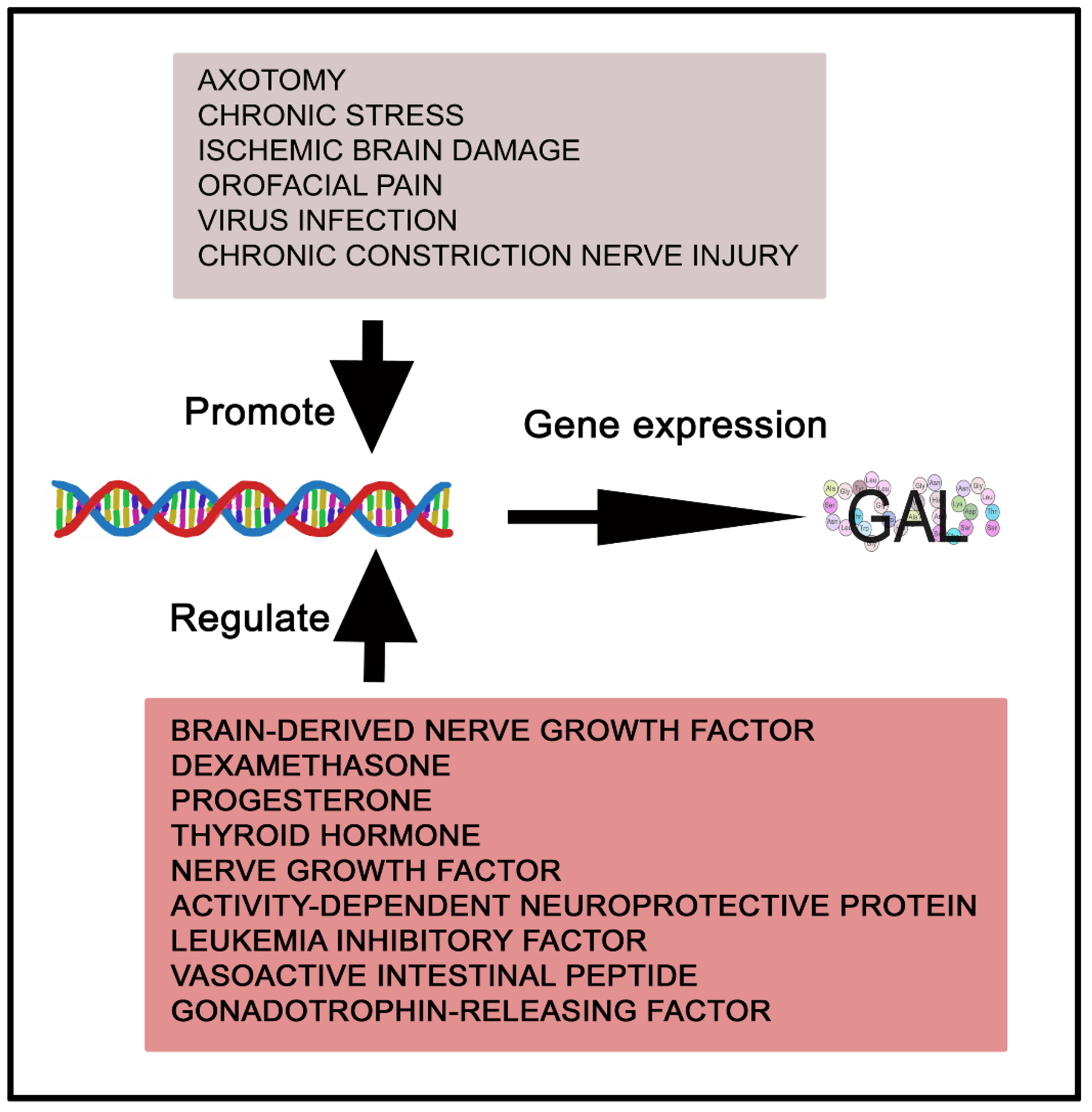
2. The Galaninergic System: Galanin and Its Receptors
3. The Galaninergic System and Cancer
3.1. Galanin and Neuroendocrine Tumors
3.1.1. Phaeochromocytoma
3.1.2. Insulinoma
3.1.3. Neuroblastic Tumor
3.1.4. Pituitary Tumor
3.1.5. Small-Cell Lung Cancer
3.2. Galanin and Gastric Cancer
3.3. Galanin and Colorectal Cancer
3.4. Galanin and Head and Neck Squamous Cell Carcinoma
3.5. Galanin and Glioma
3.6. Galanin and Other Cancers
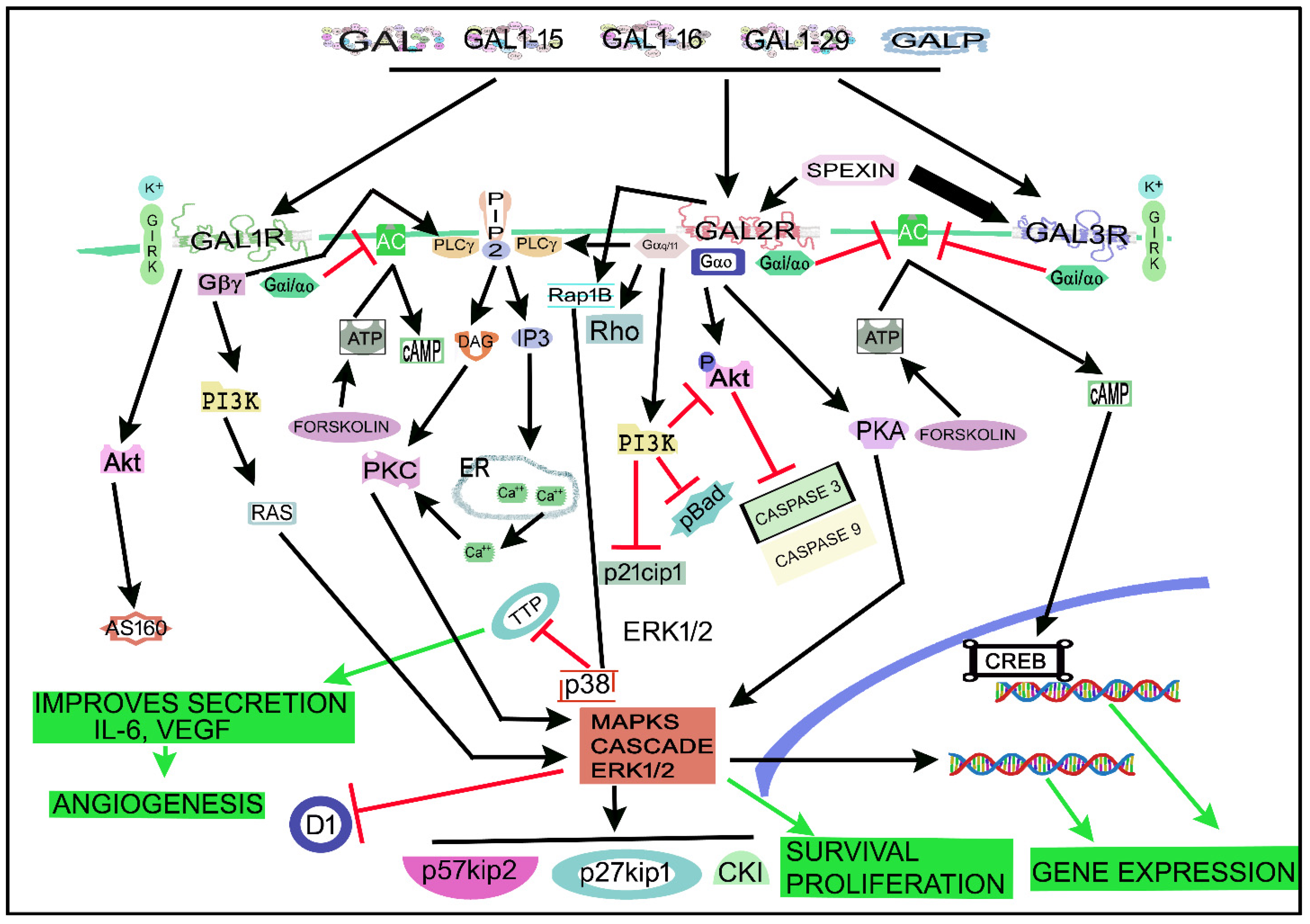
4. The Galaninergic System and Cancer: Signaling Pathways
5. Therapeutic Strategies
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
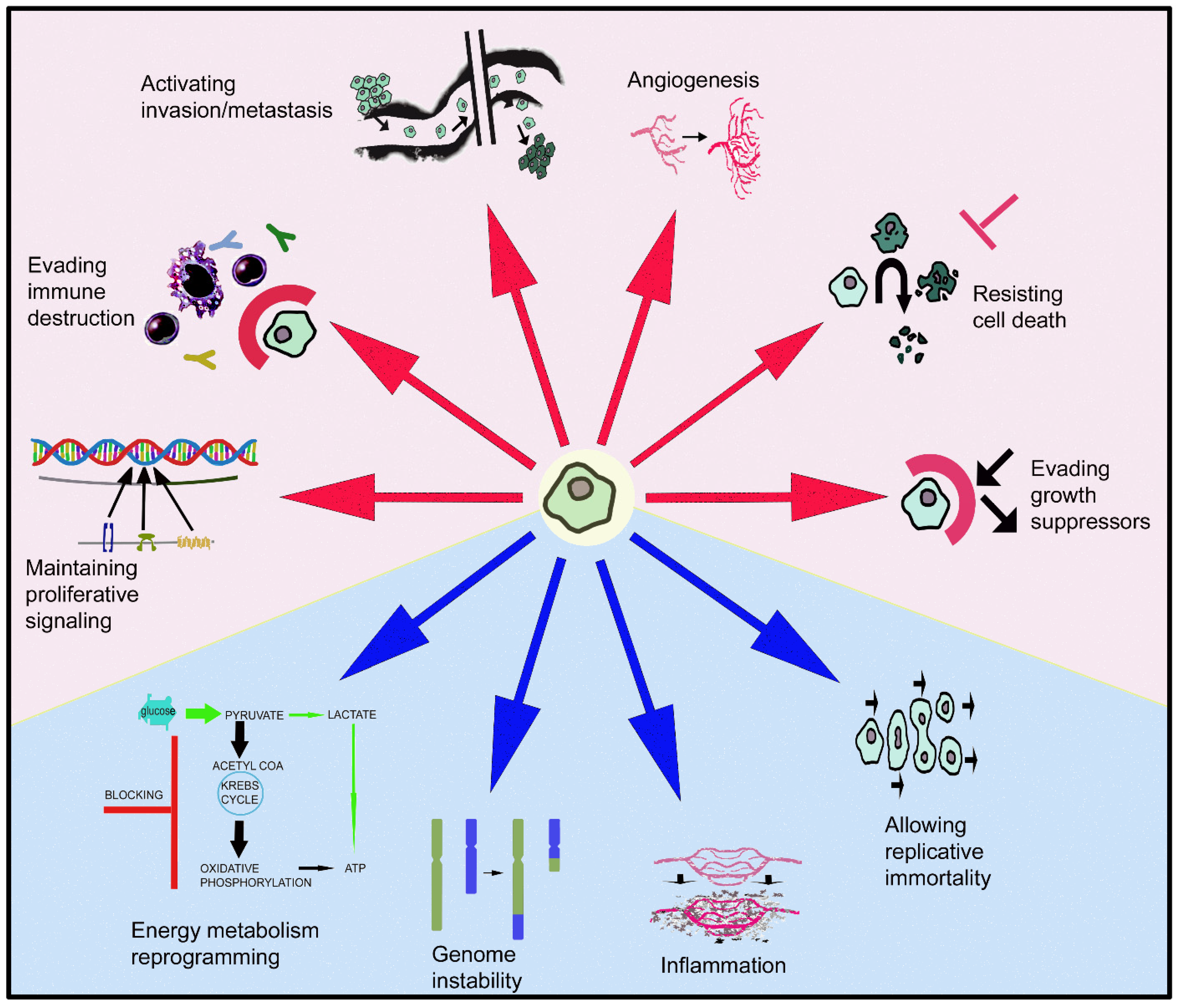
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. Involvement of Substance P and the NK-1 Receptor in Human Pathology. Amino Acids 2014, 46, 1727–1750. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A.J. Handbook of Biologically Active Peptides, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhao, M.; Wang, T.; Liu, Q.; Cummins, S. Copy Number Alteration of Neuropeptides and Receptors in Multiple Cancers. Sci. Rep. 2017, 7, 4598. [Google Scholar] [CrossRef]
- Kasprzak, A.; Adamek, A. The Neuropeptide System and Colorectal Cancer Liver Metastases: Mechanisms and Management. Int. J. Mol. Sci. 2020, 21, 3494. [Google Scholar] [CrossRef]
- Colucci, R.; Blandizzi, C.; Ghisu, N.; Florio, T.; Del Tacca, M. Somatostatin Inhibits Colon Cancer Cell Growth through Cyclooxygenase-2 Downregulation: Somatostatin and Cyclooxygenase-2 in Colon Cancer. Br. J. Pharmacol. 2008, 155, 198–209. [Google Scholar] [CrossRef]
- Godlewski, J.; Kmiec, Z. Colorectal Cancer Invasion and Atrophy of the Enteric Nervous System: Potential Feedback and Impact on Cancer Progression. Int. J. Mol. Sci. 2020, 21, 3391. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Coveñas, R. The Neurotensinergic System: A Target for Cancer Treatment. Curr. Med. Chem. 2022, 29, 3231–3260. [Google Scholar] [CrossRef]
- Muñoz, M.F.; Argüelles, S.; Rosso, M.; Medina, R.; Coveñas, R.; Ayala, A.; Muñoz, M. The Neurokinin-1 Receptor Is Essential for the Viability of Human Glioma Cells: A Possible Target for Treating Glioblastoma. BioMed Res. Int. 2022, 2022, 6291504 . [Google Scholar] [CrossRef]
- Kanazawa, T.; Misawa, K.; Carey, T.E. Galanin Receptor Subtypes 1 and 2 as Therapeutic Targets in Head and Neck Squamous Cell Carcinoma. Expert Opin. Ther. Targets 2010, 14, 289–302. [Google Scholar] [CrossRef]
- Berger, A.; Lang, R.; Moritz, K.; Santic, R.; Hermann, A.; Sperl, W.; Kofler, B. Galanin Receptor Subtype GalR2 Mediates Apoptosis in SH-SY5Y Neuroblastoma Cells. Endocrinology 2004, 145, 500–507. [Google Scholar] [CrossRef]
- Iishi, H.; Tatsuta, M.; Baba, M.; Uehara, H.; Nakaizumi, A. Protection by Galanin against Gastric Carcinogenesis Induced by N-Methyl-N’-Nitro-N-Nitrosoguanidine in Wistar Rats. Cancer Res. 1994, 54, 3167–3170. [Google Scholar]
- Tatemoto, K.; Rökaeus, Å.; Jörnvall, H.; McDonald, T.J.; Mutt, V. Galanin-a Novel Biologically Active Peptide from Porcine Intestine. FEBS Lett. 1983, 164, 124–128. [Google Scholar] [CrossRef]
- Bersani, M.; Johnsen, A.H.; Højrup, P.; Dunning, B.E.; Andreasen, J.J.; Holst, J.J. Human Galanin: Primary Structure and Indentification of Two Molecular Forms. FEBS Lett. 1991, 283, 189–194. [Google Scholar] [CrossRef]
- Schmidt, W.E.; Kratzin, H.; Eckart, K.; Drevs, D.; Mundkowski, G.; Clemens, A.; Katsoulis, S.; Schäfer, H.; Gallwitz, B.; Creutzfeldt, W. Isolation and Primary Structure of Pituitary Human Galanin, a 30-Residue Nonamidated Neuropeptide. Proc. Natl. Acad. Sci. USA 1991, 88, 11435–11439. [Google Scholar] [CrossRef]
- Chen, D.; Kodama, Y.; Kulseng, B.; Johannessen, H.; Zhao, C. Galanin. In Handbook of BiologicallyActive Peptides; Kastin, A.J., Ed.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 1210–1218. [Google Scholar]
- Maria, E.; Vrontakis, B.S.P. Galanin: A Biologically Active Peptide. Curr. Drug Target-CNS Neurol. Disord. 2002, 1, 531–541. [Google Scholar] [CrossRef]
- Katsoulis, S.; Clemens, A.; Morys-Wortmann, C.; Schwörer, H.; Schaube, H.; Klomp, H.-J.; Fölsch, U.R.; Schmidt, W.E. Human Galanin Modulates Human Colonic Motility in Vitro Characterization of Structural Requirements. Scand. J. Gastroenterol. 1996, 31, 446–451. [Google Scholar] [CrossRef]
- Lang, R.; Gundlach, A.L.; Holmes, F.E.; Hobson, S.A.; Wynick, D.; Hökfelt, T.; Kofler, B. Physiology, Signaling, and Pharmacology of Galanin Peptides and Receptors: Three Decades of Emerging Diversity. Pharmacol. Rev. 2015, 67, 118–175. [Google Scholar] [CrossRef]
- Tran, A.; He, W.; Chen, J.T.C.; Belsham, D.D. Spexin: Its Role, Regulation, and Therapeutic Potential in the Hypothalamus. Pharmacol. Ther. 2022, 233, 108033. [Google Scholar] [CrossRef]
- Ohtaki, T.; Kumano, S.; Ishibashi, Y.; Ogi, K.; Matsui, H.; Harada, M.; Kitada, C.; Kurokawa, T.; Onda, H.; Fujino, M. Isolation and CDNA Cloning of a Novel Galanin-like Peptide (GALP) from Porcine Hypothalamus. J. Biol. Chem. 1999, 274, 37041–37045. [Google Scholar] [CrossRef]
- Lawrence, C.; Fraley, G.S. Galanin-like Peptide (GALP) Is a Hypothalamic Regulator of Energy Homeostasis and Reproduction. Front. Neuroendocrinol. 2011, 32, 1–9. [Google Scholar] [CrossRef]
- Santic, R.; Fenninger, K.; Graf, K.; Schneider, R.; Hauser-Kronberger, C.; Schilling, F.H.; Kogner, P.; Ratschek, M.; Jones, N.; Sperl, W.; et al. Gangliocytes in Neuroblastic Tumors Express Alarin, a Novel Peptide Derived by Differential Splicing of the Galanin-Like Peptide Gene. J. Mol. Neurosci. 2006, 29, 145–152. [Google Scholar] [CrossRef]
- Marcos, P.; Coveñas, R. Neuropeptidergic Control of Feeding: Focus on the Galanin Family of Peptides. Int. J. Mol. Sci. 2021, 22, 2544. [Google Scholar] [CrossRef]
- Evans, H.; Baumgartner, M.; Shine, J.; Herzog, H. Genomic Organization and Localization of the Gene Encoding Human Preprogalanin. Genomics 1993, 18, 473–477. [Google Scholar] [CrossRef]
- Kofler, B.; Evans, H.F.; Liu, M.L.; Falls, V.; Iismaa, T.P.; Shine, J.; Herzog, H. Characterization of the 5′-Flanking Region of the Human Preprogalanin Gene. DNA Cell Biol. 1995, 14, 321–329. [Google Scholar] [CrossRef]
- Cunningham, M.J.; Scarlett, J.M.; Steiner, R.A. Cloning and Distribution of Galanin-Like Peptide MRNA in the Hypothalamus and Pituitary of the Macaque. Endocrinology 2002, 143, 755–763. [Google Scholar] [CrossRef]
- Landry, M.; Åman, K.; Dostrovsky, J.; Lozano, A.M.; Carlstedt, T.; Spenger, C.; Josephson, A.; Wiesenfeld-Hallin, Z.; Hökfelt, T. Galanin Expression in Adult Human Dorsal Root Ganglion Neurons: Initial Observations. Neuroscience 2003, 117, 795–809. [Google Scholar] [CrossRef]
- Falkenstetter, S.; Leitner, J.; Brunner, S.M.; Rieder, T.N.; Kofler, B.; Weis, S. Galanin System in Human Glioma and Pituitary Adenoma. Front. Endocrinol. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, L.; Stella, S.L.; Lakhter, A.; Hirano, A.; Tonini, M. Catia Sternini Galanin Receptors in the Rat Gastrointestinal Tract. Neuropeptides 2005, 39, 349–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parker, E.M.; Izzarelli, D.G.; Nowak, H.P.; Mahle, C.D.; Iben, L.G.; Wang, J.; Goldstein, M.E. Cloning and Characterization of the Rat GALR1 Galanin Receptor from Rin14B Insulinoma Cells. Mol. Brain Res. 1995, 34, 179–189. [Google Scholar] [CrossRef]
- Waters, S.M.; Krause, J.E. Distribution of Galanin-1, -2 and -3 Receptor Messenger RNAs in Central and Peripheral Rat Tissues. Neuroscience 1999, 95, 265–271. [Google Scholar] [CrossRef]
- Gustafson, E.L.; Smith, K.E.; Durkin, M.M.; Gerald, C.; Branchek, T.A. Distribution of a Rat Galanin Receptor MRNA in Rat Brain. NeuroReport 1996, 7, 953–957. [Google Scholar] [CrossRef]
- Smith, K.E.; Forray, C.; Walker, M.W.; Jones, K.A.; Tamm, J.A.; Bard, J.; Branchek, T.A.; Linemeyer, D.L.; Gerald, C. Expression Cloning of a Rat Hypothalamic Galanin Receptor Coupled to Phosphoinositide Turnover. J. Biol. Chem. 1997, 272, 24612–24616. [Google Scholar] [CrossRef]
- Bennet, W.M.; Hill, S.F.; Ghatei, M.A.; Bloom, S.R. Galanin in the Normal Human Pituitary and Brain and in Pituitary Adenomas. J. Endocrinol. 1991, 130, 463–467. [Google Scholar] [CrossRef]
- Zhang, X.; Verge, V.M.K.; Wiesenfeld-Hallin, Z.; Piehl, F.; Hökfelt, T. Expression of Neuropeptides and Neuropeptide MRNAs in Spinal Cord after Axotomy in the Rat, with Special Reference to Motoneurons and Galanin. Exp. Brain Res. 1993, 93, 450–461. [Google Scholar] [CrossRef]
- Perumal, P.; Vrontakis, M. Transgenic Mice Over-Expressing Galanin Exhibit Pituitary Adenomas and Increased Secretion of Galanin, Prolactin and Growth Hormone. J. Endocrinol. 2003, 179, 145–154. [Google Scholar] [CrossRef][Green Version]
