Blood Immune Cell Biomarkers in Lung Cancer Patients Undergoing Treatment with a Combination of Chemotherapy and Immune Checkpoint Blockade
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics and General Outcome
3.2. Blood Cells and Therapy Response
3.3. Comparison of Baseline and Third-Cycle Blood Cell Markers
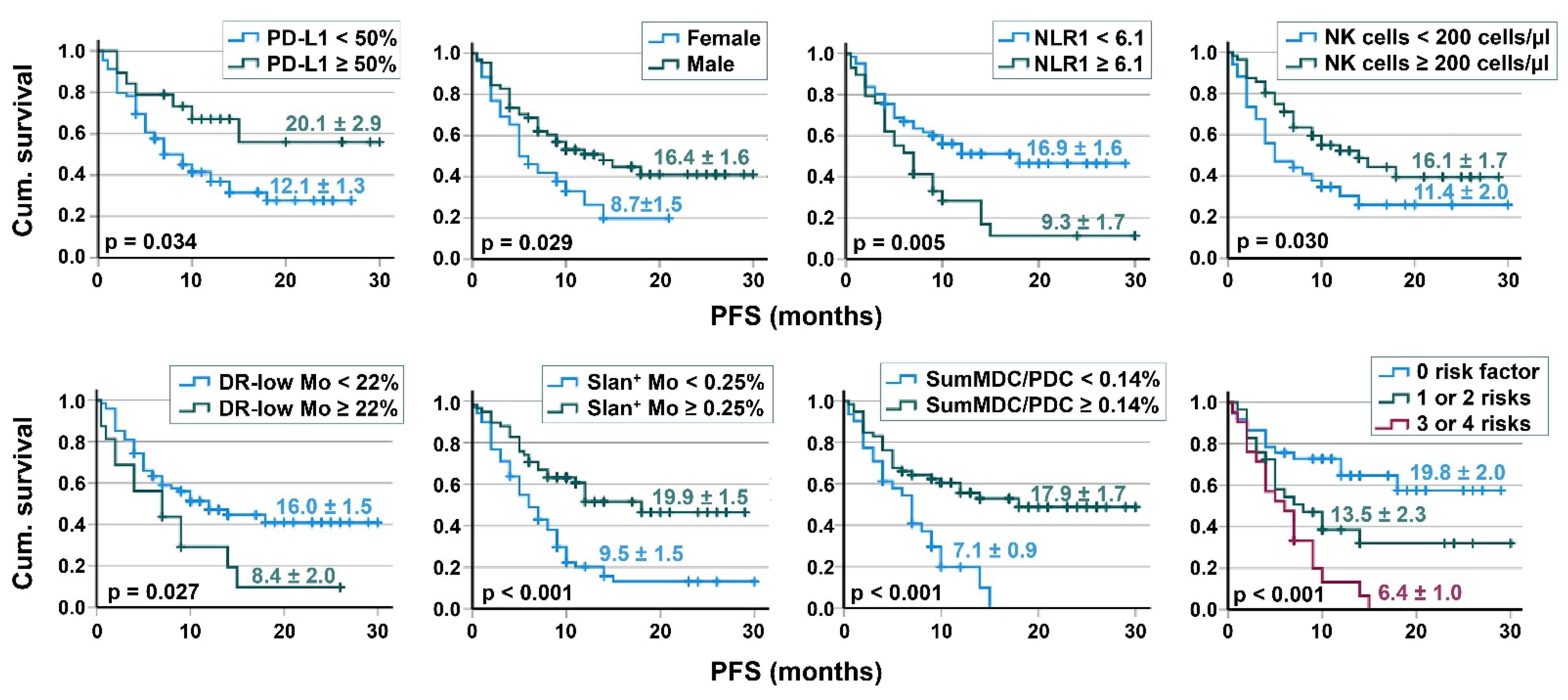
3.4. Survival Analyses
3.5. Correlation of Immune Cell Subpopulations
3.6. Comparison of Female and Male Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, S.; Badheeb, A. Prognostic factors in advanced non-small-cell lung cancer patients: Patient characteristics and type of chemotherapy. Lung Cancer Int. 2011, 2011, 152125. [Google Scholar] [PubMed] [Green Version]
- Pujol, J.L.; Breton, J.L.; Gervais, R.; Rebattu, P.; Depierre, A.; Morere, J.F.; Milleron, B.; Debieuvre, D.; Castera, D.; Souquet, P.J.; et al. Gemcitabine-docetaxel versus cisplatin-vinorelbine in advanced or metastatic non-small-cell lung cancer: A phase iii study addressing the case for cisplatin. Ann. Oncol. 2005, 16, 602–610. [Google Scholar] [PubMed]
- Scagliotti, G.; Brodowicz, T.; Shepherd, F.A.; Zielinski, C.; Vansteenkiste, J.; Manegold, C.; Simms, L.; Fossella, F.; Sugarman, K.; Belani, C.P. Treatment-by-histology interaction analyses in three phase iii trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Zhang, L.; Yu, J.; Zhang, Y.; Pang, X.; Ma, C.; Shen, M.; Ruan, S.; Wasan, H.S.; Qiu, S. Clinical efficacy and safety of anti-pd-1/pd-l1 inhibitors for the treatment of advanced or metastatic cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 2083. [Google Scholar]
- Bodor, J.N.; Kasireddy, V.; Borghaei, H. First-line therapies for metastatic lung adenocarcinoma without a driver mutation. J. Oncol. Pract. 2018, 14, 529–535. [Google Scholar] [CrossRef]
- Aerts, J.G.; Lievense, L.A.; Hoogsteden, H.C.; Hegmans, J.P. Immunotherapy prospects in the treatment of lung cancer and mesothelioma. Transl. Lung Cancer Res. 2014, 3, 34–45. [Google Scholar]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar]
- Moller, M.; Turzer, S.; Schutte, W.; Seliger, B.; Riemann, D. Blood immune cell biomarkers in patient with lung cancer undergoing treatment with checkpoint blockade. J. Immunother. 2020, 43, 57–66. [Google Scholar] [CrossRef]
- Ahmad, F.; Dobel, T.; Schmitz, M.; Schakel, K. Current concepts on 6-sulfo lacnac expressing monocytes (slanmo). Front. Immunol. 2019, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Cay Senler, F.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Frost, N.; Zhamurashvili, T.; von Laffert, M.; Klauschen, F.; Ruwwe-Glosenkamp, C.; Raspe, M.; Brunn, M.; Ochsenreither, S.; Temmesfeld-Wollbruck, B.; Suttorp, N.; et al. Pemetrexed-based chemotherapy is inferior to pemetrexed-free regimens in thyroid transcription factor 1 (ttf-1)-negative, egfr/alk-negative lung adenocarcinoma: A propensity score matched pairs analysis. Clin. Lung Cancer 2020, 21, e607–e621. