Adult Medulloblastoma: Updates on Current Management and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecular Genetics
2.1. Medulloblastoma Histologically Defined
2.2. Medulloblastoma Molecularly Defined
2.3. Medulloblastoma SHH-Activated and TP53-Wild Type
2.4. Medulloblastoma SHH-Activated TP53-Mutant
2.5. Medulloblastoma Non-WNT/Non-SHH-MB
2.6. Medulloblastoma WNT-Activated
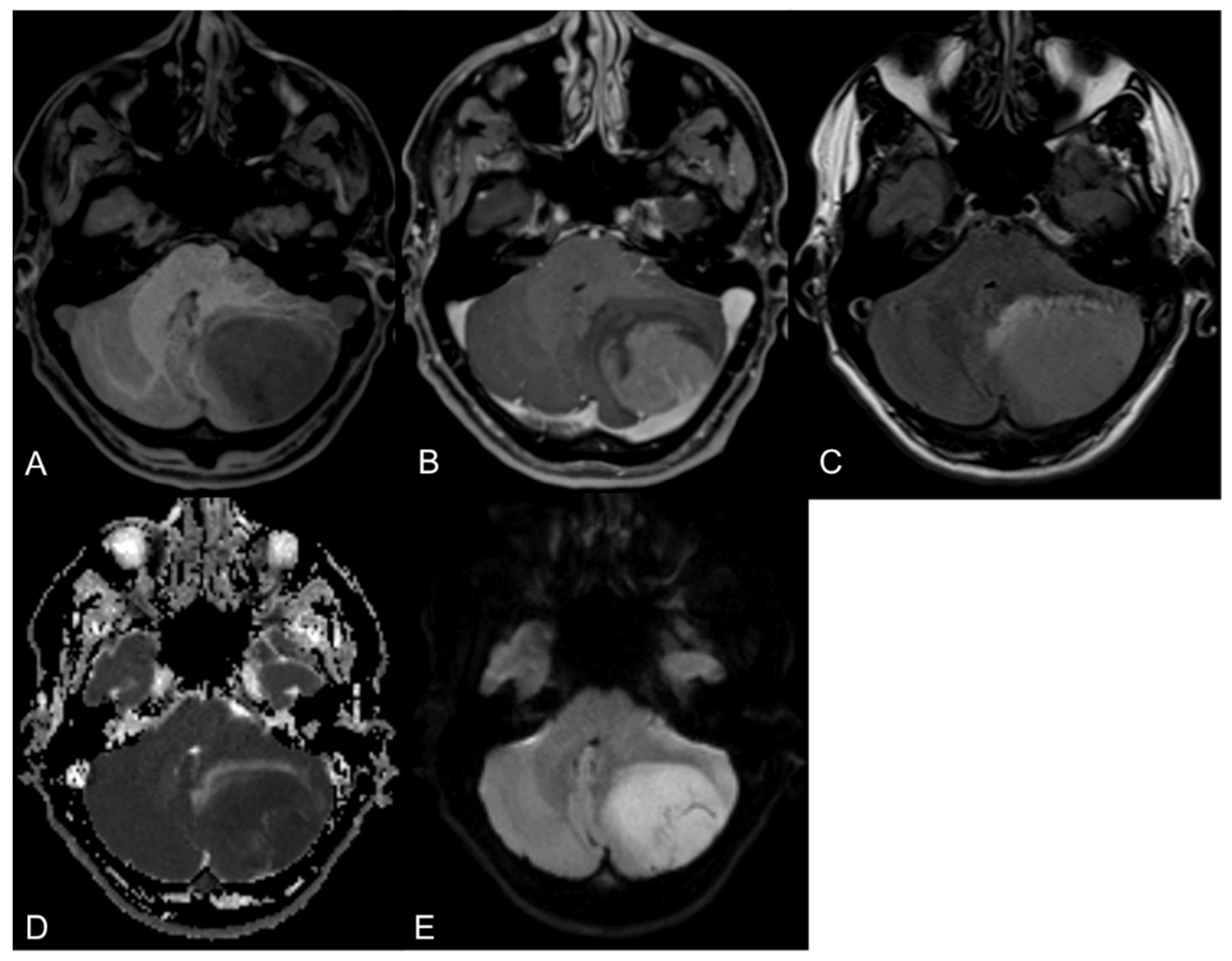
3. Diagnostic Imaging
4. Neurosurgical Aspects in Adult Medulloblastoma Surgery
4.1. Preoperative Management
4.2. Surgical Considerations
5. Role of Chemotherapy and Radiotherapy
5.1. Why It Is Difficult Translating Pediatric Experiences in the Adult Counterpart
5.1.1. Molecular Differences between Tumors
5.1.2. Differences between Toxicity in Children and Adults
5.2. Results of German and Italian Trials for Adult MB
5.3. New Techniques of Radiotherapy
5.4. Proton Treatment
5.5. EORTC 1634-BTG Trial/Alliance AMBUSH Trial
5.6. New Drugs
6. Importance of Centralizing Adult Medulloblastoma Patients in Hub Centers
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bailey, P.; Cushing, H. Medulloblastoma Cerebelli: A common type of midcerebellar glioma of childhood. Arch. Neurol. Psychiatry 1925, 14, 192–224. [Google Scholar] [CrossRef]
- Raffel, C. Medulloblastoma: Molecular genetics and animal models. Neoplasia 2004, 6, 310–322. [Google Scholar] [CrossRef]
- Smoll, N.R.; Drummond, K.J. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J. Clin. Neurosci. 2012, 19, 1541–1544. [Google Scholar] [CrossRef]
- Smoll, N.R. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer 2012, 118, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Wooley, J.R.; Penas-Prado, M. Pediatric versus Adult Medulloblastoma: Towards a Definition That Goes beyond Age. Cancers 2021, 13, 6313. [Google Scholar] [CrossRef] [PubMed]
- Brandes, A.A.; Ermani, M.; Amista, P.; Basso, U.; Vastola, F.; Gardiman, M.; Iuzzolino, P.; Turazzi, S.; Rotilio, A.; Volpin, L.; et al. The treatment of adults with medulloblastoma: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 755–761. [Google Scholar] [CrossRef]
- Franceschi, E.; Hofer, S.; Brandes, A.A.; Frappaz, D.; Kortmann, R.D.; Bromberg, J.; Dangouloff-Ros, V.; Boddaert, N.; Hattingen, E.; Wiestler, B.; et al. EANO-EURACAN clinical practice guideline for diagnosis, treatment, and follow-up of post-pubertal and adult patients with medulloblastoma. Lancet. Oncol. 2019, 20, e715–e728. [Google Scholar] [CrossRef]
- Hau, P.; Frappaz, D.; Hovey, E.; McCabe, M.G.; Pajtler, K.W.; Wiestler, B.; Seidel, C.; Combs, S.E.; Dirven, L.; Klein, M.; et al. Development of Randomized Trials in Adults with Medulloblastoma-The Example of EORTC 1634-BTG/NOA-23. Cancers 2021, 13, 3451. [Google Scholar] [CrossRef] [PubMed]
- Beier, D.; Proescholdt, M.; Reinert, C.; Pietsch, T.; Jones, D.T.W.; Pfister, S.M.; Hattingen, E.; Seidel, C.; Dirven, L.; Luerding, R.; et al. Multicenter pilot study of radiochemotherapy as first-line treatment for adults with medulloblastoma (NOA-07). Neuro Oncol. 2018, 20, 400–410. [Google Scholar] [CrossRef]
- Friedrich, C.; von Bueren, A.O.; von Hoff, K.; Kwiecien, R.; Pietsch, T.; Warmuth-Metz, M.; Hau, P.; Deinlein, F.; Kuehl, J.; Kortmann, R.D.; et al. Treatment of adult nonmetastatic medulloblastoma patients according to the paediatric HIT 2000 protocol: A prospective observational multicentre study. Eur. J. Cancer 2013, 49, 893–903. [Google Scholar] [CrossRef]
- Remke, M.; Hielscher, T.; Northcott, P.A.; Witt, H.; Ryzhova, M.; Wittmann, A.; Benner, A.; von Deimling, A.; Scheurlen, W.; Perry, A.; et al. Adult medulloblastoma comprises three major molecular variants. J. Clin. Oncol. 2011, 29, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Hielscher, T.; Bouffet, E.; Remke, M.; Luu, B.; Gururangan, S.; McLendon, R.E.; Bigner, D.D.; Lipp, E.S.; Perreault, S.; et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: A retrospective integrated clinical and molecular analysis. Lancet. Oncol. 2016, 17, 484–495. [Google Scholar] [CrossRef]
- Korshunov, A.; Remke, M.; Werft, W.; Benner, A.; Ryzhova, M.; Witt, H.; Sturm, D.; Wittmann, A.; Schöttler, A.; Felsberg, J.; et al. Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J. Clin. Oncol. 2010, 28, 3054–3060. [Google Scholar] [CrossRef]
- Kocakaya, S.; Beier, C.P.; Beier, D. Chemotherapy increases long-term survival in patients with adult medulloblastoma--a literature-based meta-analysis. Neuro Oncol. 2016, 18, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Blatt, V.; Ermani, M. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer 2007, 110, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Northcott, P.A.; Korshunov, A.; Witt, H.; Hielscher, T.; Eberhart, C.G.; Mack, S.; Bouffet, E.; Clifford, S.C.; Hawkins, C.E.; French, P.; et al. Medulloblastoma comprises four distinct molecular variants. J. Clin. Oncol. 2011, 29, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Garancher, A.; Ramaswamy, V.; Wechsler-Reya, R.J. Medulloblastoma: From Molecular Subgroups to Molecular Targeted Therapies. Annu. Rev. Neurosci. 2018, 41, 207–232. [Google Scholar] [CrossRef]