- Hacker, G.W.; Bishop, A.E.; Terenghi, G.; Varndell, I.M.; Aghahowa, J.; Pollard, K.; Thurner, J.; Polak, J.M. Multiple Peptide Production and Presence of General Neuroendocrine Markers Detected in 12 Cases of Human Phaeochromocytoma and in Mammalian Adrenal Glands. Virchows Arch. A Pathol. Anat. Histopathol. 1988, 412, 399–411. [Google Scholar] [CrossRef]
- Melander, T.; Hijkfelt, T.; Riikaeus, A. Coexistence of Galanin-like Lmmunoreactivity with Catecholamines, 5=Hydroxytryptamine, GABA and Neuropeptides in the Rat CNS. J. Neurosci. 1986, 6, 15. [Google Scholar] [CrossRef]
- Xu, Z.-Q.D.; Shi, T.-J.S.; Hökfelt, T. Galanin/GMAP- and NPY-like Immunoreactivities in Locus Coeruleus and Noradrenergic Nerve Terminals in the Hippocampal Formation and Cortex with Notes on the Galanin-R1 and -R2 Receptors. J. Comp. Neurol. 1998, 392, 227–251. [Google Scholar] [CrossRef]
- Merchenthaler, I.; Lopez, F.J.; Negro-Vilar, A. Colocalization of Galanin and Luteinizing Hormone-Releasing Hormone in a Subset of Preoptic Hypothalamic Neurons: Anatomical and Functional Correlates. Proc. Natl. Acad. Sci. USA 1990, 87, 6326–6330. [Google Scholar] [CrossRef]
- Zhang, X.; Nicholas, A.P.; Ho¨kfelt, T. Ultrastructural Studies on Peptides in the Dorsal Horn of the Spinal Cord—I. Co-Existence of Galanin with Other Peptides in Primary Afferents in Normal Rats. Neuroscience 1993, 57, 365–384. [Google Scholar] [CrossRef]
- Zhang, X.; Nicholas, A.P.; Hökfelt, T. Ultrastructural Studies on Peptides in the Dorsal Horn of the Rat Spinal Cord—II. Co-Existence of Galanin with Other Peptides in Local Neurons. Neuroscience 1995, 64, 875–891. [Google Scholar] [CrossRef]
- Gundlach, A.L.; Ryan, P.J. Galanin and GALP. In Handbook of BiologicallyActive Peptides; Kastin, A.J., Ed.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 766–775. [Google Scholar]
- McKnight, G.L.; Karlsen, A.E.; Kowalyk, S.; Mathewes, S.L.; Sheppard, P.O.; O’Hara, P.J.; Taborsky, G.J. Sequence of Human Galanin and Its Inhibition of Glucose-Stimulated Insulin Secretion From RIN Cells. Diabetes 1992, 41, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hökfelt, T.; Tatemoto, K. Galanin—25 Years with a Multitalented Neuropeptide. Cell. Mol. Life Sci. 2008, 65, 1791–1795. [Google Scholar] [CrossRef]
- Scott, M.K.; Ross, T.M.; Lee, D.H.S.; Wang, H.-Y.; Shank, R.P.; Wild, K.D.; Davis, C.B.; Crooke, J.J.; Potocki, A.C.; Reitz, A.B. 2,3-Dihydro-Dithiin and -Dithiepine-1,1,4,4-Tetroxides: Small Molecule Non-Peptide Antagonists of the Human Galanin HGAL-1 Receptor. Bioorg. Med. Chem. 2000, 8, 1383–1391. [Google Scholar] [CrossRef]
- Hobson, S.-A.; Bacon, A.; Elliot-Hunt, C.R.; Holmes, F.E.; Kerr, N.C.H.; Pope, R.; Vanderplank, P.; Wynick, D. Galanin Acts as a Trophic Factor to the Central and Peripheral Nervous Systems. Cell. Mol. Life Sci. 2010, 102, 25–38. [Google Scholar] [CrossRef]
- Elliott-Hunt, C.R.; Marsh, B.; Bacon, A.; Pope, R.; Vanderplank, P.; Wynick, D. Galanin Acts as a Neuroprotective Factor to the Hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 5105–5110. [Google Scholar] [CrossRef]
- Quynh, N.T.T.; Islam, S.M.; Florén, A.; Bartfai, T.; Langel, Ü.; Östenson, C.-G. Effects of Galnon, a Non-Peptide Galanin-Receptor Agonist, on Insulin Release from Rat Pancreatic Islets. Biochem. Biophys. Res. Commun. 2005, 328, 213–220. [Google Scholar] [CrossRef]
- Giustina, A.; Schettino, M.; Bodini, C.; Doga, M.; Licini, M.; Giustina, G. Effect of Galanin on the Growth Hormone Response to Growth Hormone-Releasing Hormone in Acromegaly. Metabolism 1992, 41, 1291–1294. [Google Scholar] [CrossRef]
- Mazancourt, P.D.; Goldsmith, P.K.; Weinstein, L.S. Inhibition of Adenylate Cyclase Activity by Galanin in Rat Insulinoma Cells Is Mediated by the G-Protein G13. Biochem. J. 1994, 303, 369–375. [Google Scholar] [CrossRef]
- Guipponi, M.; Chentouf, A.; Webling, K.E.B.; Freimann, K.; Crespel, A.; Nobile, C.; Lemke, J.R.; Hansen, J.; Dorn, T.; Lesca, G.; et al. Galanin Pathogenic Mutations in Temporal Lobe Epilepsy. Hum. Mol. Genet. 2015, 24, 3082–3091. [Google Scholar] [CrossRef]
- Webling, K.E.B.; Runesson, J.; Bartfai, T.; Langel, Ü. Galanin Receptors and Ligands. Front. Endocrinol. 2012, 3, 1–14. [Google Scholar] [CrossRef]
- Chan-Palay, V. Galanin Hyperinnervates Surviving Neurons of the Human Basal Nucleus of Meynert in Dementias of Alzheimer’s and Parkinson’s Disease: A Hypothesis for the Role of Galanin in Accentuating Cholinergic Dysfunction in Dementia. J. Comp. Neurol. 1988, 273, 543–557. [Google Scholar] [CrossRef]
- Wiesenfeld-Hallin, Z.; Villar, M.J.; Ho¨kfelt, T. The Effects of Intrathecal Galanin and C-Fiber Stimulation on the Flexor Reflex in the Rat. Brain Res. 1989, 486, 205–213. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Barde, S.; Zhang, M.-D.; Sun, J.; Wang, T.; Zhang, P.; Luo, H.; Wang, Y.; Yang, Y.; et al. Depression-like Behavior in Rat: Involvement of Galanin Receptor Subtype 1 in the Ventral Periaqueductal Gray. Proc. Natl. Acad. Sci. USA 2016, 113, 4726–4735. [Google Scholar] [CrossRef]
- Koller, A.; Brunner, S.M.; Bianchini, R.; Ramspacher, A.; Emberger, M.; Locker, F.; Schlager, S.; Kofler, B. Galanin Is a Potent Modulator of Cytokine and Chemokine Expression in Human Macrophages. Sci. Rep. 2019, 9, 7237. [Google Scholar] [CrossRef]
- Jurkowski, W.; Yazdi, S.; Elofsson, A. Ligand Binding Properties of Human Galanin Receptors. Mol. Membr. Biol. 2013, 30, 206–216. [Google Scholar] [CrossRef]
- Probst, W.C.; Snyder, L.A.; Schuster, D.I.; Brosius, J.; Sealfon, S.C. Sequence Alignment of the G-Protein Coupled Receptor Superfamily. DNA Cell Biol. 1992, 11, 1–20. [Google Scholar] [CrossRef]
- Krishna, A.G.; Menon, S.T.; Terry, T.J.; Sakmar, T.P. Evidence That Helix 8 of Rhodopsin Acts as a Membrane-Dependent Conformational Switch. Biochemistry 2002, 41, 8298–8309. [Google Scholar] [CrossRef]
- Kolakowski, L.F.; O’Neill, G.P.; Howard, A.D.; Broussard, S.R.; Sullivan, K.A.; Feighner, S.D.; Sawzdargo, M.; Nguyen, T.; Kargman, S.; Shiao, L.-L.; et al. Molecular Characterization and Expression of Cloned Human Galanin Receptors GALR2 and GALR3. J. Neurochem. 2002, 71, 2239–2251. [Google Scholar] [CrossRef]
- Lang, R.; Gundlach, A.; Kofler, B. The Galanin Peptide Family: Receptor Pharmacology, Pleiotropic Biological Actions, and Implications in Health and Disease. Pharmacol. Ther. 2007, 115, 177–207. [Google Scholar] [CrossRef]
- Fathi, Z.; Battaglino, P.M.; Iben, L.G.; Li, H.; Baker, E.; Zhang, D.; McGovern, R.; Mahle, C.D.; Sutherland, G.R.; Iismaa, T.P.; et al. Molecular Characterization, Pharmacological Properties and Chromosomal Localization of the Human GALR2 Galanin Receptor. Mol. Brain Res. 1998, 58, 156–169. [Google Scholar] [CrossRef]
- Wang, S.; Hashemi, T.; He, C.; Strader, C.; Bayne, M. Molecular Cloning and Pharmacological Characterization of a New Galanin Receptor Subtype. Mol. Pharmacol. 1997, 52, 337–343. [Google Scholar] [CrossRef]
- Habert-Ortoli, E.; Amiranoff, B.; Loquet, I.; Laburthe, M.; Mayaux, J.F. Molecular Cloning of a Functional Human Galanin Receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 9780–9783. [Google Scholar] [CrossRef]
- Wang, S.; Hashemi, T.; Fried, S.; Clemmons, A.L.; Hawes, B.E. Differential Intracellular Signaling of the GalR1 and GalR2 Galanin Receptor Subtypes. Biochemistry 1998, 37, 6711–6717. [Google Scholar] [CrossRef]
- Wang, S.; He, C.; Maguire, M.T.; Clemmons, A.L.; Burrier, R.E.; Guzzi, M.F.; Strader, C.D.; Parker, E.M.; Bayne, M.L. Genomic Organization and Functional Characterization of the Mouse GalR1 Galanin Receptor. FEBS Lett. 1997, 411, 225–230. [Google Scholar] [CrossRef]
- Badie-Mahdavi, H.; Lu, X.; Behrens, M.M.; Bartfai, T. Role of Galanin Receptor 1 and Galanin Receptor 2 Activation in Synaptic Plasticity Associated with 3′,5′-Cyclic AMP Response Element-Binding Protein Phosphorylation in the Dentate Gyrus: Studies with a Galanin Receptor 2 Agonist and Galanin Receptor 1 Knockout Mice. Neuroscience 2005, 133, 591–604. [Google Scholar] [CrossRef]
- Zachariou, V.; Georgescu, D.; Kansal, L.; Merriam, P.; Picciotto, M.R. Galanin Receptor 1 Gene Expression Is Regulated by Cyclic AMP through a CREB-Dependent Mechanism: Galanin Receptor I Gene Expression. J. Neurochem. 2008, 76, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hawes, J.J.; Brunzell, D.H.; Wynick, D.; Zachariou, V.; Picciotto, M.R. GalR1, but Not GalR2 or GalR3, Levels Are Regulated by Galanin Signaling in the Locus Coeruleus through a Cyclic AMP-Dependent Mechanism: GalR Regulation in the Locus Coeruleus. J. Neurochem. 2005, 93, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, A.S.; Webb, G.C.; Liu, M.L.; Kofler, B.; Hort, Y.J.; Fathi, Z.; Bottema, C.D.K.; Shine, J.; Iismaa, T.P. Structural Organization of the Mouse and Human GALR1 Galanin Receptor Genes (GalnrandGALNR) and Chromosomal Localization of the Mouse Gene. Genomics 1997, 45, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, D.D.; Benya, R.V. Cloning and Quantification of Galanin-1 Receptor Expression by Mucosal Cells Lining the Human Gastrointestinal Tract. Biochem. Biophys. Res. Commun. 1996, 222, 379–385. [Google Scholar] [CrossRef]