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for first-line treatment of metastatic nonsquamous nsclc. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef] [Green Version]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local consolidative therapy vs. Maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase ii, randomized study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. Bdca-2, bdca-3, and bdca-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000, 165, 6037–6046. [Google Scholar] [CrossRef] [Green Version]
- Docke, W.D.; Hoflich, C.; Davis, K.A.; Rottgers, K.; Meisel, C.; Kiefer, P.; Weber, S.U.; Hedwig-Geissing, M.; Kreuzfelder, E.; Tschentscher, P.; et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic hla-dr expression: A multicenter standardized study. Clin. Chem. 2005, 51, 2341–2347. [Google Scholar] [CrossRef] [Green Version]
- Riemann, D.; Cwikowski, M.; Turzer, S.; Giese, T.; Grallert, M.; Schutte, W.; Seliger, B. Blood immune cell biomarkers in lung cancer. Clin. Exp. Immunol. 2019, 195, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Li, X.; Huang, Q.; Zheng, X.; Liu, M. Progress and challenges of predictive biomarkers for immune checkpoint blockade. Front. Oncol. 2021, 11, 617335. [Google Scholar] [CrossRef]
- Ushio, R.; Murakami, S.; Saito, H. Predictive markers for immune checkpoint inhibitors in non-small cell lung cancer. J. Clin. Med. 2022, 11, 1855. [Google Scholar] [CrossRef]
- Chen, J.A.; Ma, W.; Yuan, J.; Li, T. Translational biomarkers and rationale strategies to overcome resistance to immune checkpoint inhibitors in solid tumors. Cancer Treat. Res. 2020, 180, 251–279. [Google Scholar]
- Sacdalan, D.B.; Lucero, J.A.; Sacdalan, D.L. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: A review and meta-analysis. Onco. Targets Ther. 2018, 11, 955–965. [Google Scholar] [CrossRef] [Green Version]
- Olingy, C.; Alimadadi, A.; Araujo, D.J.; Barry, D.; Gutierrez, N.A.; Werbin, M.H.; Arriola, E.; Patel, S.P.; Ottensmeier, C.H.; Dinh, H.Q.; et al. Cd33 expression on peripheral blood monocytes predicts efficacy of anti-pd-1 immunotherapy against non-small cell lung cancer. Front. Immunol. 2022, 13, 842653. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 keynot.te-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Vitale, I.; Shema, E.; Loi, S.; Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 2021, 27, 212–224. [Google Scholar]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar]
- Galluzzi, L.; Humeau, J.; Buque, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef]