- Remke, M.; Ramaswamy, V.; Taylor, M.D. Medulloblastoma molecular dissection: The way toward targeted therapy. Curr. Opin. Oncol. 2013, 25, 674–681. [Google Scholar] [CrossRef]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef]
- Shih, D.J.; Northcott, P.A.; Remke, M.; Korshunov, A.; Ramaswamy, V.; Kool, M.; Luu, B.; Yao, Y.; Wang, X.; Dubuc, A.M.; et al. Cytogenetic prognostication within medulloblastoma subgroups. J. Clin. Oncol. 2014, 32, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Coltin, H.; Sundaresan, L.; Smith, K.S.; Skowron, P.; Massimi, L.; Eberhart, C.G.; Schreck, K.C.; Gupta, N.; Weiss, W.A.; Tirapelli, D.; et al. Subgroup and subtype-specific outcomes in adult medulloblastoma. Acta Neuropathol. 2021, 142, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ohgaki, H.; Xu, L.; Giangaspero, F.; Li, C.; Li, P.; Yang, Z.; Wang, B.; Wang, X.; Wang, Z.; et al. Molecular subgroups of adult medulloblastoma: A long-term single-institution study. Neuro Oncol. 2016, 18, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Frost, P.J.; Laperriere, N.J.; Wong, C.S.; Milosevic, M.F.; Simpson, W.J.; Pintilie, M. Medulloblastoma in adults. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 951–957. [Google Scholar] [CrossRef]
- Becker, R.L.; Becker, A.D.; Sobel, D.F. Adult medulloblastoma: Review of 13 cases with emphasis on MRI. Neuroradiology 1995, 37, 104–108. [Google Scholar] [CrossRef]
- Kool, M.; Korshunov, A.; Pfister, S.M. Update on molecular and genetic alterations in adult medulloblastoma. Memo 2012, 5, 228–232. [Google Scholar] [CrossRef][Green Version]
- Rodon, J.; Tawbi, H.A.; Thomas, A.L.; Stoller, R.G.; Turtschi, C.P.; Baselga, J.; Sarantopoulos, J.; Mahalingam, D.; Shou, Y.; Moles, M.A.; et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 1900–1909. [Google Scholar] [CrossRef]
- LoRusso, P.M.; Rudin, C.M.; Reddy, J.C.; Tibes, R.; Weiss, G.J.; Borad, M.J.; Hann, C.L.; Brahmer, J.R.; Chang, I.; Darbonne, W.C.; et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin. Cancer Res. 2011, 17, 2502–2511. [Google Scholar] [CrossRef]
- Gajjar, A.; Stewart, C.F.; Ellison, D.W.; Kaste, S.; Kun, L.E.; Packer, R.J.; Goldman, S.; Chintagumpala, M.; Wallace, D.; Takebe, N.; et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: A pediatric brain tumor consortium study. Clin. Cancer Res. 2013, 19, 6305–6312. [Google Scholar] [CrossRef]
- Robinson, G.W.; Orr, B.A.; Wu, G.; Gururangan, S.; Lin, T.; Qaddoumi, I.; Packer, R.J.; Goldman, S.; Prados, M.D.; Desjardins, A.; et al. Vismodegib Exerts Targeted Efficacy Against Recurrent Sonic Hedgehog-Subgroup Medulloblastoma: Results From Phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J. Clin. Oncol. 2015, 33, 2646–2654. [Google Scholar] [CrossRef]
- Kieran, M.W.; Chisholm, J.; Casanova, M.; Brandes, A.A.; Aerts, I.; Bouffet, E.; Bailey, S.; Leary, S.; MacDonald, T.J.; Mechinaud, F.; et al. Phase I study of oral sonidegib (LDE225) in pediatric brain and solid tumors and a phase II study in children and adults with relapsed medulloblastoma. Neuro Oncol. 2017, 19, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Frappaz, D.; Barritault, M.; Montané, L.; Laigle-Donadey, F.; Chinot, O.; Le Rhun, E.; Bonneville-Levard, A.; Hottinger, A.F.; Meyronnet, D.; Bidaux, A.S.; et al. MEVITEM-a phase I/II trial of vismodegib + temozolomide vs. temozolomide in patients with recurrent/refractory medulloblastoma with Sonic Hedgehog pathway activation. Neuro Oncol. 2021, 23, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Jones, D.T.; Jäger, N.; Northcott, P.A.; Pugh, T.J.; Hovestadt, V.; Piro, R.M.; Esparza, L.A.; Markant, S.L.; Remke, M.; et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014, 25, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Shih, H.; Penas-Prado, M.; Ligon, K.; Aldape, K.; Hu, L.S.; Loughan, A.R.; Basso, M.R.; Leeper, H.E.; Nahed, B.V.; et al. The Alliance AMBUSH Trial: Rationale and Design. Cancers 2022, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Goschzik, T.; Zur Muehlen, A.; Doerner, E.; Waha, A.; Friedrich, C.; Hau, P.; Pietsch, T. Medulloblastoma in Adults: Cytogenetic Phenotypes Identify Prognostic Subgroups. J. Neuropathol. Exp. Neurol. 2021, 80, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.Y.; Priesterbach-Ackley, L.P.; Orr, B.A.; Li, B.K.; Gudenas, B.; Reddingius, R.E.; Suñol, M.; Lavarino, C.E.; Olaciregui, N.G.; Santa-María López, V.; et al. WNT-activated embryonal tumors of the pineal region: Ectopic medulloblastomas or a novel pineoblastoma subgroup? Acta Neuropathol. 2020, 140, 595–597. [Google Scholar] [CrossRef]