- Sullivan, K.A.; Shiao, L.-L.; Cascieri, M.A. Pharmacological Characterization and Tissue Distribution of the Human and Rat GALR1 Receptors. Biochem. Biophys. Res. Commun. 1997, 233, 823–828. [Google Scholar] [CrossRef]
- Howard, A.D.; Tan, C.; Shiao, L.-L.; Palyha, O.C.; McKee, K.K.; Weinberg, D.H.; Feighner, S.D.; Cascieri, M.A.; Smith, R.G.; Van Der Ploeg, L.H.T.; et al. Molecular Cloning and Characterization of a New Receptor for Galanin. FEBS Lett. 1997, 405, 285–290. [Google Scholar] [CrossRef]
- Demsie, D.G.; Altaye, B.M.; Weldekidan, E.; Gebremedhin, H.; Alema, N.M.; Tefera, M.M.; Tilahun, A. Galanin Receptors as Drug Target for Novel Antidepressants: Review. Biol. Targets Ther. 2020, 14, 37–45. [Google Scholar] [CrossRef]
- Bloomquist, B.T.; Beauchamp, M.R.; Zhelnin, L.; Brown, S.-E.; Gore-Willse, A.R.; Gregor, P.; Cornfield, L.J. Cloning and Expression of the Human Galanin Receptor GalR2. Biochem. Biophys. Res. Commun. 1998, 243, 474–479. [Google Scholar] [CrossRef]
- Mitchell, V.; Bouret, S.; Howard, A.D.; Beauvillain, J.-C. Expression of the Galanin Receptor Subtype Gal-R2 MRNA in the Rat Hypothalamus. J. Chem. Neuroanat. 1999, 16, 265–277. [Google Scholar] [CrossRef]
- O’Donnell, D.; Ahmad, S.; Wahlestedt, C.; Walker, P. Expression of the Novel Galanin Receptor Subtype GALR2 in the Adult Rat CNS: Distinct Distribution from GALR. J. Comp. Neurol. 1999, 409, 2. [Google Scholar] [CrossRef]
- Mons, N.; Decorte, L.; Jaffard, R.; Cooper, D. Ca2+-Sensitive Adenylyl Cyclases, Key Integrators of Cellular Signalling. Life Sci. 1998, 62, 1647–1652. [Google Scholar] [CrossRef]
- Burazin, T.C.D.; Larm, J.A.; Ryan, M.C.; Gundlach, A.L. Galanin-R1 and -R2 Receptor MRNA Expression during the Development of Rat Brain Suggests Differential Subtype Involvement in Synaptic Transmission and Plasticity: Gal-R1 and Gal-R2 MRNA Localization in Developing Rat Brain. Eur. J. Neurosci. 2000, 12, 2901–2917. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; MacTavish, D.; Kar, S.; Jhamandas, J.H. Galanin Attenuates β-Amyloid (Aβ) Toxicity in Rat Cholinergic Basal Forebrain Neurons. Neurobiol. Dis. 2006, 21, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, C.S.; Banerjee, R.; Inglehart, R.C.; Liu, M.; Russo, N.; Hariharan, A.; van Tubergen, E.A.; Corson, S.L.; Asangani, I.A.; Mistretta, C.M.; et al. Galanin Modulates the Neural Niche to Favour Perineural Invasion in Head and Neck Cancer. Nat. Commun. 2015, 6, 6885. [Google Scholar] [CrossRef]
- Fathi, Z.; Cunningham, A.M.; Iben, L.G.; Battaglino, P.B.; Ward, S.A.; Nichol, K.A.; Pine, K.A.; Wang, J.; Goldstein, M.E.; Iismaa, T.P.; et al. Cloning, Pharmacological Characterization and Distribution of a Novel Galanin Receptor. Mol. Brain Res. 1997, 51, 49–59. [Google Scholar] [CrossRef]
- Elliott-Hunt, C.R.; Pope, R.J.P.; Vanderplank, P.; Wynick, D. Activation of the Galanin Receptor 2 (GalR2) Protects the Hippocampus from Neuronal Damage: GalR2 and Hippocampal Neuroprotection. J. Neurochem. 2007, 100, 780–789. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Chatterjee, O.; Raj, C.; Pullimamidi, D.; Advani, J.; Mahadevan, A.; Keshava Prasad, T.S. An Assembly of Galanin–Galanin Receptor Signaling Network. J. Cell Commun. Signal. 2021, 15, 269–275. [Google Scholar] [CrossRef]
- Wittau, N.; Grosse, R.; Kalkbrenner, F.; Gohla, A.; Schultz, G.; Gudermann, T. The Galanin Receptor Type 2 Initiates Multiple Signaling Pathways in Small Cell Lung Cancer Cells by Coupling to Gq, Gi and G12 Proteins. Oncogene 2000, 19, 4199–4209. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, C.; Hashemi, T.; Bayne, M. Cloning and Expressional Characterization of a Novel Galanin Receptor. J. Biol. Chem. 1997, 272, 31949–31952. [Google Scholar] [CrossRef]
- Smith, K.E.; Walker, M.W.; Artymyshyn, R.; Bard, J.; Borowsky, B.; Tamm, J.A.; Yao, W.-J.; Vaysse, P.J.-J.; Branchek, T.A.; Gerald, C.; et al. Cloned Human and Rat Galanin GALR3 Receptors. J. Biol. Chem. 1998, 273, 23321–23326. [Google Scholar] [CrossRef]
- Mennicken, F.; Hoffert, C.; Pelletier, M.; Ahmad, S.; O’Donnell, D. Restricted Distribution of Galanin Receptor 3 (GalR3) MRNA in the Adult Rat Central Nervous System. J. Chem. Neuroanat. 2002, 24, 257–268. [Google Scholar] [CrossRef]
- Kim, D.-K.; Yun, S.; Son, G.H.; Hwang, J.-I.; Park, C.R.; Kim, J.I.; Kim, K.; Vaudry, H.; Seong, J.Y. Coevolution of the Spexin/Galanin/Kisspeptin Family: Spexin Activates Galanin Receptor Type II and III. Endocrinology 2014, 155, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Kask, K.; Berthold, M.; Bourne, J.; Andell, S.; Langel, Ü.; Bartfai, T. Binding and Agonist/Antagonist Actions of M35, Galanin(1-13)-Bradykinin(2-9) Amide Chimeric Peptide, in Rin m 5F Insulinoma Cells. Regul. Pept. 1995, 59, 341–348. [Google Scholar] [CrossRef]
- Millón, C.; Flores-Burgess, A.; Narváez, M.; Borroto-Escuela, D.O.; Gago, B.; Santín, L.; Castilla-Ortega, E.; Narváez, J.Á.; Fuxe, K.; Díaz-Cabiale, Z. The Neuropeptides Galanin and Galanin (1–15) in Depression-like Behaviours. Neuropeptides 2017, 64, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cabiale, Z.; Parrado, C.; Vela, C.; Razani, H.; Coveñas, R.; Fuxe, K.; Narváez, J.A. Role of Galanin and Galanin (1–15) on Central Cardiovascular Control. Neuropeptides 2005, 39, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Millón, C.; Flores-Burgess, A.; Castilla-Ortega, E.; Gago, B.; García-Fernandez, M.; Serrano, A.; Rodriguez de Fonseca, F.; Narváez, J.A.; Fuxe, K.; Santín, L.; et al. Central Administration of Galanin N-Terminal Fragment 1–15 Decreases the Voluntary Alcohol Intake in Rats: Galanin (1–15) and Alcohol. Addict. Biol. 2019, 24, 76–87. [Google Scholar] [CrossRef]
- Díaz-Cabiale, Z.; Parrado, C.; Narváez, M.; Millón, C.; Puigcerver, A.; Fuxe, K.; Narváez, J.A. The Galanin N-Terminal Fragment (1–15) Interacts with Neuropeptide Y in Central Cardiovascular Control: Involvement of the NPY Y2 Receptor Subtype. Regul. Pept. 2010, 163, 130–136. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Narvaez, M.; Di Palma, M.; Calvo, F.; Rodriguez, D.; Millon, C.; Carlsson, J.; Agnati, L.F.; Garriga, P.; Díaz-Cabiale, Z.; et al. Preferential Activation by Galanin 1–15 Fragment of the GalR1 Protomer of a GalR1–GalR2 Heteroreceptor Complex. Biochem. Biophys. Res. Commun. 2014, 452, 347–353. [Google Scholar] [CrossRef]
- Fuxe, K.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Calvo, F.; Garriga, P.; Tena, M.; Narvaez, M.; Millón, C.; Parrado, C.; et al. On the Existence and Function of Galanin Receptor Heteromers in the Central Nervous System. Front. Endocrinol. 2012, 3, 127. [Google Scholar] [CrossRef]
- Flores-Burgess, A.; Millón, C.; Gago, B.; García-Durán, L.; Cantero-García, N.; Coveñas, R.; Narváez, J.A.; Fuxe, K.; Santín, L.; Díaz-Cabiale, Z. Galanin (1–15)-Fluoxetine Interaction in the Novel Object Recognition Test. Involvement of 5-HT1A Receptors in the Prefrontal Cortex of the Rats. Neuropharmacology 2019, 155, 104–112. [Google Scholar] [CrossRef]
- Rauch, I.; Kofler, B. The Galanin System in Cancer. In Galanin; Experientia Supplementum; Hökfelt, T., Ed.; Springer Basel: Basel, Switzerland, 2010; Volume 102, pp. 223–241. ISBN 978-3-0346-0227-3. [Google Scholar]
- Grenbäck, E.; Bjellerup, P.; Wallerman, E.; Lundblad, L.; Änggård, A.; Ericson, K.; Åman, K.; Landry, M.; Schmidt, W.E.; Hökfelt, T.; et al. Galanin in Pituitary Adenomas. Regul. Pept. 2004, 117, 127–139. [Google Scholar] [CrossRef]
- Hsu, D.W.; Hooi, S.C.; Tessa, E.; Strauss, R.M.; Kaplan, L.M. Coexpression of Galanin and Adrenocorticotropic Hormone in Human Pituitary and Pituitary Adenomas. Am. J. Pathol. 1991, 138, 13. [Google Scholar]
- Tuechler, C.; Hametner, R.; Jones, N.; Jones, R.; Iismaa, T.P.; Sperl, W.; Kofler, B. Galanin and Galanin Receptor Expression in Neuroblastoma a. Ann. N. Y. Acad. Sci. 1998, 863, 438–441. [Google Scholar] [CrossRef]
- Leung, B.; Lismaa, T.P.; Leung, K.-C.; Hort, Y.J.; Turner, J.; Sheehy, J.P.; Ho, K.K.Y. Galanin in Human Pituitary Adenomas: Frequency and Clinical Significance. Clin. Endocrinol. 2002, 56, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Kee, M.K.; Chong, S.A.; Nam, M.J. Galanin Is Up-Regulated in Colon Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Vrontakis, M.E.; Kovacs, K.; Asa, S.L.; Friesen, H.G. Galanin Immunoreactivity in Neuroendocrine Tumors. Arch. Pathol. Lab. Med. 1991, 1, 926–929. [Google Scholar]