- Soliman, H.H. Nab-paclitaxel as a potential partner with checkpoint inhibitors in solid tumors. OncoTargets Ther. 2017, 10, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindoni, A.; Minutoli, F.; Ascenti, G.; Pergolizzi, S. Combination of immune checkpoint inhibitors and radiotherapy: Review of the literature. Crit. Rev. Oncol. Hematol. 2017, 113, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Derer, A.; Frey, B.; Fietkau, R.; Gaipl, U.S. Immune-modulating properties of ionizing radiation: Rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol. Immunother. 2016, 65, 779–786. [Google Scholar] [CrossRef] [PubMed]
- de Biasi, A.R.; Villena-Vargas, J.; Adusumilli, P.S. Cisplatin-induced antitumor immunomodulation: A review of preclinical and clinical evidence. Clin. Cancer Res. 2014, 20, 5384–5391. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Kumar, A.B.; Finnes, H.; Markovic, S.N.; Park, S.; Dronca, R.S.; Dong, H. Combining immune checkpoint inhibitors with conventional cancer therapy. Front. Immunol. 2018, 9, 1739. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Singh, S.; Qian, P.R.; Guo, N.L. Immune-omics networks of cd27, pd1, and pdl1 in non-small cell lung cancer. Cancers 2021, 13, 4296. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Malanchi, I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, S.; Shi, Y. Prognostic significance of baseline neutrophil-lymphocyte ratio in patients with non-small-cell lung cancer: A pooled analysis of open phase iii clinical trial data. Future Oncol. 2022, 18, 1679–1689. [Google Scholar] [CrossRef]
- Mazzaschi, G.; Minari, R.; Zecca, A.; Cavazzoni, A.; Ferri, V.; Mori, C.; Squadrilli, A.; Bordi, P.; Buti, S.; Bersanelli, M.; et al. Soluble pd-l1 and circulating cd8+pd-1+ and nk cells enclose a prognostic and predictive immune effector score in immunotherapy treated nsclc patients. Lung Cancer 2020, 148, 1–11. [Google Scholar] [CrossRef]
- Youn, J.I.; Park, S.M.; Park, S.; Kim, G.; Lee, H.J.; Son, J.; Hong, M.H.; Ghaderpour, A.; Baik, B.; Islam, J.; et al. Peripheral natural killer cells and myeloid-derived suppressor cells correlate with anti-pd-1 responses in non-small cell lung cancer. Sci. Rep. 2020, 10, 9050. [Google Scholar] [CrossRef]
- Greten, T.F.; Manns, M.P.; Korangy, F. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 2011, 11, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Vetsika, E.K.; Koinis, F.; Gioulbasani, M.; Aggouraki, D.; Koutoulaki, A.; Skalidaki, E.; Mavroudis, D.; Georgoulias, V.; Kotsakis, A. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J. Immunol. Res. 2014, 2014, 659294. [Google Scholar] [CrossRef]
- Docke, W.D.; Randow, F.; Syrbe, U.; Krausch, D.; Asadullah, K.; Reinke, P.; Volk, H.D.; Kox, W. Monocyte deactivation in septic patients: Restoration by ifn-gamma treatment. Nat. Med. 1997, 3, 678–681. [Google Scholar] [CrossRef]