- Kool, M.; Koster, J.; Bunt, J.; Hasselt, N.E.; Lakeman, A.; van Sluis, P.; Troost, D.; Meeteren, N.S.; Caron, H.N.; Cloos, J.; et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS ONE 2008, 3, e3088. [Google Scholar] [CrossRef]
- Schüller, U.; Koch, A.; Hartmann, W.; Garrè, M.L.; Goodyer, C.G.; Cama, A.; Sörensen, N.; Wiestler, O.D.; Pietsch, T. Subtype-specific expression and genetic alterations of the chemokinereceptor gene CXCR4 in medulloblastomas. Int. J. Cancer 2005, 117, 82–89. [Google Scholar] [CrossRef]
- Hovestadt, V.; Remke, M.; Kool, M.; Pietsch, T.; Northcott, P.A.; Fischer, R.; Cavalli, F.M.; Ramaswamy, V.; Zapatka, M.; Reifenberger, G.; et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013, 125, 913–916. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Schwalbe, E.C.; Williamson, D.; Lindsey, J.C.; Hamilton, D.; Ryan, S.L.; Megahed, H.; Garami, M.; Hauser, P.; Dembowska-Baginska, B.; Perek, D.; et al. DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathol. 2013, 125, 359–371. [Google Scholar] [CrossRef]
- Schwalbe, E.C.; Lindsey, J.C.; Nakjang, S.; Crosier, S.; Smith, A.J.; Hicks, D.; Rafiee, G.; Hill, R.M.; Iliasova, A.; Stone, T.; et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: A cohort study. Lancet Oncol. 2017, 18, 958–971. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Capper, D.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Dutt, M.; Suraweera, N.; Pfister, S.M.; von Deimling, A.; Brandner, S. Methylation array profiling of adult brain tumours: Diagnostic outcomes in a large, single centre. Acta Neuropathol. Commun. 2019, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.C.; Li, K.K.; Wang, W.W.; Liu, A.P.; Huang, Q.J.; Chan, A.K.; Poon, M.F.; Chung, N.Y.; Wong, Q.H.; Chen, H.; et al. Clinical and mutational profiles of adult medulloblastoma groups. Acta Neuropathol. Commun. 2020, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Juraschka, K.; Taylor, M.D. Medulloblastoma in the age of molecular subgroups: A review. J. Neurosurg. Pediatr. 2019, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Korshunov, A.; Remke, M.; Jones, D.T.; Schlanstein, M.; Northcott, P.A.; Cho, Y.J.; Koster, J.; Schouten-van Meeteren, A.; van Vuurden, D.; et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012, 123, 473–484. [Google Scholar] [CrossRef]
- Jones, D.T.; Jäger, N.; Kool, M.; Zichner, T.; Hutter, B.; Sultan, M.; Cho, Y.J.; Pugh, T.J.; Hovestadt, V.; Stütz, A.M.; et al. Dissecting the genomic complexity underlying medulloblastoma. Nature 2012, 488, 100–105. [Google Scholar] [CrossRef]
- Hicks, D.; Rafiee, G.; Schwalbe, E.C.; Howell, C.I.; Lindsey, J.C.; Hill, R.M.; Smith, A.J.; Adidharma, P.; Steel, C.; Richardson, S.; et al. The molecular landscape and associated clinical experience in infant medulloblastoma: Prognostic significance of second-generation subtypes. Neuropathol. Appl. Neurobiol. 2021, 47, 236–250. [Google Scholar] [CrossRef]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754.e6. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.W.; Rudneva, V.A.; Buchhalter, I.; Billups, C.A.; Waszak, S.M.; Smith, K.S.; Bowers, D.C.; Bendel, A.; Fisher, P.G.; Partap, S.; et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): Therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018, 19, 768–784. [Google Scholar] [CrossRef]
- Suzuki, H.; Kumar, S.A.; Shuai, S.; Diaz-Navarro, A.; Gutierrez-Fernandez, A.; De Antonellis, P.; Cavalli, F.M.G.; Juraschka, K.; Farooq, H.; Shibahara, I.; et al. Recurrent noncoding U1 snRNA mutations drive cryptic splicing in SHH medulloblastoma. Nature 2019, 574, 707–711. [Google Scholar] [CrossRef]
- Waszak, S.M.; Robinson, G.W.; Gudenas, B.L.; Smith, K.S.; Forget, A.; Kojic, M.; Garcia-Lopez, J.; Hadley, J.; Hamilton, K.V.; Indersie, E.; et al. Germline Elongator mutations in Sonic Hedgehog medulloblastoma. Nature 2020, 580, 396–401. [Google Scholar] [CrossRef]
- Remke, M.; Ramaswamy, V.; Peacock, J.; Shih, D.J.; Koelsche, C.; Northcott, P.A.; Hill, N.; Cavalli, F.M.; Kool, M.; Wang, X.; et al. TERT promoter mutations are highly recurrent in SHH subgroup medulloblastoma. Acta Neuropathol. 2013, 126, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Buchhalter, I.; Morrissy, A.S.; Hovestadt, V.; Weischenfeldt, J.; Ehrenberger, T.; Gröbner, S.; Segura-Wang, M.; Zichner, T.; Rudneva, V.A.; et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017, 547, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Schwalbe, E.C.; Williamson, D.; Sill, M.; Hovestadt, V.; Mynarek, M.; Rutkowski, S.; Robinson, G.W.; Gajjar, A.; Cavalli, F.; et al. Second-generation molecular subgrouping of medulloblastoma: An international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol. 2019, 138, 309–326. [Google Scholar] [CrossRef]
- Bourgouin, P.M.; Tampieri, D.; Grahovac, S.Z.; Léger, C.; Del Carpio, R.; Melançon, D. CT and MR imaging findings in adults with cerebellar medulloblastoma: Comparison with findings in children. AJR Am. J. Roentgenol. 1992, 159, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, E.; Seidel, C.; Sahm, F.; Pajtler, K.W.; Hau, P. How we treat medulloblastoma in adults. ESMO Open 2021, 6, 100173. [Google Scholar] [CrossRef]