- Tadros, T.S.; Strauss, R.M.; Cohen, C.; Gal, A.A. Galanin Immunoreactivity in Paragangliomas but Not in Carcinoid Tumors. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 250–252. [Google Scholar] [CrossRef]
- Hulting, A.-L.; Meister, B.; Grimetlius, L.; Wersäll, J.; Änggård, A.; Hökfelt, T. Production of a Galanin-like Peptide by a Human Pituitary Adenoma: ImmunohistochemicaI Evidence. Acta Physiol. Scand. 1989, 137, 561–562. [Google Scholar] [CrossRef]
- Vrontakis, M.E.; Sano, T.; Kovacs, K.; Friesen, H.G. Presence of Galanin-Like Immunoreactivity in Nontumorous Corticotrophs and Corticotroph Adenomas of the Human Pituitary. J. Clin. Endocrinol. Metab. 1990, 70, 747–751. [Google Scholar] [CrossRef]
- Felix, I.; Bilbao, J.M.; Asa, S.L.; Tyndel, F.; Kovacs, K.; Becker, L.E. Cerebral and Cerebellar Gangliocytomas: A Morphological Study of Nine Cases. Acta Neuropathol. 1994, 88, 246–251. [Google Scholar] [CrossRef]
- Fried, G.; Wikström, L.-M.; Höög, A.; Arver, S.; Cedermark, B.; Hamberger, B.; Grimelius, L.; Meister, B. Multiple Neuropetide Immunoreactivities in a Renin-Producing Human Paraganglioma. Cancer 1994, 74, 142–151. [Google Scholar] [CrossRef]
- Kanazawa, T.; Kommareddi, P.K.; Iwashita, T.; Kumar, B.; Misawa, K.; Misawa, Y.; Jang, I.; Nair, T.S.; Iino, Y.; Carey, T.E. Galanin Receptor Subtype 2 Suppresses Cell Proliferation and Induces Apoptosis in P53 Mutant Head and Neck Cancer Cells. Clin. Cancer Res. 2009, 15, 2222–2230. [Google Scholar] [CrossRef]
- Skotheim, R.I.; Lind, G.E.; Monni, O.; Nesland, J.M.; Abeler, V.M.; Fosså, S.D.; Duale, N.; Brunborg, G.; Kallioniemi, O.; Andrews, P.W.; et al. Differentiation of Human Embryonal Carcinomas In Vitro and In Vivo Reveals Expression Profiles Relevant to Normal Development. Cancer Res. 2005, 65, 5588–5598. [Google Scholar] [CrossRef]
- Kepron, C.; Reis, P.; Bharadwaj, R.; Shaw, J.; Kamel-Reid, S.; Ghazarian, D. Identification of Genomic Predictors of Non-Melanoma Skin Cancer in Solid Organ Transplant Recipients. Eur. J. Dermatol. 2009, 19, 278–280. [Google Scholar] [CrossRef]
- Berger, A.; Santic, R.; Hauser-Kronberger, C.; Schilling, F.H.; Kogner, P.; Ratschek, M.; Gamper, A.; Jones, N.; Sperl, W.; Kofler, B. Galanin and Galanin Receptors in Human Cancers. Neuropeptides 2005, 39, 353–359. [Google Scholar] [CrossRef]
- Stevenson, L.; Allen, W.L.; Turkington, R.; Jithesh, P.V.; Proutski, I.; Stewart, G.; Lenz, H.-J.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Identification of Galanin and Its Receptor GalR1 as Novel Determinants of Resistance to Chemotherapy and Potential Biomarkers in Colorectal Cancer. Clin. Cancer Res. 2012, 18, 5412–5426. [Google Scholar] [CrossRef]
- Berger, A.; Santic, R.; Almer, D.; Hauser-Kronberger, C.; Huemer, M.; Humpel, C.; Stockhammer, G.; Sperl, W.; Kofler, B. Galanin and Galanin Receptors in Human Gliomas. Acta Neuropathol. 2003, 105, 555–560. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Vera, J.; Coscojuela, C.; Roca, M.J.; Parrado, C.; González, S. Expression of Galanin in Melanocytic Tumors. Actas Dermo-Sifiliogr. Engl. Ed. 2007, 98, 24–34. [Google Scholar] [CrossRef]
- Sugimoto, T.; Seki, N.; Shimizu, S.; Kikkawa, N.; Tsukada, J.; Shimada, H.; Sasaki, K.; Hanazawa, T.; Okamoto, Y.; Hata, A. The Galanin Signaling Cascade Is a Candidate Pathway Regulating Oncogenesis in Human Squamous Cell Carcinoma. Genes. Chromosomes Cancer 2009, 48, 132–142. [Google Scholar] [CrossRef]
- Godlewski, J.; Pidsudko, Z. Characteristic of Galaninergic Components of the Enteric Nervous System in the Cancer Invasion of Human Large Intestine. Ann. Anat.-Anat. Anz. 2012, 194, 368–372. [Google Scholar] [CrossRef]
- Banerjee, R.; Henson, B.S.; Russo, N.; Tsodikov, A.; D’Silva, N.J. Rap1 Mediates Galanin Receptor 2-Induced Proliferation and Survival in Squamous Cell Carcinoma. Cell. Signal. 2011, 23, 1110–1118. [Google Scholar] [CrossRef]
- Henson, B.S.; Neubig, R.R.; Jang, I.; Ogawa, T.; Zhang, Z.; Carey, T.E.; D’Silva, N.J. Galanin Receptor 1 Has Anti-Proliferative Effects in Oral Squamous Cell Carcinoma. J. Biol. Chem. 2005, 280, 22564–22571. [Google Scholar] [CrossRef]
- Barakat, M.T.; Meeran, K.; Bloom, S.R. Neuroendocrine Tumours. Endocr. Relat. Cancer 2004, 11, 1–18. [Google Scholar] [CrossRef]
- Reubi, J.C. Regulatory Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Q. J. Nucl. Med. 1997, 41, 63–70. [Google Scholar]
- Wood, S.M.; Polak, J.M.; Bloom, S.R. Gut Hormone Secreting Tumours. Scand. J. Gastroenterol. 1983, 82, 165–179. [Google Scholar]
- Kogner, P. Neuropeptides in Neuroblastomas Andganglioneuromas. Prog. Brain Res. 1995, 104, 325–338. [Google Scholar] [CrossRef]
- Gregg, D.W.; Galkin, M.; Gorski, J. Effect of Estrogen on the Expression of Galanin MRNA in Pituitary Tumor-Sensitive and Tumor-Resistant Rat Strains. Steroids 1996, 61, 468–472. [Google Scholar] [CrossRef]
- Hsu, D.W.; El-Azouzi, M.; Black, P.M.; Chin, W.W.; Hedley-Whyte, E.T.; Kaplan, L.M. Estrogen Increases Galanin Immunoreactivity in Hyperplastic Prolactin-Secreting Cells in Fisher 344 Rats. Endocrinology 1990, 126, 3159–3167. [Google Scholar] [CrossRef]
- Hyde, J.F.; Howard, G. Regulation of Galanin Gene Expression in the Rat Anterior Pituitary Gland by the Somatostatin Analog SMS 201-995. Endocrinology 1992, 131, 2097–2102. [Google Scholar] [CrossRef]
- Wynick, D.; Hammond, P.J.; Akinsanya, K.O.; Bloom, S.R. Galanin Regulates Basal and Oestrogen-Stimulated Lactotroph Function. Nature 1993, 364, 529–532. [Google Scholar] [CrossRef]
- Wynick, D.; Small, C.J.; Bacon, A.; Holmes, F.E.; Norman, M.; Ormandy, C.J.; Kilic, E.; Kerr, N.C.H.; Ghatei, M.; Talamantes, F.; et al. Galanin Regulates Prolactin Release and Lactotroph Proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 12671–12676. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Bartfai, T. Analyzing the Validity of GalR1 and GalR2 Antibodies Using Knockout Mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2009, 379, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Beermann, S.; Seifert, R.; Neumann, D. Commercially Available Antibodies against Human and Murine Histamine H4-Receptor Lack Specificity. Naunyn. Schmiedebergs Arch. Pharmacol. 2012, 385, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Invitti, C.; Giraldi, F.P.; Dubini, A.; Moroni, P.; Losa, M.; Piccoletti, R.; Cavagnini, F. Galanin Is Released by Adrenocorticotropin-Secreting Pituitary Adenomas In Vivo and In Vitro. J. Clin. Endocrinol. Metab. 1999, 84, 1351–1356. [Google Scholar] [CrossRef]
- Berger, A.; Tuechler, C.; Almer, D.; Kogner, P.; Ratschek, M.; Kerbl, R.; Iismaa, T.P.; Jones, N.; Sperl, W.; Kofler, B. Elevated Expression of Galanin Receptors in Childhood Neuroblastic Tumors. Neuroendocrinology 2002, 75, 130–138. [Google Scholar] [CrossRef]
- Perel, Y.; Amrein, L.; Dobremez, E.; Rivel, J.; Daniel, J.Y.; Landry, M. Galanin and Galanin Receptor Expression in Neuroblastic Tumours: Correlation with Their Differentiation Status. Br. J. Cancer 2002, 86, 117–122. [Google Scholar] [CrossRef]
- Sjöholm, Å. Regulation of Insulinoma Cell Proliferation and Insulin Accumulation by Peptides and Second Messengers. Ups. J. Med. Sci. 1995, 100, 201–216. [Google Scholar] [CrossRef]
- Fehmann, H.C.; Habener, J.F. Galanin Inhibits Proinsulin Gene Expression Stimulated by the Insulinotropic Hormone Glucagon-like Peptide-I(7-37) in Mouse Insulinoma Beta TC-1 Cells. Endocrinology 1992, 130, 2890–2896. [Google Scholar] [CrossRef]
- Toneff, T.; Funkelstein, L.; Mosier, C.; Abagyan, A.; Ziegler, M.; Hook, V. Beta-Amyloid Peptides Undergo Regulated Co-Secretion with Neuropeptide and Catecholamine Neurotransmitters. Peptides 2013, 46, 126–135. [Google Scholar] [CrossRef]
- Cheng, S.; Yuan, C.-G. Differential Effect of Galanin on Proliferation of PC12 and B104 Cells. NeuroReport 2007, 18, 1379–1383. [Google Scholar] [CrossRef]
- Squillaci, S.; Gal, A.A. Galanin Immunoreactivity in a Laryngealparaganglioma: Case Report and Literature Review. Pathologica 2004, 96, 111–116. [Google Scholar]
- Tofighi, R.; Joseph, B.; Xia, S.; Xu, Z.-Q.D.; Hamberger, B.; Hökfelt, T.; Ceccatelli, S. Galanin Decreases Proliferation of PC12 Cells and Induces Apoptosis via Its Subtype 2 Receptor (GalR2). Proc. Natl. Acad. Sci. USA 2008, 105, 2717–2722. [Google Scholar] [CrossRef]
- Bauer, F.E.; Hacker, G.W.; Terenghi, G.; Adrian, T.E.; Polak, J.M.; Bloom, S.R. Localization and Molecular Forms of Galanin in Human Adrenals: Elevated Levels in Pheochromocytomas. J. Clin. Endocrinol. Metab. 1986, 63, 1372–1378. [Google Scholar] [CrossRef]
- Tofighi, R.; Barde, S.; Palkovits, M.; Höög, A.; Hökfelt, T.; Ceccatelli, S.; Hulting, A.-L. Galanin and Its Three Receptors in Human Pituitary Adenoma. Neuropeptides 2012, 46, 195–201. [Google Scholar] [CrossRef]