- Hershman, M.J.; Cheadle, W.G.; Wellhausen, S.R.; Davidson, P.F.; Polk, H.C., Jr. Monocyte hla-dr antigen expression characterizes clinical outcome in the trauma patient. Br. J. Surg. 1990, 77, 204–207. [Google Scholar] [CrossRef]
- Passlick, B.; Flieger, D.; Ziegler-Heitbrock, H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989, 74, 2527–2534. [Google Scholar] [CrossRef] [Green Version]
- Gren, S.T.; Rasmussen, T.B.; Janciauskiene, S.; Hakansson, K.; Gerwien, J.G.; Grip, O. A single-cell gene-expression profile reveals inter-cellular heterogeneity within human monocyte subsets. PLoS ONE 2015, 10, e0144351. [Google Scholar] [CrossRef] [Green Version]
- Hofer, T.P.; Zawada, A.M.; Frankenberger, M.; Skokann, K.; Satzl, A.A.; Gesierich, W.; Schuberth, M.; Levin, J.; Danek, A.; Rotter, B.; et al. Slan-defined subsets of cd16-positive monocytes: Impact of granulomatous inflammation and m-csf receptor mutation. Blood 2015, 126, 2601–2610. [Google Scholar] [CrossRef] [Green Version]
- Hofer, T.P.; van de Loosdrecht, A.A.; Stahl-Hennig, C.; Cassatella, M.A.; Ziegler-Heitbrock, L. 6-sulfo lacnac (slan) as a marker for non-classical monocytes. Front. Immunol. 2019, 10, 2052. [Google Scholar] [CrossRef] [Green Version]
- Hanna, R.N.; Cekic, C.; Sag, D.; Tacke, R.; Thomas, G.D.; Nowyhed, H.; Herrley, E.; Rasquinha, N.; McArdle, S.; Wu, R.; et al. Patrolling monocytes control tumor metastasis to the lung. Science 2015, 350, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Romano, E.; Kusio-Kobialka, M.; Foukas, P.G.; Baumgaertner, P.; Meyer, C.; Ballabeni, P.; Michielin, O.; Weide, B.; Romero, P.; Speiser, D.E. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory t cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl. Acad. Sci. USA 2015, 112, 6140–6145. [Google Scholar] [CrossRef] [Green Version]
- Wehner, R.; Lobel, B.; Bornhauser, M.; Schakel, K.; Cartellieri, M.; Bachmann, M.; Rieber, E.P.; Schmitz, M. Reciprocal activating interaction between 6-sulfo lacnac+ dendritic cells and nk cells. Int. J. Cancer 2009, 124, 358–366. [Google Scholar] [CrossRef]
- Tabarkiewicz, J.; Rybojad, P.; Jablonka, A.; Rolinski, J. Cd1c+ and cd303+ dendritic cells in peripheral blood, lymph nodes and tumor tissue of patients with non-small cell lung cancer. Oncol. Rep. 2008, 19, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and activation of cd103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic pd-l1 and braf inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef] [Green Version]
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S.; Chen, S.; Sum, E.; Jost, C.; Fransen, M.F.; Buser, R.B.; Kowanetz, M.; et al. Dendritic cells dictate responses to pd-l1 blockade cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaav7431. [Google Scholar] [CrossRef]