- Warren, K.E.; Vezina, G.; Poussaint, T.Y.; Warmuth-Metz, M.; Chamberlain, M.C.; Packer, R.J.; Brandes, A.A.; Reiss, M.; Goldman, S.; Fisher, M.J.; et al. Response assessment in medulloblastoma and leptomeningeal seeding tumors: Recommendations from the Response Assessment in Pediatric Neuro-Oncology committee. Neuro Oncol. 2018, 20, 13–23. [Google Scholar] [CrossRef]
- Keil, V.C.; Warmuth-Metz, M.; Reh, C.; Enkirch, S.J.; Reinert, C.; Beier, D.; Jones, D.T.W.; Pietsch, T.; Schild, H.H.; Hattingen, E.; et al. Imaging Biomarkers for Adult Medulloblastomas: Genetic Entities May Be Identified by Their MR Imaging Radiophenotype. AJNR Am. J. Neuroradiol. 2017, 38, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Buch, K.; Caruso, P.; Ebb, D.; Rincon, S. Balanced Steady-State Free Precession Sequence (CISS/FIESTA/3D Driven Equilibrium Radiofrequency Reset Pulse) Increases the Diagnostic Yield for Spinal Drop Metastases in Children with Brain Tumors. AJNR Am. J. Neuroradiol. 2018, 39, 1355–1361. [Google Scholar] [CrossRef]
- Luque, R.; Benavides, M.; Del Barco, S.; Egaña, L.; García-Gómez, J.; Martínez-García, M.; Pérez-Segura, P.; Pineda, E.; Sepúlveda, J.M.; Vieito, M. SEOM clinical guideline for management of adult medulloblastoma (2020). Clin. Transl. Oncol. 2021, 23, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wong, S.W.; Wright, J.N.; Wagner, M.W.; Toescu, S.; Han, M.; Tam, L.T.; Zhou, Q.; Ahmadian, S.S.; Shpanskaya, K.; et al. MRI Radiogenomics of Pediatric Medulloblastoma: A Multicenter Study. Radiology 2022, 304, 406–416. 212137. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.R.; Figueiredo, E.G.; Deshmukh, P.; Crawford, N.R.; Preul, M.C.; Spetzler, R.F. Quantification and comparison of telovelar and transvermian approaches to the fourth ventricle. Neurosurgery 2006, 58 (Suppl. S2), ONS-202-6, discussion ONS-206-7. [Google Scholar] [CrossRef]
- Zhang, N.; Ouyang, T.; Kang, H.; Long, W.; Thomas, B.; Zhu, S. Adult medulloblastoma: Clinical characters, prognostic factors, outcomes and patterns of relapse. J. Neurooncol. 2015, 124, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Tarbell, N.J.; Black, P.M.; Louis, D.N.; Frosch, M.P.; Ancukiewicz, M.; Chapman, P.; Loeffler, J.S. Adult medulloblastoma: Prognostic factors and patterns of relapse. Neurosurgery 2000, 47, 623–631; discussion 631–632. [Google Scholar]
- Yilmaz, H.; Akcay, E.; Benek, H.B. Medulloblastoma in Adults: Surgical Outcomes and Survival—A Single Center Analysis of 16 Patients. Turk Neurosurg. 2021, 31, 980–985. [Google Scholar] [CrossRef]
- Due-Tønnessen, B.J.; Helseth, E. Management of hydrocephalus in children with posterior fossa tumors: Role of tumor surgery. Pediatr. Neurosurg. 2007, 43, 92–96. [Google Scholar] [CrossRef]
- Albright, A.L.; Wisoff, J.H.; Zeltzer, P.M.; Boyett, J.M.; Rorke, L.B.; Stanley, P. Effects of medulloblastoma resections on outcome in children: A report from the Children’s Cancer Group. Neurosurgery 1996, 38, 265–271. [Google Scholar] [CrossRef]
- Hadi, I.; Roengvoraphoj, O.; Niyazi, M.; Roeder, F.; Schüller, U.; Belka, C.; Nachbichler, S.B. Medulloblastoma in adults: A retrospective single institution analysis. Strahlenther. Onkol. 2018, 194, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Hoffman, H.J.; Hendrick, E.B.; Humphreys, R.P.; Becker, L.E. Medulloblastoma: Clinical presentation and management. Experience at the hospital for sick children, toronto, 1950-1980. J. Neurosurg. 1983, 58, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Koeller, K.K.; Rushing, E.J. From the archives of the AFIP: Medulloblastoma: A comprehensive review with radiologic-pathologic correlation. Radiographics 2003, 23, 1613–1637. [Google Scholar] [CrossRef]
- Wu, T.; Qu, P.R.; Zhang, S.; Li, S.W.; Zhang, J.; Wang, B.; Liu, P.; Li, C.D.; Zhao, F. The clinical treatment and outcome of cerebellopontine angle medulloblastoma: A retrospective study of 15 cases. Sci. Rep. 2020, 10, 9769. [Google Scholar] [CrossRef] [PubMed]
- Schwake, M.; Schipmann, S.; Müther, M.; Köchling, M.; Brentrup, A.; Stummer, W. 5-ALA fluorescence-guided surgery in pediatric brain tumors-a systematic review. Acta Neurochir. 2019, 161, 1099–1108. [Google Scholar] [CrossRef]
- Onorini, N.; Spennato, P.; Orlando, V.; Savoia, F.; Calì, C.; Russo, C.; De Martino, L.; de Santi, M.S.; Mirone, G.; Ruggiero, C.; et al. The Clinical and Prognostic Impact of the Choice of Surgical Approach to Fourth Ventricular Tumors in a Single-Center, Single-Surgeon Cohort of 92 Consecutive Pediatric Patients. Front. Oncol. 2022, 12, 821738. [Google Scholar] [CrossRef]
- Ildan, F.; Tuna, M.; Erman, T.; Göçer, A.I.; Zeren, M.; Cetinalp, E. The evaluation and comparison of cerebellar mutism in children and adults after posterior fossa surgery: Report of two adult cases and review of the literature. Acta Neurochir. (Wien) 2002, 144, 463–473. [Google Scholar] [CrossRef]