- Hyde, J.F.; Morrison, D.G.; Moore, J.P.; Howard, G. MtTW-10 Pituitary Tumor Cells: Galanin Gene Expression and Peptide Secretion. Endocrinology 1993, 133, 2588–2593. [Google Scholar] [CrossRef]
- Hyde, J.F.; Moore, J.P.; Drake, K.W.; Morrison, D.G. Galanin Gene Expression in Radiothyroidectomy-Induced Thyrotroph Adenomas. Am. J. Physiol.-Endocrinol. Metab. 1996, 271, E24–E30. [Google Scholar] [CrossRef]
- Vrontakis, M.E.; Peden, L.M.; Duckworth, M.L.; Friesen, H.G. Isolation and Characterization of a Complementary DNA (Galanin) Clone from Estrogen-Induced Pituitary Tumor Messenger RNA. J. Biol. Chem. 1987, 262, 16755–16758. [Google Scholar] [CrossRef]
- Piroli, G.G.; Cassataro, J.; Pietranera, L.; Grillo, C.A.; Ferrini, M.; Lux-Lantos, V.; De Nicola, A.F. Progestin Regulation of Galanin and Prolactin Gene Expression in Oestrogen-Induced Pituitary Tumours: Progestin Regulation of Galanin Expression in Prolactinomas. J. Neuroendocrinol. 2001, 13, 302–309. [Google Scholar] [CrossRef]
- Sethi, T.; Rozengurt, E. Multiple Neuropeptides Stimulate Clonal Growth of Small Cell Lung Cancer: Effects of Bradykinin, Vasopressin, Cholecystokinin, Galanin, and Neurotensin. Cancer Res. 1991, 51, 3621–3623. [Google Scholar]
- Sethi, T.; Rozengurt’, E. Galanin Stimulates Ca2+Mobilization, Inositol Phosphate Accumulation, and Clonal Growth in Small Cell Lung Cancer Cells. Cancer Res. 1991, 51, 1674–1679. [Google Scholar] [PubMed]
- Sethi, T.; Langdon, S.; Smyth, J.; Rozengurt, E. Growth of Small Cell Lung Cancer Cells: Stimulation by Multiple Neuropeptides and Inhibition by Broad Spectrum Antagonists In Vitro and In Vivo. Cancer Res. 1992, 25 (Suppl. 9), 2737S–2743S. [Google Scholar]
- Seufferlein, T.; Rozengurt, E. Galanin, Neurotensin, and Phorbol Esters Rapidly Stimulate Activation of Mitogen-Activated Protein Kinase in Small Cell Lung Cancer Cells. Cancer Res. 1996, 56, 5758–5764. [Google Scholar] [PubMed]
- Gudermann, T.; Roelle, S. Calcium-Dependent Growth Regulation of Small Cell Lung Cancer Cells by Neuropeptides. Endocr. Relat. Cancer 2006, 13, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Ben, S.; Saitoh, S.; Kamata, K.; Iguchi, K.; Hoshino, M. Plasmin: Its Role in the Extracellular Processing of Progalanin in Tumor Tissue. Protein Pept. Lett. 2011, 18, 1204–1211. [Google Scholar] [CrossRef]
- Yamamoto, H.; Iguchi, K.; Ohno, S.; Yokogawa, T.; Nishikawa, K.; Hoshino, M. Activation of Large Form Galanin-LI by Extracellular Processing in Small Cell Lung Carcinoma Tissue. Protein Pept. Lett. 2011, 18, 1058–1064. [Google Scholar] [CrossRef]
- Hulting, A.-L.; Land, T.; Berthold, M.; Langel, Ü.; Hökfelt, T.; Bartfai, T. Galanin Receptors from Human Pituitary Tumors Assayed with Human Galanin as Ligand. Brain Res. 1993, 625, 173–176. [Google Scholar] [CrossRef]
- Giustina, A.; Ragni, G.; Bollati, A.; Cozzi, R.; Licini, M.; Poiesi, C.; Turazzi, S.; Bonfanti, C. Inhibitory Effects of Galanin on Growth Hormone (GH) Release in Cultured GH-Secreting Adenoma Cells: Comparative Study with Octreotide, GH-Releasing Hormone, and Thyrotropin-Releasing Hormone. Metabolism 1997, 46, 425–430. [Google Scholar] [CrossRef]
- Giustina, A.; Bonfanti, C.; Licini, M.; De Rango, C.; Milani, G. Inhibitory Effect of Galanin on Growth Hormone Release from Rat Pituitary Tumor Cells (GH1) in Culture. Life Sci. 1994, 55, 1845–1851. [Google Scholar] [CrossRef]
- Hyde, J.F.; Moore, J.P.; Cai, A. Galanin in Normal and Hyperplastic Anterior Pituitary Cells: From Pituitary Tumor Cell Lines to Transgenic Mice. Ann. N. Y. Acad. Sci. 1998, 863, 48–55. [Google Scholar] [CrossRef]
- Mathur, A.; Gorden, P.; Libutti, S.K. Insulinoma. Surg. Clin. N. Am. 2009, 89, 1105–1121. [Google Scholar] [CrossRef]
- Gallwitz, B.; Schmidt, W.E.; Schwarzhoff, R.; Creutzfeldt, W. Galanin: Structural Requirements for Binding and Signal Transduction in RINm5F Insulinoma Cells. Biochem. Biophys. Res. Commun. 1990, 172, 268–275. [Google Scholar] [CrossRef]
- Fridolf, T.; Ahren, B. Dual Action of the Neuropeptide Galanin on the Cytoplasmic Free Calcium Concentration in RIN M5F Cells. Biochem. Biophys. Res. Commun. 1993, 191, 1224–1229. [Google Scholar] [CrossRef]
- Maris, J.M. Recent Advances in Neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef]
- London, W.B.; Castleberry, R.P.; Matthay, K.K.; Look, A.T.; Seeger, R.C.; Shimada, H.; Thorner, P.; Brodeur, G.; Maris, J.M.; Reynolds, C.P.; et al. Evidence for an Age Cutoff Greater Than 365 Days for Neuroblastoma Risk Group Stratification in the Children’s Oncology Group. J. Clin. Oncol. 2005, 23, 6459–6465. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services; National Cancer Institute. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995; National Cancer Institute: Bethesda, MD, USA, 1999.
- Hyde, J.F.; Keller, B.K. Galanin Secretion from Anterior Pituitary Cells in Vitro Is Regulated by Dopamine, Somatostatin, and Thyrotropin-Releasing Hormone. Endocrinology 1991, 128, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Piroli, G.G.; Pietranera, L.; Grillo, C.A.; De Nicola, A.F. Gender Differences in the Expression of Galanin and Vasoactive Intestinal Peptide in Oestrogen-Induced Prolactinomas of Fischer 344 Rats. J. Neuroendocrinol. 2004, 16, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Hayes, J.D.; Patel, N.; Hyde, J.F. Targeted Overexpression of Galanin in Lactotrophs of Transgenic Mice Induces Hyperprolactinemia and Pituitary Hyperplasia. Endocrinology 1999, 140, 10. [Google Scholar] [CrossRef][Green Version]
- Schneider, B.J.; Kalemkerian, G.P. Personalized Therapy of Small Cell Lung Cancer. In Lung Cancer and Personalized Medicine: Novel Therapies and Clinical Management; Advances in Experimental Medicine and Biology; Ahmad, A., Gadgeel, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 890, pp. 149–174. ISBN 978-3-319-24931-5. [Google Scholar]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. In Lung Cancer and Personalized Medicine; Advances in Experimental Medicine and Biology; Ahmad, A., Gadgeel, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 893, pp. 1–19. ISBN 978-3-319-24221-7. [Google Scholar]
- Kalemkerian, G.P.; Akerley, W.; Bogner, P.; Borghaei, H.; Chow, L.Q.; Downey, R.J.; Gandhi, L.; Ganti, A.K.P.; Govindan, R.; Grecula, J.C.; et al. Small Cell Lung Cancer. J. Natl. Compr. Canc. Netw. 2013, 11, 78–98. [Google Scholar] [CrossRef]
- Roelle, S.; Grosse, R.; Buech, T.; Chubanov, V.; Gudermann, T. Essential Role of Pyk2 and Src Kinase Activation in Neuropeptide-Induced Proliferation of Small Cell Lung Cancer Cells. Oncogene 2008, 27, 1737–1748. [Google Scholar] [CrossRef][Green Version]
- Kozłowska, A.; Kozera, P.; Majewski, M.; Godlewski, J. Co-Expression of Caspase-3 or Caspase-8 with Galanin in the Human Stomach Section Affected by Carcinoma. Apoptosis 2018, 23, 484–491. [Google Scholar] [CrossRef]
- Kozłowska, A.; Godlewski, J.; Majewski, M. Distribution Patterns of Cocaine- and Amphetamine-Regulated Transcript- and/or Galanin-Containing Neurons and Nerve Fibers Located in the Human Stomach Wall Affected by Tumor. Int. J. Mol. Sci. 2018, 19, 3357. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, P.; Chai, C.; Shao, L.; Mao, H.; Qiao, D.; Kong, G.; Dong, X.; Shi, M.; Zhang, Z.; et al. Galanin Expression Is Down-Regulated in Patients with Gastric Cancer. J. Int. Med. Res. 2019, 47, 1241–1249. [Google Scholar] [CrossRef]
- Yoon, D.; Bae, K.; Lee, M.-K.; Kim, J.H.; Yoon, K.-A. Galanin Is an Epigenetically Silenced Tumor Suppressor Gene in Gastric Cancer Cells. PLoS ONE 2018, 13, e0193275. [Google Scholar] [CrossRef]
- El-Salhy, M. Effects of Octreotide, Galanin and Serotonin on a Human Gastric Cancer Cell Line. Oncol. Rep. 2005. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Godlewski, J.; Kieżun, J.; Kraziński, B.E.; Kmieć, Z. Colorectal Cancer Patients Exhibit Increased Levels of Galanin in Serum and Colon Tissues. Oncol. Lett. 2016, 12, 3323–3329. [Google Scholar] [CrossRef]
- Nagayoshi, K.; Ueki, T.; Tashiro, K.; Mizuuchi, Y.; Manabe, T.; Araki, H.; Oda, Y.; Kuhara, S.; Tanaka, M. Galanin Plays an Important Role in Cancer Invasiveness and Is Associated with Poor Prognosis in Stage II Colorectal Cancer. Oncol. Rep. 2015, 33, 539–546. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, H.C.; Cho, D.H.; Choi, E.Y.; Cho, Y.K.; Ha, Y.J.; Choi, P.W.; Roh, S.A.; Kim, S.Y.; Kim, Y.S. Genome-Wide Identification of Possible Methylation Markers Chemosensitive to Targeted Regimens in Colorectal Cancers. J. Cancer Res. Clin. Oncol. 2011, 137, 1571–1580. [Google Scholar] [CrossRef]