- Riemann, D.; Schütte, W.; Turzer, S.; Seliger, B.; Moller, M. High pd-l1/cd274 expression of monocytes and blood dendritic cells is a risk factor in lung cancer patients undergoing treatment with pd1 inhibitor therapy. Cancers 2020, 12, 2966. [Google Scholar] [CrossRef]
- Murgaski, A.; Bardet, P.M.R.; Arnouk, S.M.; Clappaert, E.J.; Laoui, D. Unleashing tumour-dendritic cells to fight cancer by tackling their three a’s: Abundance, activation and antigen-delivery. Cancers 2019, 11, 670. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, pd-l1-positive nsclc (keynote-024): A multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017, 18, 1600–1609. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Polanczyk, M.J.; Hopke, C.; Vandenbark, A.A.; Offner, H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (pd-1). Int. Immunol. 2007, 19, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanjanapan, Y.; Day, D.; Wang, L.; Al-Sawaihey, H.; Abbas, E.; Namini, A.; Siu, L.L.; Hansen, A.; Razak, A.A.; Spreafico, A.; et al. Hyperprogressive disease in early-phase immunotherapy trials: Clinical predictors and association with immune-related toxicities. Cancer 2019, 125, 1341–1349. [Google Scholar] [CrossRef] [PubMed]


| Parameters | AC | SqC | NSCLC Other Than AC and SqC |
|---|---|---|---|
| Number | 56 | 27 | 7 |
| Age, median (IQR) | 64 (15) | 67 (8) | 59 (17) |
| Sex | |||
| Male, n (%) | 35 (62.5) | 25 (92.6) | 4 |
| Female, n (%) | 21 (37.5) | 2 (7.4) | 3 |
| ECOG | |||
| 0 | 34 (61.4) | 14 (51.85) | 3 |
| 1 | 22 (38.6) | 13 (48.15) | 4 |
| 2 | 0 | 0 | 0 |
| PD-L1 expression, n (%) | |||
| <1% | 25 (44.6) | 12 (44.4) | 3 (42.9) |
| 1–49% | 18 (32.1) | 9 (33.3) | 2 (28.6) |
| ≥50 | 11 (19.6) | 6 (22.2) | 2 (28.6) |
| missing | 2 | ||
| Smoker status | |||
| 11 (19.6) | 1 (3.7) | 0 |
| 45 (80.4) | 26 (96.3) | 7 (100) |
| Metastases | |||
| <3, n (%) | 26 (46.4) | 17 (63) | 1 (14.3) |
| ≥3, n (%) | 30 (53.6) | 10 (37) | 6 (85.7) |
| Brain and/or liver metastases | 16 (28.6) | 6 (22.2) | 3 (42.9) |
| Therapy setting: | Carboplatin | Carboplatin | Carboplatin |
| Chemotherapy | + pemetrexed (TTF-1-pos.) or + nab-Paclitaxel (TTF-1neg.) | + nab-Paclitaxel | + nab-Paclitaxel |
| Therapy setting: | Pembrolizumab | Pembrolizumab | Pembrolizumab |
| ICI + others | or (if liver metastasis) Atezolizumab + Bevacizumab | ||
| Radiatio before ICI, n (%) | 6 | 3 | 1 |
| Radiatio after ICI, n (%) | 9 | 7 | 1 |
| Patients with tumor recurrence, n (%) | 10 (17.8) | 7 (25.9) | 1 |
| Patients with primary advanced state, n (%) | 46 (82.1) | 20 (74.1) | 6 |
| Clinical response, n (%) | |||
| 14 (25) | 8 (29.6) | 1 |
| 10 (17.9) | 2 (7.4) | 0 |
| 32 (57.1) | 17 (63) | 6 (85.7) |
| Parameters | Baseline Values | Third Cycle Values | ||||
|---|---|---|---|---|---|---|
| Progressive Disease/ Discontinuation | Clinical Response | p-Value | Progressive Disease/ Discontinuation | Clinical Response | p-Value | |