- Gajjar, A.; Fouladi, M.; Walter, A.W.; Thompson, S.J.; Reardon, D.A.; Merchant, T.E.; Jenkins, J.J.; Liu, A.; Boyett, J.M.; Kun, L.E.; et al. Comparison of lumbar and shunt cerebrospinal fluid specimens for cytologic detection of leptomeningeal disease in pediatric patients with brain tumors. J. Clin. Oncol. 1999, 17, 1825–1828. [Google Scholar] [CrossRef]
- Jenkin, R.D. Medulloblastoma in childhood: Radiation therapy. Can. Med. Assoc. J. 1969, 100, 51–53. [Google Scholar]
- Deutsch, M.; Thomas, P.R.; Krischer, J.; Boyett, J.M.; Albright, L.; Aronin, P.; Langston, J.; Allen, J.C.; Packer, R.J.; Linggood, R.; et al. Results of a prospective randomized trial comparing standard dose neuraxis irradiation (3600 cGy/20) with reduced neuraxis irradiation (2340 cGy/13) in patients with low-stage medulloblastoma. A Combined Children’s Cancer Group-Pediatric Oncology Group Study. Pediatr. Neurosurg. 1996, 24, 167–176; discussion 176–177. [Google Scholar]
- Packer, R.J.; Sutton, L.N.; Goldwein, J.W.; Perilongo, G.; Bunin, G.; Ryan, J.; Cohen, B.H.; D’Angio, G.; Kramer, E.D.; Zimmerman, R.A.; et al. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. J. Neurosurg. 1991, 74, 433–440. [Google Scholar] [CrossRef]
- Packer, R.J.; Sutton, L.N.; Elterman, R.; Lange, B.; Goldwein, J.; Nicholson, H.S.; Mulne, L.; Boyett, J.; D’Angio, G.; Wechsler-Jentzsch, K.; et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J. Neurosurg. 1994, 81, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Goldwein, J.; Nicholson, H.S.; Vezina, L.G.; Allen, J.C.; Ris, M.D.; Muraszko, K.; Rorke, L.B.; Wara, W.M.; Cohen, B.H.; et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J. Clin. Oncol. 1999, 17, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Gajjar, A.; Vezina, G.; Rorke-Adams, L.; Burger, P.C.; Robertson, P.L.; Bayer, L.; LaFond, D.; Donahue, B.R.; Marymont, M.H.; et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J. Clin. Oncol. 2006, 24, 4202–4208. [Google Scholar] [CrossRef]
- Lannering, B.; Rutkowski, S.; Doz, F.; Pizer, B.; Gustafsson, G.; Navajas, A.; Massimino, M.; Reddingius, R.; Benesch, M.; Carrie, C.; et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: Results from the randomized multicenter HIT-SIOP PNET 4 trial. J. Clin. Oncol. 2012, 30, 3187–3193. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.; Sunyach, M.P.; Barretta, F.; Gandola, L.; Garegnani, A.; Pecori, E.; Spreafico, F.; Bonneville-Levard, A.; Meyronet, D.; Mottolese, C.; et al. Reduced-dose craniospinal irradiation is feasible for standard-risk adult medulloblastoma patients. J. Neurooncol. 2020, 148, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dai, Z.; Cao, Y.; Wang, L. Comparing children and adults with medulloblastoma: A SEER based analysis. Oncotarget 2018, 9, 30189–30198. [Google Scholar] [CrossRef] [PubMed]
- Tabori, U.; Sung, L.; Hukin, J.; Laperriere, N.; Crooks, B.; Carret, A.S.; Silva, M.; Odame, I.; Mpofu, C.; Strother, D.; et al. Medulloblastoma in the second decade of life: A specific group with respect to toxicity and management: A Canadian Pediatric Brain Tumor Consortium Study. Cancer 2005, 103, 1874–1880. [Google Scholar] [CrossRef]
- Friedrich, C.; Müller, K.; von Hoff, K.; Kwiecien, R.; Pietsch, T.; Warmuth-Metz, M.; Gerber, N.U.; Hau, P.; Kuehl, J.; Kortmann, R.D.; et al. Adults with CNS primitive neuroectodermal tumors/pineoblastomas: Results of multimodal treatment according to the pediatric HIT 2000 protocol. J. Neurooncol. 2014, 116, 567–575. [Google Scholar] [CrossRef]
- Silber, J.H.; Radcliffe, J.; Peckham, V.; Perilongo, G.; Kishnani, P.; Fridman, M.; Goldwein, J.W.; Meadows, A.T. Whole-brain irradiation and decline in intelligence: The influence of dose and age on IQ score. J. Clin. Oncol. 1992, 10, 1390–1396. [Google Scholar] [CrossRef]
- Mabbott, D.J.; Spiegler, B.J.; Greenberg, M.L.; Rutka, J.T.; Hyder, D.J.; Bouffet, E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J. Clin. Oncol. 2005, 23, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- King, A.A.; Seidel, K.; Di, C.; Leisenring, W.M.; Perkins, S.M.; Krull, K.R.; Sklar, C.A.; Green, D.M.; Armstrong, G.T.; Zeltzer, L.K.; et al. Long-term neurologic health and psychosocial function of adult survivors of childhood medulloblastoma/PNET: A report from the Childhood Cancer Survivor Study. Neuro Oncol. 2017, 19, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Heikens, J.; Michiels, E.M.; Behrendt, H.; Endert, E.; Bakker, P.J.; Fliers, E. Long-term neuro-endocrine sequelae after treatment for childhood medulloblastoma. Eur. J. Cancer 1998, 34, 1592–1597. [Google Scholar] [CrossRef]