- Iishi, H.; Tatsuta, M.; Baba, M.; Uehara, H.; Yano, H.; Nakaizumi, A. Chemoprevention by Galanin against Colon Carcinogenesis Induced by Azoxymethane in Wistar Rats. Int. J. Cancer 1995, 61, 861–863. [Google Scholar] [CrossRef]
- Talaat, I.M.; Yakout, N.M.; Soliman, A.S.A.; Venkatachalam, T.; Vinod, A.; Eldohaji, L.; Nair, V.; Hareedy, A.; Kandil, A.; Abdel-Rahman, W.M.; et al. Evaluation of Galanin Expression in Colorectal Cancer: An Immunohistochemical and Transcriptomic Study. Front. Oncol. 2022, 12, 877147. [Google Scholar] [CrossRef]
- Kiezun, J.; Godlewski, J.; Krazinski, B.E.; Kozielec, Z.; Kmiec, Z. Galanin Receptors (GalR1, GalR2, and GalR3) Expression in Colorectal Cancer Tissue and Correlations to the Overall Survival and Poor Prognosis of CRC Patients. Int. J. Mol. Sci. 2022, 23, 3735. [Google Scholar] [CrossRef]
- Kozlowska, A.; Kwiatkowski, P.; Oponowicz, A.; Majewski, M.; Kmiec, Z.; Godlewski, J. Myenteric Plexuses Atrophy in the Vicinity of Colorectal Cancer Tissue Is Not Caused by Apoptosis or Necrosis. Folia Histochem. Cytobiol. 2016, 54, 99–107. [Google Scholar] [CrossRef]
- Jung, K.; Narwal, M.; Min, S.Y.; Keam, B.; Kang, H. Squamous Cell Carcinoma of Head and Neck: What Internists Should Know. Korean J. Intern. Med. 2020, 35, 1031–1044. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural Invasion in Cancer: A Review of the Literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-Related Axonogenesis and Neurogenesis in Prostate Cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, F.; Piemonti, L.; Mantovani, A.; Allavena, P. Molecular Mechanisms of Perineural Invasion, a Forgotten Pathway of Dissemination and Metastasis. Cytokine Growth Factor Rev. 2010, 21, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of Cancer Dissemination along Nerves. Nat. Rev. Cancer 2016, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Misawa, K.; Mochizuki, D.; Imai, A.; Endo, S.; Mima, M.; Misawa, Y.; Kanazawa, T.; Carey, T.E.; Mineta, H. Prognostic Value of Aberrant Promoter Hypermethylation of Tumor-Related Genes in Early-Stage Head and Neck Cancer. Oncotarget 2016, 7, 26087–26098. [Google Scholar] [CrossRef][Green Version]
- Misawa, K.; Mima, M.; Imai, A.; Mochizuki, D.; Misawa, Y.; Endo, S.; Ishikawa, R.; Kanazawa, T.; Mineta, H. The Neuropeptide Genes SST, TAC1, HCRT, NPY, and GAL Are Powerful Epigenetic Biomarkers in Head and Neck Cancer: A Site-Specific Analysis. Clin. Epigenetics 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Kanazawa, T.; Misawa, K.; Shinmura, K.; Misawa, Y.; Kusaka, G.; Maruta, M.; Sasaki, T.; Watanabe, Y.; Carey, T.E. Promoter Methylation of Galanin Receptors as Epigenetic Biomarkers for Head and Neck Squamous Cell Carcinomas. Expert Rev. Mol. Diagn. 2019, 19, 137–148. [Google Scholar] [CrossRef]
- Misawa, K.; Mochizuki, D.; Endo, S.; Mima, M.; Misawa, Y.; Imai, A.; Shinmura, K.; Kanazawa, T.; Carey, T.E.; Mineta, H. Site-Specific Methylation Patterns of the GAL and GALR1/2 Genes in Head and Neck Cancer: Potential Utility as Biomarkers for Prognosis: Methylation Patterns of the GAL and GALR1/2 Genes. Mol. Carcinog. 2017, 56, 1107–1116. [Google Scholar] [CrossRef]
- Misawa, K.; Misawa, Y.; Kanazawa, T.; Mochizuki, D.; Imai, A.; Endo, S.; Carey, T.E.; Mineta, H. Epigenetic Inactivation of Galanin and GALR1/2 Is Associated with Early Recurrence in Head and Neck Cancer. Clin. Exp. Metastasis 2016, 33, 187–195. [Google Scholar] [CrossRef]
- Kanazawa, T.; Iwashita, T.; Kommareddi, P.; Nair, T.; Misawa, K.; Misawa, Y.; Ueda, Y.; Tono, T.; Carey, T.E. Galanin and Galanin Receptor Type 1 Suppress Proliferation in Squamous Carcinoma Cells: Activation of the Extracellular Signal Regulated Kinase Pathway and Induction of Cyclin-Dependent Kinase Inhibitors. Oncogene 2007, 26, 5762–5771. [Google Scholar] [CrossRef]
- Kanazawa, T.; Misawa, K.; Misawa, Y.; Maruta, M.; Uehara, T.; Kawada, K.; Nagatomo, T.; Ichimura, K. Galanin Receptor 2 Utilizes Distinct Signaling Pathways to Suppress Cell Proliferation and Induce Apoptosis in HNSCC. Mol. Med. Rep. 2014, 10, 1289–1294. [Google Scholar] [CrossRef]
- Misawa, K.; Ueda, Y.; Kanazawa, T.; Misawa, Y.; Jang, I.; Brenner, J.C.; Ogawa, T.; Takebayashi, S.; Grenman, R.A.; Herman, J.G.; et al. Epigenetic Inactivation of Galanin Receptor 1 in Head and Neck Cancer. Clin. Cancer Res. 2008, 14, 7604–7613. [Google Scholar] [CrossRef]
- Uehara, T.; Kanazawa, T.; Mizukami, H.; Uchibori, R.; Tsukahara, T.; Urabe, M.; Kume, A.; Misawa, K.; Carey, T.E.; Suzuki, M.; et al. Novel Anti-Tumor Mechanism of Galanin Receptor Type 2 in Head and Neck Squamous Cell Carcinoma Cells. Cancer Sci. 2014, 105, 72–80. [Google Scholar] [CrossRef]
- Kanazawa, T.; Misawa, K.; Misawa, Y.; Uehara, T.; Fukushima, H.; Kusaka, G.; Maruta, M.; Carey, T. G-Protein-Coupled Receptors: Next Generation Therapeutic Targets in Head and Neck Cancer? Toxins 2015, 7, 2959–2984. [Google Scholar] [CrossRef]
- Banerjee, R.; Van Tubergen, E.A.; Scanlon, C.S.; Vander Broek, R.; Lints, J.P.; Liu, M.; Russo, N.; Inglehart, R.C.; Wang, Y.; Polverini, P.J.; et al. The G Protein–Coupled Receptor GALR2 Promotes Angiogenesis in Head and Neck Cancer. Mol. Cancer Ther. 2014, 13, 1323–1333. [Google Scholar] [CrossRef]
- de Medeiros, M.C.; Liu, M.; Banerjee, R.; Bellile, E.; D’Silva, N.J.; Rossa, C. Galanin Mediates Tumor-Induced Immunosuppression in Head and Neck Squamous Cell Carcinoma. Cell. Oncol. 2022, 45, 241–256. [Google Scholar] [CrossRef]
- Shen, F.; Zhang, Y.; Yao, Y.; Hua, W.; Zhang, H.; Wu, J.; Zhong, P.; Zhou, L. Proteomic Analysis of Cerebrospinal Fluid: Toward the Identification of Biomarkers for Gliomas. Neurosurg. Rev. 2014, 37, 367–380. [Google Scholar] [CrossRef]
- Locarno, C.V.; Simonelli, M.; Carenza, C.; Capucetti, A.; Stanzani, E.; Lorenzi, E.; Persico, P.; Della Bella, S.; Passoni, L.; Mavilio, D.; et al. Role of Myeloid Cells in the Immunosuppressive Microenvironment in Gliomas. Immunobiology 2020, 225, 151853. [Google Scholar] [CrossRef]
- Mei, Z.; Yang, Y.; Li, Y.; Yang, F.; Li, J.; Xing, N.; Xu, Z.-Q.D. Galanin Suppresses Proliferation of Human U251 and T98G Glioma Cells via Its Subtype 1 Receptor. Biol. Chem. 2017, 398, 1127–1139. [Google Scholar] [CrossRef]
- Ormandy, C.J.; Lee, C.S.L.; Ormandy, H.F.; Fanti, V.; Shine, J.; Peters, G.; Sutherland, R.L. Amplification, Expression, and Steroid Regulation of the Preprogalanin Gene in Human Breast Cancer. Cancer Res. 1998, 6, 1353–1357. [Google Scholar]
- Lü, S.-H. Peptidergic Innervation of Human Esophageal and Cardiac Carcinoma. World J. Gastroenterol. 2003, 9, 399. [Google Scholar] [CrossRef]
- Doufekas, K.; Hadwin, R.; Kandimalla, R.; Jones, A.; Mould, T.; Crowe, S.; Olaitan, A.; Macdonald, N.; Fiegl, H.; Wik, E.; et al. GALR1 Methylation in Vaginal Swabs Is Highly Accurate in Identifying Women With Endometrial Cancer. Int. J. Gynecol. Cancer 2013, 23, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Qi, Y.; Wang, F.; Li, S.; Jia, Z.; Yang, J. A Three Protein-Coding Gene Prognostic Model Predicts Overall Survival in Bladder Cancer Patients. BioMed Res. Int. 2020, 2020, 7272960. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Misawa, K.; Fukushima, H.; Misawa, Y.; Sato, Y.; Maruta, M.; Imayoshi, S.; Kusaka, G.; Kawabata, K.; Mineta, H.; et al. Epigenetic Inactivation of Galanin Receptors in Salivary Duct Carcinoma of the Parotid Gland: Potential Utility as Biomarkers for Prognosis. Oncol. Lett. 2018, 15, 9043–9050. [Google Scholar] [CrossRef] [PubMed]
- Tjomsland, V.; El-Salhy, M. Effects of Single, Double or Triple Combinations of Octreotide, Galanin and Serotonin on a Human Pancreatic Cancer Cell Line. Histol. Histopathol. 2005, 537–541. [Google Scholar] [CrossRef]
- Iishi, H.; Tatsuta, M.; Baba, M.; Yano, H.; Iseki, K.; Uehara, H.; Nakaizumi, A. Inhibition by Galanin of Experimental Carcinogenesis Induced by Azaserine in Rat Pancreas. Int. J. Cancer 1998, 75, 396–399. [Google Scholar] [CrossRef]
- Lang, R.; Berger, A.; Hermann, A.; Kofler, B. Biphasic Response to Human Galanin of Extracellular Acidification in Human Bowes Melanoma Cells. Eur. J. Pharmacol. 2001, 423, 135–141. [Google Scholar] [CrossRef]
- Dunne, M.J.; Bullett, M.J.; Li, G.D.; Wollheim, C.B.; Petersen, O.H. Galanin Activates Nucleotide-Dependent K+ Channels in Insulin-Secreting Cells via a Pertussis Toxin-Sensitive G-Protein. EMBO J. 1989, 8, 413–420. [Google Scholar] [CrossRef]
- Cormont, M.; Le Marchand-Brustel, Y.; Van Obberghen, E.; Spiegel, A.M.; Sharp, G.W. Identification of G Protein Alpha-Subunits in RINm5F Cells and Their Selective Interaction with Galanin Receptor. Diabetes 1991, 40, 1170–1176. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Liu, Y.; Zhang, W.; Liu, Y.; Yang, X.; Cao, Y.; Wang, S. C7 Peptide Inhibits Hepatocellular Carcinoma Metastasis by Targeting the HGF/c-Met Signaling Pathway. Cancer Biol. Ther. 2019, 20, 1430–1442. [Google Scholar] [CrossRef]