| n | 23 | 67 | 16 | 66 | ||
| Leukocyte counts (cells/μL) | 11,000 (4600) | 8910 (5550) | 10,250 (7620) | 7535 (4780) | 0.004 | |
| Neutrophil counts (cells/μL) | 8170 (5560) | 6120 (4960) | 0.016 | 7770 (7930) | 4845 (3805) | 0.002 |
| Lymphocyte counts (cells/μL) | 1890 (1250) | 1630 (690) | 1445 (1074) | 1460 (1160) | ||
| T cells (cells/μL) | 1142 (695) | 1086 (646) | 1043 (826) | 1109 (878) | ||
| B cells (cells/μL) | 209 (239) | 109 (103) | 160 (212) | 75 (58) | ||
| NK cells (cells/μL) | 188 (396) | 268 (276) | 202 (340) | 235 (196) | ||
| NLR | 4.54 (5.26) | 3.88 (4.19) | 6.77 (5.74) | 3.46 (3.12) | 0.006 | |
| Monocytes (cells/μL) | 840 (340) | 660 (280) | 807 (352) | 710 (423) | ||
| CD16+ monocytes (% of monocytes) | 9.3 (8.6) | 13 (7.3) | 10.2 (6.8) | 14.6 (8.9) | ||
| Slan+ monocytes (% leukocytes) | 0.16 (0.32) | 0.26 (0.54) | 0.13 (0.21) | 0.32 (0.52) | 0.014 | |
| HLA-DRlow MDSC (% of monocytes) | 7.9 (22.1) | 6.9 (13.4) | 11.4 (17.1) | 6.65 (8.7) | 0.026 | |
| CD1c+ MDC (% of leukocytes) | 0.062 (0.074) | 0.105 (0.091) | 0.04 | 0.070 (0.063) | 0.162 (0.143) | <0.001 |
| CD141+ MDC (% of leukocytes) | 0.004 (0.005) | 0.007 (0.006) | 0.022 | 0.004 (0.004) | 0.008 (0.006) | 0.001 |
| PDC (% of leukocytes) | 0.067 (0.068) | 0.093 (0.092) | 0.051 (0.047) | 0.116 (0.134) | 0.011 | |
| Sum of MDC/PDC (% of leukocytes) | 0.142 (0.167) | 0.198 (0.162) | 0.043 | 0.149 (0.142) | 0.313 (0.268) | 0.001 |
| 3A | Cutoff | n | Kaplan–Meier PFS | Cox Regression, PFS | ||||
|---|---|---|---|---|---|---|---|---|
| % Cen-sored | PFS (months) | p-Value | HR | 95% CI | p-Value | |||
| Neutrophil counts(cells/μL) | ≤10,000 | 67 | 50.7 | 16.7 ± 1.6 | 0.013 | 1 | 1.11–3.48 | 0.019 |
| >10,000 | 23 | 17.4 | 8.7 ± 1.6 | 1.98 | ||||
| NLR | <6.1 | 61 | 52.5 | 16.9 ± 1.6 | 0.005 | 1 | 1.21–3.64 | 0.009 |
| ≥6.1 | 29 | 20.7 | 9.3 ± 1.7 | 2.10 | ||||
| NK cells (cells/μL) | <200 | 34 | 29.4 | 11.4 ± 2.05 | 0.030 | 1 | 0.32–0.97 | 0.038 |
| ≥200 | 56 | 50.0 | 16.1 ± 1.67 | 0.56 | ||||
| HLA-DRlow MDSC (% of monocytes) | <22 | 74 | 47.3 | 16.0 ± 1.52 | 0.027 | 1 | 1.05–3.69 | 0.036 |
| ≥22 | 16 | 18.8 | 8.4 ± 1.98 | 1.96 | ||||
| CD16+ monocytes (% of monocytes) | <10 | 28 | 28.6 | 10.2 ± 2.1 | 0.024 | 1 | 0.30–0.95 | 0.031 |
| ≥10 | 60 | 48.3 | 16.4 ± 1.67 | 0.54 | ||||
| Slan+ monocytes (% of leukocytes) | <0.25 | 35 | 17.1 | 6.97 ± 0.87 | <0.001 | 1 | 0.18–0.58 | <0.001 |
| ≥0.25 | 52 | 59.6 | 19.3 ± 1.78 | 0.32 | ||||
| Sum of MDC/PDC (% of leukocytes) | <0.14 | 31 | 19.4 | 7.1 ± 0.88 | <0.001 | 1 | ||
| ≥0.14 | 59 | 54.2 | 17.9 ± 1.70 | 0.38 | 0.22-0.68 | <0.001 | ||
| 3B | Cutoff | n | Kaplan-Meier OS | Cox Regression, OS | ||||
| % censored | OS (months) | p-Value | HR | 95% CI | p-Value | |||
| Neutrophil counts(cells/μL) | ≤10,000 | 67 | 50.7 | 17.9 ± 1.47 | 0.012 | 1 | 1.14–3.53 | 0.016 |