- Kortmann, R.D.; Kuhl, J.; Timmermann, B.; Mittler, U.; Urban, C.; Budach, V.; Richter, E.; Willich, N.; Flentje, M.; Berthold, F.; et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: Results of the German prospective randomized trial HIT ‘91. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 269–279. [Google Scholar] [CrossRef]
- Franceschi, E.; Bartolotti, M.; Paccapelo, A.; Marucci, G.; Agati, R.; Volpin, L.; Danieli, D.; Ghimenton, C.; Gardiman, M.P.; Sturiale, C.; et al. Adjuvant chemotherapy in adult medulloblastoma: Is it an option for average-risk patients? J. Neurooncol. 2016, 128, 235–240. [Google Scholar] [CrossRef]
- Kann, B.H.; Lester-Coll, N.H.; Park, H.S.; Yeboa, D.N.; Kelly, J.R.; Baehring, J.M.; Becker, K.P.; Yu, J.B.; Bindra, R.S.; Roberts, K.B. Adjuvant chemotherapy and overall survival in adult medulloblastoma. Neuro. Oncol. 2017, 19, 259–269. [Google Scholar] [CrossRef]
- Seravalli, E.; Bosman, M.; Lassen-Ramshad, Y.; Vestergaard, A.; Oldenburger, F.; Visser, J.; Koutsouveli, E.; Paraskevopoulou, C.; Horan, G.; Ajithkumar, T.; et al. Dosimetric comparison of five different techniques for craniospinal irradiation across 15 European centers: Analysis on behalf of the SIOP-E-BTG (radiotherapy working group). Acta Oncol. 2018, 57, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.; Filion, E.; Roberge, D.; Freeman, C.R. Intensity-modulated radiotherapy for craniospinal irradiation: Target volume considerations, dose constraints, and competing risks. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 251–257. [Google Scholar] [CrossRef]
- Pai Panandiker, A.; Ning, H.; Likhacheva, A.; Ullman, K.; Arora, B.; Ondos, J.; Karimpour, S.; Packer, R.; Miller, R.; Citrin, D. Craniospinal irradiation with spinal IMRT to improve target homogeneity. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1402–1409. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.K.; Brooks, C.J.; Bedford, J.L.; Warrington, A.P.; Saran, F.H. Development and evaluation of multiple isocentric volumetric modulated arc therapy technique for craniospinal axis radiotherapy planning. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1006–1012. [Google Scholar] [CrossRef]
- Yock, T.I.; Yeap, B.Y.; Ebb, D.H.; Weyman, E.; Eaton, B.R.; Sherry, N.A.; Jones, R.M.; MacDonald, S.M.; Pulsifer, M.B.; Lavally, B.; et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016, 17, 287–298. [Google Scholar] [CrossRef]
- Eaton, B.R.; Esiashvili, N.; Kim, S.; Weyman, E.A.; Thornton, L.T.; Mazewski, C.; MacDonald, T.; Ebb, D.; MacDonald, S.M.; Tarbell, N.J.; et al. Clinical Outcomes Among Children With Standard-Risk Medulloblastoma Treated With Proton and Photon Radiation Therapy: A Comparison of Disease Control and Overall Survival. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 133–138. [Google Scholar] [CrossRef]
- Howell, R.M.; Giebeler, A.; Koontz-Raisig, W.; Mahajan, A.; Etzel, C.J.; D’Amelio, A.M., Jr.; Homann, K.L.; Newhauser, W.D. Comparison of therapeutic dosimetric data from passively scattered proton and photon craniospinal irradiations for medulloblastoma. Radiat. Oncol. 2012, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Miralbell, R.; Lomax, A.; Russo, M. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuro-ectodermal tumors: Spinal theca irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 805–811. [Google Scholar] [CrossRef]
- St Clair, W.H.; Adams, J.A.; Bues, M.; Fullerton, B.C.; La Shell, S.; Kooy, H.M.; Loeffler, J.S.; Tarbell, N.J. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 727–734. [Google Scholar] [CrossRef]
- Lee, C.T.; Bilton, S.D.; Famiglietti, R.M.; Riley, B.A.; Mahajan, A.; Chang, E.L.; Maor, M.H.; Woo, S.Y.; Cox, J.D.; Smith, A.R. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: How do protons compare with other conformal techniques? Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 362–372. [Google Scholar] [CrossRef]
- Ho, E.S.Q.; Barrett, S.A.; Mullaney, L.M. A review of dosimetric and toxicity modeling of proton versus photon craniospinal irradiation for pediatrics medulloblastoma. Acta. Oncol. 2017, 56, 1031–1042. [Google Scholar] [CrossRef]
- Liu, I.C.; Holtzman, A.L.; Rotondo, R.L.; Indelicato, D.J.; Gururangan, S.; Cavaliere, R.; Carter, B.; Morris, C.G.; Tavanaiepour, D.; Rutenberg, M.S. Proton therapy for adult medulloblastoma: Acute toxicity and disease control outcomes. J. Neurooncol. 2021, 153, 467–476. [Google Scholar] [CrossRef]
- Liu, K.X.; Ioakeim-Ioannidou, M.; Susko, M.S.; Rao, A.D.; Yeap, B.Y.; Snijders, A.M.; Ladra, M.M.; Vogel, J.; Zaslowe-Dude, C.; Marcus, K.J.; et al. A Multi-institutional Comparative Analysis of Proton and Photon Therapy-Induced Hematologic Toxicity in Patients With Medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 726–735. [Google Scholar] [CrossRef]
- Eaton, B.R.; Esiashvili, N.; Kim, S.; Patterson, B.; Weyman, E.A.; Thornton, L.T.; Mazewski, C.; MacDonald, T.J.; Ebb, D.; MacDonald, S.M.; et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016, 18, 881–887. [Google Scholar] [CrossRef]