- Koller, A.; Rid, R.; Beyreis, M.; Bianchini, R.; Holub, B.S.; Lang, A.; Locker, F.; Brodowicz, B.; Velickovic, O.; Jakab, M.; et al. In Vitro Toxicity of the Galanin Receptor 3 Antagonist SNAP 37889. Neuropeptides 2016, 56, 83–88. [Google Scholar] [CrossRef]
- El-Salhy, M. Effects of Triple Therapy with Octreotide, Galanin and Serotonin on a Human Colon Cancer Cell Line Implanted in Mice: Comparison between Different Routes of Administration. Histol. Histopathol. 2005, 20, 19–25. [Google Scholar] [CrossRef]
- El-Salhy, M. Comparison between Triple Therapy with Octreotide, Galanin and Serotonin, 5-Fluorouracil/Leucovorin, and Sequential Treatment with Both, on Human Colon Cancer. Oncol. Rep. 2004, 11, 11611161168. [Google Scholar] [CrossRef]
- El-Salhy, M.; Dennerqvist, V. Effects of Triple Therapy with Octreotide, Galanin and Serotonin on Liver Metastasis of Human Colon Cancer in Xenografts. Oncol. Rep. 2004, 6, 1177–1182. [Google Scholar] [CrossRef]
- Sitohy, B.; El-Salhy, M. A Comparison between Double and Triple Therapies of Octreotide, Galanin and Serotonin on a Rat Colon Carcinoma. Histol. Histopathol. 2003, 1, 103–110. [Google Scholar] [CrossRef]
- El-Salhy, M.; Sitohy, B.; Norrgård, Ö. Triple Therapy with Octreotide, Galanin, and Serotonin Reduces the Size and Blood Vessel Density and Increases Apoptosis of a Rat Colon Carcinoma. Regul. Pept. 2003, 111, 145–152. [Google Scholar] [CrossRef]
- El-Salhy, M. Triple Treatment with Octreotide, Galanin and Serotonin Is a Promising Therapy for Colorectal Cancer. Curr. Pharm. Des. 2005, 11, 2107–2117. [Google Scholar] [CrossRef]
- El-Salhy, M.; Starefeldt, A. Direct Effects of Octreotide, Galanin and Serotonin on Human Colon Cancer Cells. Oncol. Rep. 2003, 6, 1723–1728. [Google Scholar] [CrossRef]
- Tjomsland, V.; El-Salhy, M. Anti-Tumour Effects of Triple Therapy with Octreotide, Galanin and Serotonin in Comparison with Those of 5-Fluorouracil/Leukovorin on Human Colon Cancer. Int. J. Oncol. 2005, 2, 427–432. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hilding, L.; Royson, H.; Tjomsland, V. Comparison between Triple Therapy with Octreotide, Galanin and Serotonin vs. Irinotecan or Oxaliplatin in Combination with 5-Fluorouracil/Leukovorin in Human Colon Cancer. Int. J. Oncol. 2005, 27, 1. [Google Scholar]
- Muñoz, M.; Berger, M.; Rosso, M.; Gonzalez-Ortega, A.; Carranza, A.; Coveñas, R. Antitumor Activity of Neurokinin-1 Receptor Antagonists in MG-63 Human Osteosarcoma Xenografts. Int. J. Oncol. 2014, 44, 137–146. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Salinas-Martín, M.V.; Carranza, A.; Garcia-Recio, S.; Almendro, V.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant Is a Promising Candidate for the Treatment of Breast Cancer. Int. J. Oncol. 2014, 45, 1658–1672. [Google Scholar] [CrossRef]
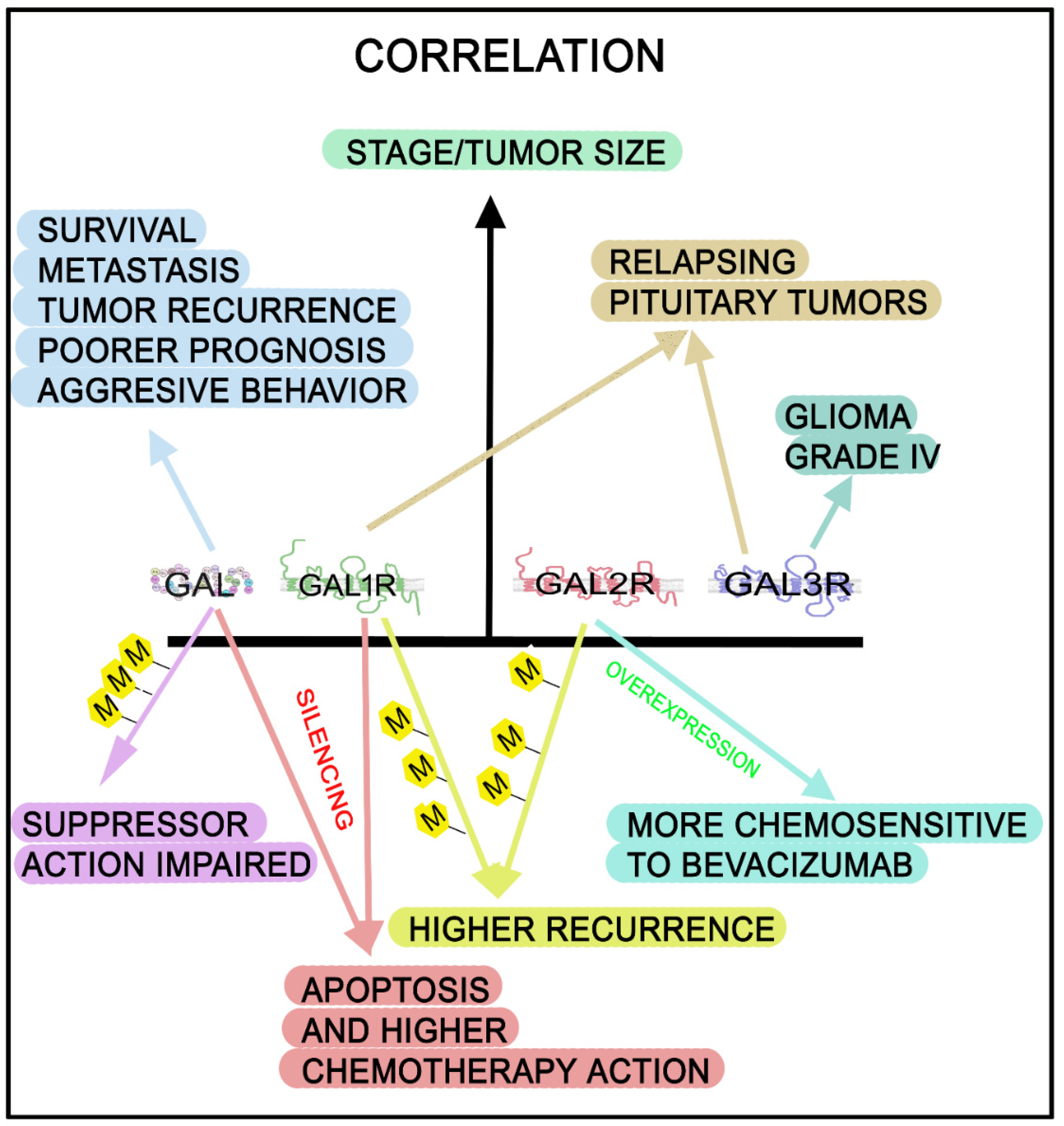
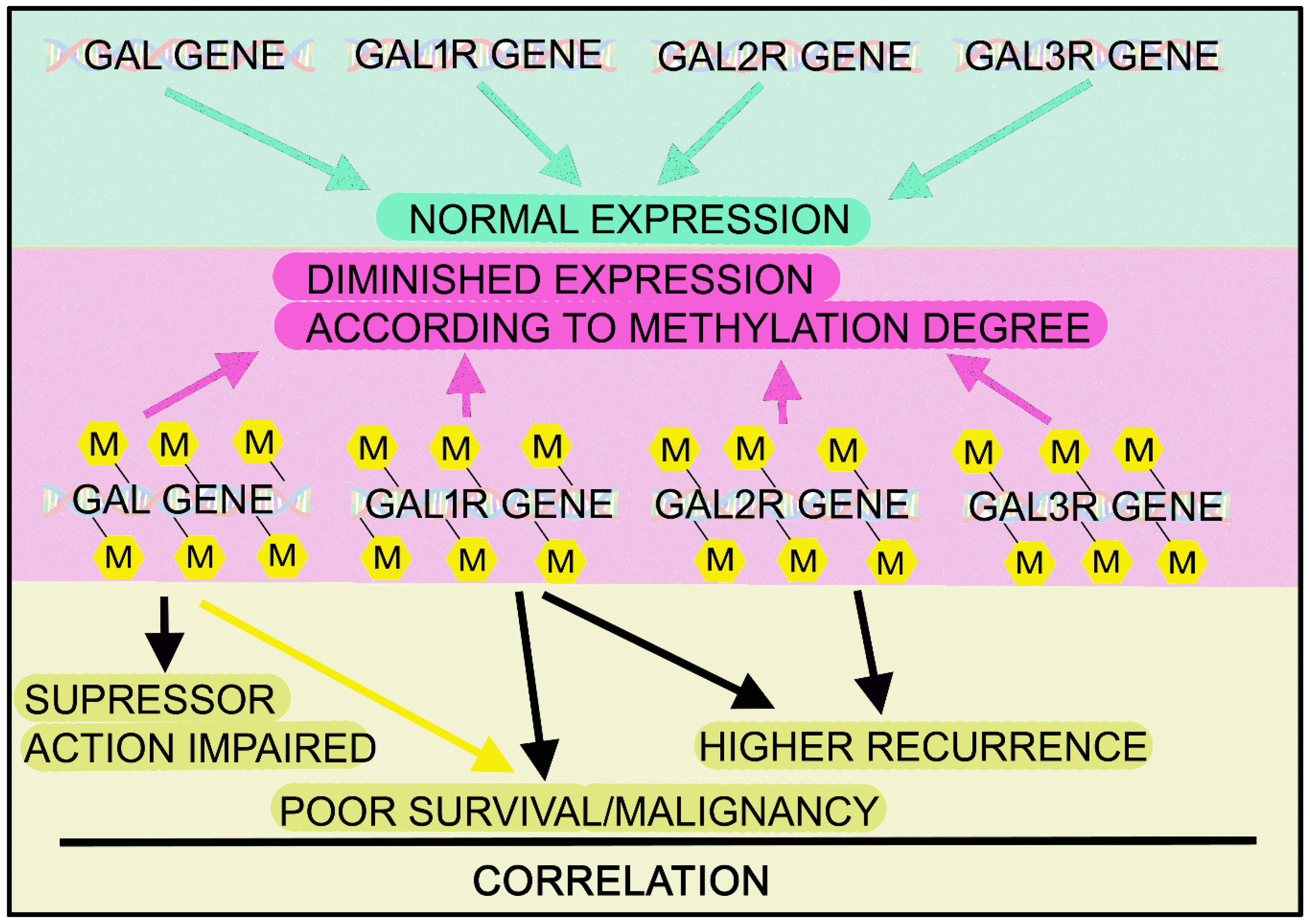
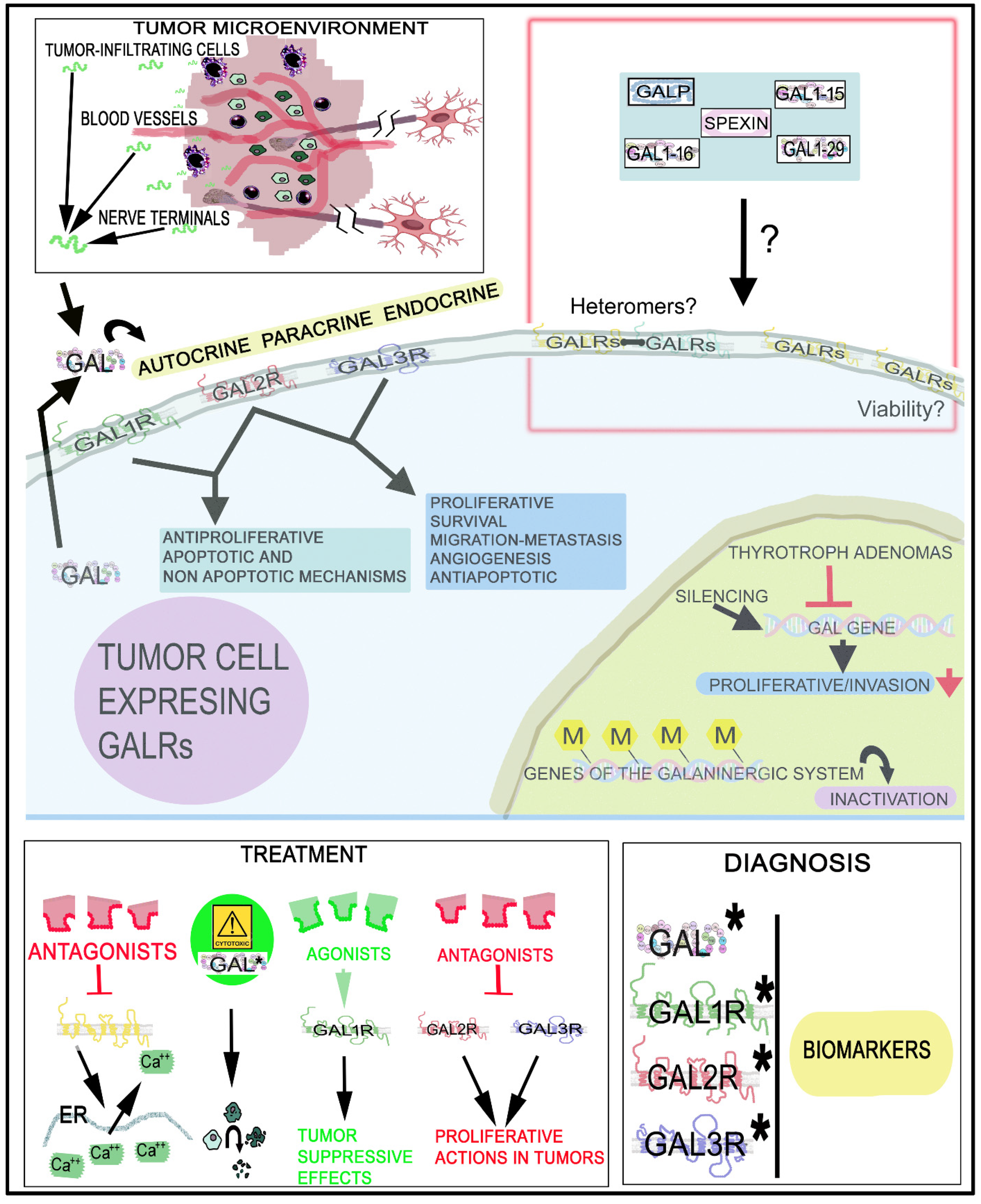
| Cancer | Actions/Presence | References |
|---|---|---|
| Corticotroph adenoma Human | - High GAL expression (RIA) | [102] |
| - GAL in 84% of tumors (IH) | [103] | |
| - GAL expression: smaller adenomas and better prognosis (IH) | [105] | |
| - GAL release and responded to corticotropin-releasing factor | [135] | |
| Ganglioneuroma Human | - No correlation between prognosis/tumor markers and GAL level (RIA) | [136] |
| - GAL1R/GAL3R immunoreactivity decrease (IH) | [137] | |
| Insulinoma Rat Rin14B cell line | - GAL1R expression (Northern blot, in situ hybridization) | [32] |
| Insulinoma Rat RINm5F cell line | - GAL moderately suppressed insulin accumulation, but did not affect cell proliferation | [138] |
| - Pancreatic beta-cells: GAL inhibited adenylate cyclase activity and insulin secretion | [53] | |
| Insulinoma Mouse | - Beta TC-1 cells: GAL, released from sympathetic nerve terminals, inhibited pro-insulin gene expression stimulated by glucagon-like peptide-I (Northern blot) | [139] |
| Neuroblastic tumors Human | - GAL mRNA, GAL immunoreactivity and GAL binding sites expression (IH, in situ hybridization) | [137] |
| - Low level of GAL binding sites correlated with survival; GAL/GALR expression related to tumor differentiation stage (RIA, IH, in situ hybridization) | [136,137] | |
| Neuroblastoma Human | - No correlation between prognosis/tumor markers and GAL concentration | [136] |
| - GAL expression; GAL2R mRNA was less common than GAL1R mRNA (IH, in situ hybridization) | [104] | |
| - GAL1R/GAL3R highly expressed; GAL promoted tumor growth (IH, in situ hybridization) | [137] | |
| Neuroblastoma Human IMR32 cell line | - Dense core secretory vesicles: coexistence of GAL and beta-amyloid (IH) | [140] |
| Neuroblastoma Human SH-SY5Y cell line | - GAL2R mediated apoptosis. GAL antiproliferative potency: 100-fold higher in SY5Y/GAL2R cells than in SY5Y/GAL1R cells | [12] |
| - GAL2R transfection: cell proliferation was blocked and caspase-dependent apoptotic mechanisms induced | [12] | |
| Neuroblastoma Rat B104 cell line | - GAL, GAL2R and GAL3R mRNAs were detected, but not GAL1R mRNA (reverse transcription-PCR) | [141] |
| - GAL promoted cell proliferation | ||
| Paraganglioma Human | - GAL expression (IH) | [108,112,142] |
| Paraganglioma Human carotid body | - GAL was detected in 18% of tumors (IH) | [108] |
| Paraganglioma Human jugulo tympanic | - GAL was detected in 40% of tumors (IH) | [108] |
| Phaeochromocytoma Human | - High GAL2R mRNA expression (Western blot) | [143] |
| - Higher GAL concentration than in normal adrenal glands (RIA) | [144] | |
| Phaeochromocytoma Rat PC12 cell line | - GAL inhibited cell proliferation and GAL1R, GAL2R and GAL3R mRNA expression, but not GAL mRNA (reverse transcription-PCR) | [141] |
| Pituitary adenoma Human | - GAL/GALR expression correlated with tumor stage (IH) | [101] |
| Pituitary adenoma Human | - High GAL3R levels found in some patients who relapsed shortly after surgical intervention (q-PCR) | [145] |
| Pituitary adenoma Rat | - GAL promoted pituitary cell proliferation and tumor development | [38] |
| Pituitary adenoma Rat MtTW-10 cell line | - Estradiol increased GAL mRNA level | [146] |
| Prolactinoma Rat | - GAL concentration increased and GAL promoted tumor development | [147,148] |
| - Levonorgestrel decreased GAL mRNA expression and GAL-expressing cells (IH, in situ hybridization) | [149] | |
| Small-cell lung cancer Human H345, H510 cell lines | - GAL, via GAL2R, mediated cell proliferation | [88,150] |
| Small-cell lung cancer Human H69, H510 cell lines | - GAL, via GAL2R, activated G proteins and promoted cell proliferation | [88] |
| - GAL increased the levels of inositol phosphate and intracellular Ca2+ and promoted cell growth | [151] | |
| Small-cell lung cancer Human H345, H510 cell lines | - Ca2+-mobilizing peptides (e.g., GAL) promoted cell growth. Broad spectrum antagonists directed against multiple Ca2+-mobilizing receptors inhibited cell growth | [150,152] |
| Small-cell lung cancer Human H69, H345, H510 cell lines | - GAL, via the p42MAPK pathway, promoted cell growth. Protein kinase C inhibitors blocked cell growth induced by GAL | [153,154] |
| Small-cell lung cancer Human SBC-3A cell line, mouse SBC-3A tumor | - SBC-3A cells secreted the pre-pro-GAL precursor which was extracellular processed to GAL1-20 by plasmin | [155,156] |
| Somatotroph adenoma Human | - Low GAL level (RIA) | [102] |
| - GAL increased circulating growth hormone level and growth hormone-producing tumors expressed GAL (IH) | [157] | |
| - GAL blocked growth hormone release | [158] | |
| Somatotroph adenoma Rat GH1 cell line | - GAL inhibited growth hormone release | [159] |
| Somatotroph adenoma Mouse | - GAL mRNA level and peptide concentration increased | [147] |
| - GAL secretion increased | [160] | |
| Thyrotroph adenoma Rat | - GAL gene expression blocked | [147] |
| Thyrotroph adenoma Mouse | - GAL synthesis inhibited | [160] |
| Actions/Presence | References | |
|---|---|---|
| Gastric Cancer | ||
| Human | - Fibers containing GAL: increased in longitudinal muscle layer, lamina muscularis mucosae and neoplastic proliferation vicinity (IH) | [175] |
| - Myenteric plexus: neurons showed a high expression of caspases 3/8 and low GAL expression (IH) | [175] | |
| - GAL/GAL1R level reduced | [176] | |
| - GAL2R/GAL3R level unchanged (RT-PCR) | [176] | |
| - Lower level of GAL in pre-operative samples (and plasma) when compared with that found in post-operative samples or in healthy donors. Gastric cancer tissues: GAL/GAL1R level was lower compared with that found in adjacent regions GAL2R/GAL3R: no change (Western blot; RT-PCR; ELISA) | [176] | |