| >10,000 | 23 | 17.4 | 10.5 ± 1.49 | 2.00 | ||||
| NLR | <6.1 | 61 | 52.5 | 17.9 ± 1.49 | 0.008 | 1 | 1.18–3.53 | 0.011 |
| ≥6.1 | 29 | 20.7 | 11.3 ± 1.55 | 2.03 | ||||
| NK cells (cells/μL) | <200 | 34 | 29.4 | 13.1 ± 1.87 | 0.044 | 1 | 0.34–1.01 | 0.053 |
| ≥200 | 56 | 50.0 | 17.4 ± 1.5 | 0.58 | ||||
| HLA-DRlow MDSC (% of monocytes) | <22 | 74 | 47.3 | 17.2 ± 1.39 | 0.033 | 1 | 1.03–3.61 | 0.041 |
| ≥22 | 16 | 18.8 | 10.3 ± 1.96 | 1.93 | ||||
| CD16+ monocytes (% of monocytes) | <10 | 28 | 28.6 | 11.3 ± 1.72 | 0.030 | 1 | 0.31–0.97 | 0.038 |
| ≥10 | 60 | 48.3 | 17.6 ± 1.52 | 0.55 | ||||
| Slan+ monocytes(% of leukocytes) | <0.25 | 35 | 17.1 | 11.8 ± 1.38 | <0.001 | 1 | 0.19–0.66 | <0.001 |
| ≥0.25 | 52 | 59.6 | 20.6 ± 1.8 | 0.35 | ||||
| Sum of MDC/PDC (% of leukocytes) | <0.14 | 31 | 19.4 | 8.95 ± 0.81 | <0.001 | 1 | 0.20–0.64 | <0.001 |
| ≥0.14 | 59 | 54.2 | 18.9 ± 1.55 | 0.36 | ||||
| Baseline Blood Immune Cells | Correlation Coefficient | p-Value |
|---|---|---|
| Neutrophil number with monocyte count | 0.420 | <0.001 |
| Neutrophil number with the frequency of HLA-DRlow MDSC | 0.598 | <0.001 |
| Neutrophil number with the frequency of CD16+ monocytes | −0.477 | <0.001 |
| Neutrophil number with the frequency of slan+CD16+ monocytes | −0.599 | <0.001 |
| Neutrophil number with the frequency of MDC/PDC | −0.662 | <0.001 |
| HLA-DRlow MDSC with the frequency of MDC/PDC | −0.600 | <0.001 |
| HLA-DRlow MDSC with the frequency of CD16+ monocytes | −0.548 | <0.001 |
| HLA-DRlow MDSC with the frequency of slan+CD16+ monocytes | −0.440 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, M.; Turzer, S.; Ganchev, G.; Wienke, A.; Schütte, W.; Seliger, B.; Riemann, D. Blood Immune Cell Biomarkers in Lung Cancer Patients Undergoing Treatment with a Combination of Chemotherapy and Immune Checkpoint Blockade. Cancers 2022, 14, 3690. https://doi.org/10.3390/cancers14153690
Möller M, Turzer S, Ganchev G, Wienke A, Schütte W, Seliger B, Riemann D. Blood Immune Cell Biomarkers in Lung Cancer Patients Undergoing Treatment with a Combination of Chemotherapy and Immune Checkpoint Blockade. Cancers. 2022; 14(15):3690. https://doi.org/10.3390/cancers14153690
Chicago/Turabian StyleMöller, Miriam, Steffi Turzer, Georgi Ganchev, Andreas Wienke, Wolfgang Schütte, Barbara Seliger, and Dagmar Riemann. 2022. "Blood Immune Cell Biomarkers in Lung Cancer Patients Undergoing Treatment with a Combination of Chemotherapy and Immune Checkpoint Blockade" Cancers 14, no. 15: 3690. https://doi.org/10.3390/cancers14153690
APA StyleMöller, M., Turzer, S., Ganchev, G., Wienke, A., Schütte, W., Seliger, B., & Riemann, D. (2022). Blood Immune Cell Biomarkers in Lung Cancer Patients Undergoing Treatment with a Combination of Chemotherapy and Immune Checkpoint Blockade. Cancers, 14(15), 3690. https://doi.org/10.3390/cancers14153690