- Zhang, R.; Howell, R.M.; Homann, K.; Giebeler, A.; Taddei, P.J.; Mahajan, A.; Newhauser, W.D. Predicted risks of radiogenic cardiac toxicity in two pediatric patients undergoing photon or proton radiotherapy. Radiat. Oncol. 2013, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Howell, R.M.; Taddei, P.J.; Giebeler, A.; Mahajan, A.; Newhauser, W.D. A comparative study on the risks of radiogenic second cancers and cardiac mortality in a set of pediatric medulloblastoma patients treated with photon or proton craniospinal irradiation. Radiother. Oncol. 2014, 113, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.C.; Mahajan, A.; Ye, R.; Grosshans, D.R.; Fatih Okcu, M.; Su, J.; McAleer, M.F.; McGovern, S.; Mangona, V.A.; Chintagumpala, M. Ototoxicity and cochlear sparing in children with medulloblastoma: Proton vs. photon radiotherapy. Radiother. Oncol. 2018, 128, 128–132. [Google Scholar] [CrossRef]
- Taddei, P.J.; Khater, N.; Zhang, R.; Geara, F.B.; Mahajan, A.; Jalbout, W.; Pérez-Andújar, A.; Youssef, B.; Newhauser, W.D. Inter-Institutional Comparison of Personalized Risk Assessments for Second Malignant Neoplasms for a 13-Year-Old Girl Receiving Proton versus Photon Craniospinal Irradiation. Cancers 2015, 7, 407–426. [Google Scholar] [CrossRef]
- Stokkevåg, C.H.; Engeseth, G.M.; Ytre-Hauge, K.S.; Röhrich, D.; Odland, O.H.; Muren, L.P.; Brydøy, M.; Hysing, L.B.; Szostak, A.; Palmer, M.B.; et al. Estimated risk of radiation-induced cancer following paediatric cranio-spinal irradiation with electron, photon and proton therapy. Acta Oncol. 2014, 53, 1048–1057. [Google Scholar] [CrossRef]
- Zhang, R.; Howell, R.M.; Giebeler, A.; Taddei, P.J.; Mahajan, A.; Newhauser, W.D. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys. Med. Biol. 2013, 58, 807–823. [Google Scholar] [CrossRef]
- Brodin, N.P.; Munck Af Rosenschöld, P.; Aznar, M.C.; Kiil-Berthelsen, A.; Vogelius, I.R.; Nilsson, P.; Lannering, B.; Björk-Eriksson, T. Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol. 2011, 50, 806–816. [Google Scholar] [CrossRef]
- Brodin, N.P.; Vogelius, I.R.; Maraldo, M.V.; Munck af Rosenschöld, P.; Aznar, M.C.; Kiil-Berthelsen, A.; Nilsson, P.; Björk-Eriksson, T.; Specht, L.; Bentzen, S.M. Life years lost--comparing potentially fatal late complications after radiotherapy for pediatric medulloblastoma on a common scale. Cancer 2012, 118, 5432–5440. [Google Scholar] [CrossRef] [PubMed]
- Miralbell, R.; Lomax, A.; Cella, L.; Schneider, U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 824–829. [Google Scholar] [CrossRef]
- Mu, X.; Björk-Eriksson, T.; Nill, S.; Oelfke, U.; Johansson, K.A.; Gagliardi, G.; Johansson, L.; Karlsson, M.; Zackrisson, D.B. Does electron and proton therapy reduce the risk of radiation induced cancer after spinal irradiation for childhood medulloblastoma? A comparative treatment planning study. Acta Oncol. 2005, 44, 554–562. [Google Scholar] [CrossRef]
- Paulino, A.C.; Ludmir, E.B.; Grosshans, D.R.; Su, J.M.; McGovern, S.L.; Okcu, M.F.; McAleer, M.F.; Baxter, P.A.; Mahajan, A.; Chintagumpala, M.M. Overall survival and secondary malignant neoplasms in children receiving passively scattered proton or photon craniospinal irradiation for medulloblastoma. Cancer 2021, 127, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Lospinoso Severini, L.; Ghirga, F.; Bufalieri, F.; Quaglio, D.; Infante, P.; Di Marcotullio, L. The SHH/GLI signaling pathway: A therapeutic target for medulloblastoma. Expert. Opin. Ther. Targets 2020, 24, 1159–1181. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.; Wicking, C.; Zaphiropoulous, P.G.; Gailani, M.R.; Shanley, S.; Chidambaram, A.; Vorechovsky, I.; Holmberg, E.; Unden, A.B.; Gillies, S.; et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 1996, 85, 841–851. [Google Scholar] [CrossRef]
- Johnson, R.L.; Rothman, A.L.; Xie, J.; Goodrich, L.V.; Bare, J.W.; Bonifas, J.M.; Quinn, A.G.; Myers, R.M.; Cox, D.R.; Epstein, E.H., Jr.; et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 1996, 272, 1668–1671. [Google Scholar] [CrossRef]
- Huang, P.; Zheng, S.; Wierbowski, B.M.; Kim, Y.; Nedelcu, D.; Aravena, L.; Liu, J.; Kruse, A.C.; Salic, A. Structural Basis of Smoothened Activation in Hedgehog Signaling. Cell 2018, 175, 295–297. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Laterra, J.; Yauch, R.L.; Callahan, C.A.; Fu, L.; Holcomb, T.; Stinson, J.; Gould, S.E.; Coleman, B.; et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 2009, 361, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Lou, E.; Schomaker, M.; Wilson, J.D.; Ahrens, M.; Dolan, M.; Nelson, A.C. Complete and sustained response of adult medulloblastoma to first-line sonic hedgehog inhibition with vismodegib. Cancer Biol. Ther. 2016, 17, 1010–1016. [Google Scholar] [CrossRef]