| - GAL low level: used as biomarker. GAL protein/mRNA level related to tumor size, tumor node metastasis stage and lymph node metastasis | [176] | |
| Human Gastric cancer cell lines | - GAL expression decreased: restored with a demethylating agent. GAL hypermethylation: impaired GAL tumor suppressor action. GAL downregulation: due to epigenetic inactivation (Q-MSP, Western blot) | [177] |
| - GAL: decreased cell proliferation | [178] | |
| Rats | - GAL blocked gastric carcinogenesis by inhibiting antral epithelial cell proliferation | [13] |
| Colorectal Cancer (CRC) | ||
| Human | - GAL/GAL1R silencing: apoptosis in drug-sensitive/resistant cell lines and enhanced the effects mediated by chemotherapy. GAL mRNA: overexpressed. High GAL level: related to poor disease-free survival of early-stage CRC patients (IH, ELISA, RT-PCR, Western blot) | [7,106,117,121] |
| - Enteric nervous system: number of neurons containing GAL increased in regions located close to the tumor (IH) (IH, RT-PCR, ELISA) | [8] | |
| - CRC patients: more GAL-immunoreactive neurons in comparison to healthy samples (IH, ELISA) | [121] | |
| - GAL in the vicinity of cancer cell invasion (IH, ELISA) | [121] | |
| - Blood samples: increased GAL concentration. High GAL level: cancer cells. Lowest GAL level: muscular layer placed distant from tumors. GAL: CRC tumor biomarker (ELISA, IH) | [179] | |
| - GAL mRNA level: related to adenocarcinoma size/stage. Correlation between higher GAL expression and shorter disease-free survival (RT-PCR) | [106,117] | |
| - CRC cells showed a high GAL expression: more malignant and involved in tumor recurrence. High GAL expression: spread of cancer stem cells (metastasis) (RT-PCR) | [180] | |
| - High GAL expression: associated with poor prognosis (stage II) and tumor recurrence. GAL expression: related to CRC aggressive behavior (RT-PCR) | [180] | |
| Human (tissue and cell lines) | - CRC cells/tissues: higher GAL levels than non-tumor cells/tissues | [106,117,179,180] |
| - CRC tissue: increased GAL gene/protein expression. CRC cell lines: GAL/GAL1R silencing promoted apoptosis. GAL1R silencing promoted FLIPL down-regulation (IH, ELISA, RT-PCR) | [106,117,121] | |
| Human HCT116 cell line | - Cells overexpressing GAL2R were more chemosensitive to bevacizumab than control cells | [181] |
| Rat | - GAL decreased the incidence of colon tumors | [182] |
| Actions/Presence | References | |
|---|---|---|
| Human | - High GAL level (RT-PCR) | [120] |
| - GAL1R gene promoter: frequently methylated (Q-MSP) | [191] | |
| - Methylation status of some peptide-encoding genes, including GAL, is related with survival and recurrence. Methylation changes: possible molecular marker for HNSCC risk/prognosis (Q-MSP) | [192] | |
| - GAL/GALR epigenetic variants: markers for prognosis prediction (Q-MSP) | [193,194] | |
| - Poor survival: associated with methylation of GAL/GAL1R genes. Hypermethylation: inactivation of GAL/GAL1R/GAL2R genes (Q-MSP) | [195] | |
| Human Cell lines | - Apoptosis: mediated by GAL2R but not by GAL1R. GAL1R/GAL2R: tumor suppressors in a p53-independent manner | [11] |
| - GAL2R transfection into HNSCC cells: cell proliferation inhibited. GAL2R re-expression: blocked cell proliferation (showing mutant p53) | [113,196,197] | |
| - GAL1R/GAL2R negative HNSCC cells: GAL1R re-expression suppressed tumor cell proliferation via ERK1/2-mediated actions on cyclin-dependent kinase inhibitors and cyclin D1 | [113,197] | |
| - GAL/GAL1R blocked HNSCC and oral tumor cell proliferation by cell-cycle arrest (RT-PCR, ELISA, Q-MSP) | [123,177,196,198] | |
| - GAL1R blocked tumor cell proliferation through the activation of ERK1/2 | [196] | |
| - GAL2R promoted an antitumor effect by inducing cell cycle arrest and apoptotic mechanisms (caspase 3-dependent) | [197] | |
| - GAL2R suppressed HNSCC cell viability. HEp-2 cells: GAL2R mediated apoptotic mechanisms (caspase-independent) by downregulating ERK1/2 and inducing Bim | [199] | |
| Human Cell lines, tumor samples | - GAL2R overexpression: favored survival/proliferation by activating PI3K/Akt and MAPK/ERK-dependent pathways. Ras-related protein 1 (Rap1): involved in HNSCC progression. | [122] |
| - GAL/GAL1R: tumor suppressor. GAL1R absent in some cell lines (Q-MSP, RT-PCR) | [177,178,198] | |
| - GAL1R promoter: widely hypermethylated and related to reduced GAL1R expression. GAL1R/GAL2R hypermethylation: associated with higher recurrence rate and reduced disease-free survival (RT-PCR, Q-MSP) | [191,194,198,200] | |
| - GAL1R methylation status: potential biomarker for predicting clinical outcomes. Methylation: related to carcinogenesis and decreased GAL1R expression (RT-PCR, Q-MSP) | [193,194,198] | |
| Human (cell lines) Mouse | - GAL (released from nerves) activated GAL2R expressed in tumor cells inducing NFATC2-mediated transcription of cyclooxygenase-2 and GAL. GAL released from tumor cells promoted neuritogenesis, favoring perineural invasion | [84] |
| Mouse | - GAL2R promoted tumor angiogenesis through the p38-MAPK-mediated inhibition of tristetraprolin (TTP), leading to an enhanced secretion of cytokines. GAL2R activated Ras-related protein 1b (Rap1B) favoring a p38-mediated inactivation of TTP, which acted as a destabilize cytokine transcript | [201] |
| Actions/Presence | References | |
|---|---|---|
| Human | - GAL/GAL3R expression: no correlation with oligodendroglial, astrocytic and mixed neural–glial tumors | [30] |
| - High-grade glioma (WHO grade IV): related to GAL3R expression | [30] | |
| - Endothelial/immune cells: GAL3R expression. Around blood vessels: GAL1R/GAL2R not observed (IH) | [30] | |
| - GAL1R, followed by GAL3R; GAL2R absent (astrocytic/oligodendroglia tumors) (IH, autoradiography, reverse transcription-PCR) | [30,118] | |
| - Glioma-associated macrophages: GAL3R expression (quantitative PCR) | [59,204] | |
| - No correlation between proliferative activity and GAL/GAL binding levels (IH, autoradiography, reverse transcription-PCR) | [118] | |
| - Cerebrospinal fluid (glioblastoma): reduced GAL level | [203] | |
| Human Mice | - GAL blocked, via GAL1R, the proliferation of glioma cells and tumor growth. These effects were mediated through ERK1/2 signal activation. No cytotoxic/apoptotic effect was observed | [205] |
| Actions/Presence | References | |
|---|---|---|
| Breast cancer Human | - GAL/pre-pro-GAL mRNA level expression. GALN gene: unlike candidate oncogene (Northern blot) | [101,206] |
| Carcinoma (cardiac, esophageal) Human | - Fibers containing GAL contacted closely with cancer cells (IH) | [207] |
| Endometrial cancer Human | - GAL1R DNA methylation indicated malignancy (q-PCR) | [208] |
| Bladder cancer Human | - GAL1R gene methylation involved in prognosis | [209] |
| Salivary duct carcinoma Human | - GAL1R/GAL2R: therapeutic targets/prognostic factors. GAL1R/GAL2R methylation rates correlated with overall survival decrease (IH, Q-MSP) | [210] |
| Melanoma Human | - GAL/GAL1R expression (IH) | [101,119] |
| Pancreas Human | - GAL promoted SW1990 cell proliferation | [211] |
| Pancreas Rat | - GAL blocked carcinogenesis and decreased norepinephrine level (IH, HPLC) | [212] |
| Cancer | GAL | GAL1R | GAL2R | GAL3R | References |
|---|---|---|---|---|---|
| A. Proliferative action | |||||
| Colorectal | + | + | [8,30,106,117,185] | ||
| Glioma | + | [30] | |||
| Head and neck squamous cell carcinoma | + | + | + | [84,122,123,201] | |
| Neuroblastoma | + | [137,141] | |||
| Pancreas | + | [211] | |||
| Pituitary adenoma | + | + | [38,145] | ||
| Prolactinoma | + | [148,160] | |||
| Small-cell lung cancer | + | + | [88,113,150,151,173] | ||
| B. Antiproliferative action | |||||
| Colorectal | + | [182] | |||
| Endometrial | + | [208] | |||
| Gastric | + | [13,177,178] | |||
| Gastrointestinal | + | [101] | |||
| Head and neck squamous cell carcinoma | + | + | + | [13,16,120,170,184,190,192] | |
| Neuroblastoma | + | [12] | |||
| Pancreas | + | [212] | |||
| Phaeochromocytoma | + | [141] | |||
| Salivary duct carcinoma | + | + | [210] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.L.; Coveñas, R. The Galaninergic System: A Target for Cancer Treatment. Cancers 2022, 14, 3755. https://doi.org/10.3390/cancers14153755
Sánchez ML, Coveñas R. The Galaninergic System: A Target for Cancer Treatment. Cancers. 2022; 14(15):3755. https://doi.org/10.3390/cancers14153755
Chicago/Turabian StyleSánchez, Manuel Lisardo, and Rafael Coveñas. 2022. "The Galaninergic System: A Target for Cancer Treatment" Cancers 14, no. 15: 3755. https://doi.org/10.3390/cancers14153755
APA StyleSánchez, M. L., & Coveñas, R. (2022). The Galaninergic System: A Target for Cancer Treatment. Cancers, 14(15), 3755. https://doi.org/10.3390/cancers14153755