- Jain, S.; Song, R.; Xie, J. Sonidegib: Mechanism of action, pharmacology, and clinical utility for advanced basal cell carcinomas. Onco Targets Ther. 2017, 10, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.; Franceschi, E.; Tosoni, A.; Di Nunno, V.; Bartolini, S.; Brandes, A.A. Molecular Targeted Therapies: Time for a Paradigm Shift in Medulloblastoma Treatment? Cancers 2022, 14, 333. [Google Scholar] [CrossRef]
- Buonamici, S.; Williams, J.; Morrissey, M.; Wang, A.; Guo, R.; Vattay, A.; Hsiao, K.; Yuan, J.; Green, J.; Ospina, B.; et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2010, 2, 51ra70. [Google Scholar] [CrossRef]
- Dijkgraaf, G.J.; Alicke, B.; Weinmann, L.; Januario, T.; West, K.; Modrusan, Z.; Burdick, D.; Goldsmith, R.; Robarge, K.; Sutherlin, D.; et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011, 71, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Filocamo, G.; Brunetti, M.; Colaceci, F.; Sasso, R.; Tanori, M.; Pasquali, E.; Alfonsi, R.; Mancuso, M.; Saran, A.; Lahm, A.; et al. MK-4101, a Potent Inhibitor of the Hedgehog Pathway, Is Highly Active against Medulloblastoma and Basal Cell Carcinoma. Mol. Cancer Ther. 2016, 15, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, E.; Minichillo, S.; Tosoni, A.; Mascarin, M.; Mura, A.; Di Battista, M.; Di Nunno, V.; Gatto, L.; Lodi, R.; Bartolini, S.; et al. Expertise is crucial to prolong survival in average risk medulloblastoma: Long-term results of a retrospective study. Tumori 2021, 108, 331–337. [Google Scholar] [CrossRef] [PubMed]
| Genetically Defined | WNT-Activated | SHH-Activated | Non-WNT/Non-SHH (Group 3 & 4) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP53-Wildtype | TP53-Mutant | |||||||||||||
| Frequency in Adults (%) ** | 14.5 | 60.7 | 2.6 | 22.2 | ||||||||||
| Histologically Defined * | Classic | Desmoplastic/ nodular | Large cell/anaplastic | Classic, Large cell/anaplastic | ||||||||||
| Immunophenotype | Cytoplasmic & Nuclear Beta catenin YAP1 positive GAB1 negative | Cytoplasmic Beta catenin YAP1 positive GAB1 positive p53 low expression/negative | Cytoplasmic Beta catenin YAP1 positive GAB1 positive p53 high expression | Cytoplasmic Beta catenin YAP1 negative GAB1 negative | ||||||||||
| Metastasis in adults ** | M0 83.3% M1–M3 16.7% | M0 92% M1–M3 8%? | M0 100% M1–M3 0% | M0 30% M1–M3 70% | ||||||||||
| Subgroups *** | SHH-1 | SHH-2 | SHH-3 | SHH-4 | SHH-3 TP53-mutant | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| SHH-I SHH-ß SHH-infant | SHH-II SHH-ƴ SHH-infant | SHH-α SHH-child | SHH-δ SHH-adult | SHH-α SHH-child | ||||||||||
| Frequency (%) | 15–20 | 15–20 | 20–25 | 30–35 | 10–15 | 3–5 | 10 | 10 | 8 | 8 | 8 | 15 | 25 | |
| Cytogenetics FISH, MIP, Methylation Array | Monosomy 6 | 2+ | 9q− 10q− | 9p+ 9q− | 3q+ 9q− 10q− 14q− | 3q+ 17p− 3p− 10q− 14q | Balanced | 8+ 10q+ i17q | 7+ i17q 10q− 16q− | 14+ 7+ 8− 10− 11− 16− | 7+ i17q 16q | 7+ i17q 8− 11− | 7+ i17q 8− | i17q |
| Driver Events Sanger-Sequencing, Pyrosequencing NGS Panel | CTNNB1 DDX3X APC | KMT2D | PTCH1 SUFU SMO | PTCH1 ELP1 DDX3X KMT2D PPM1D | U1 snRNA TERT PTCH1 DDX3X SMO CREBBP GSE1 FBXW7 | TP53 DDX3X U1 snRNA TERT MYC GLI2 | GFI/ GFI1B activation OTX2 amplification | MYC, amplification GFI/ GFI1B Activation KBTBD4, SMARCA4 CTDNEP1, KMT2D mutation | MYC MYCN amplification | Not known | MYC, MYCN amplification | PRDM6 activation MYCN amplification | KBTBD4 mutation | PRDM6 Activation KDM6A ZMYM3 KMT2C mutation |
| Molecular Subtype | 5 Years Expected Overall Survival |
|---|---|
| WNT | 100% |
| SHH TP53 MUTATED | <50% |
| SHH TP53 WILD TYPE | 76% |
| NON SHH-NON WNT | 47–50% |
| Molecular Subtype | Expected Survival |
|---|---|
| WNT | >90% |
| SHH | |
| - metastatic TP53 wild type | 50–75% |
| - non metastatic TP53 wild type | 75–90% |
| - metastatic TP53 mutated | <50% |
| GROUP 3 | |
| - metastatic | <50% |
| - non metastatic | 75–90% |
| GROUP 4 | |
| - metastatic | 50–75% |
| - non metastatic | >90% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschi, E.; Giannini, C.; Furtner, J.; Pajtler, K.W.; Asioli, S.; Guzman, R.; Seidel, C.; Gatto, L.; Hau, P. Adult Medulloblastoma: Updates on Current Management and Future Perspectives. Cancers 2022, 14, 3708. https://doi.org/10.3390/cancers14153708
Franceschi E, Giannini C, Furtner J, Pajtler KW, Asioli S, Guzman R, Seidel C, Gatto L, Hau P. Adult Medulloblastoma: Updates on Current Management and Future Perspectives. Cancers. 2022; 14(15):3708. https://doi.org/10.3390/cancers14153708
Chicago/Turabian StyleFranceschi, Enrico, Caterina Giannini, Julia Furtner, Kristian W. Pajtler, Sofia Asioli, Raphael Guzman, Clemens Seidel, Lidia Gatto, and Peter Hau. 2022. "Adult Medulloblastoma: Updates on Current Management and Future Perspectives" Cancers 14, no. 15: 3708. https://doi.org/10.3390/cancers14153708
APA StyleFranceschi, E., Giannini, C., Furtner, J., Pajtler, K. W., Asioli, S., Guzman, R., Seidel, C., Gatto, L., & Hau, P. (2022). Adult Medulloblastoma: Updates on Current Management and Future Perspectives. Cancers, 14(15), 3708. https://doi.org/10.3390/cancers14153708











