Recent Advances in the Diagnosis and Management of High-Risk Cutaneous Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Definition of High-Risk cSCC
3. Diagnosis of High-Risk cSCC
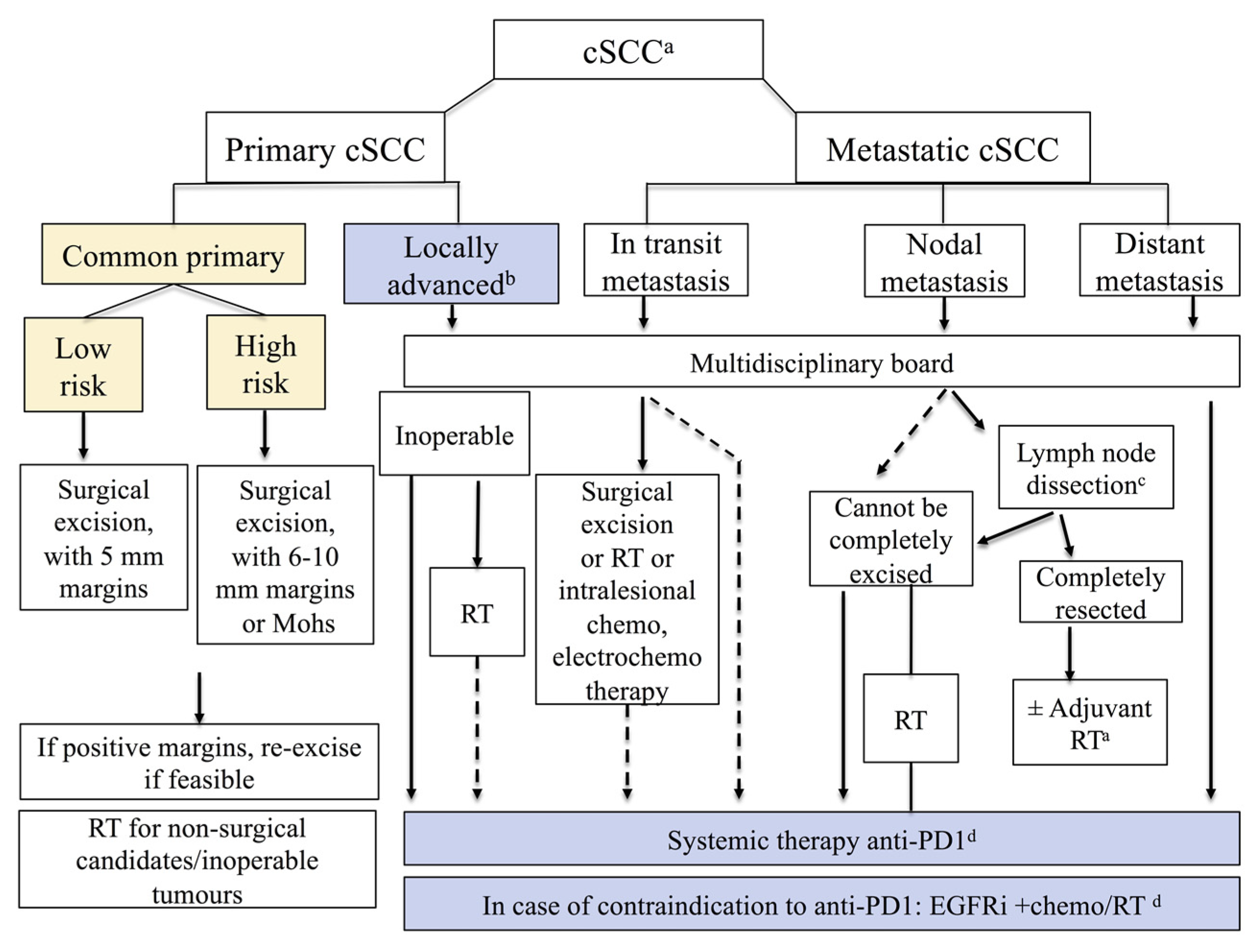
4. Primary Treatment of High-Risk cSCC
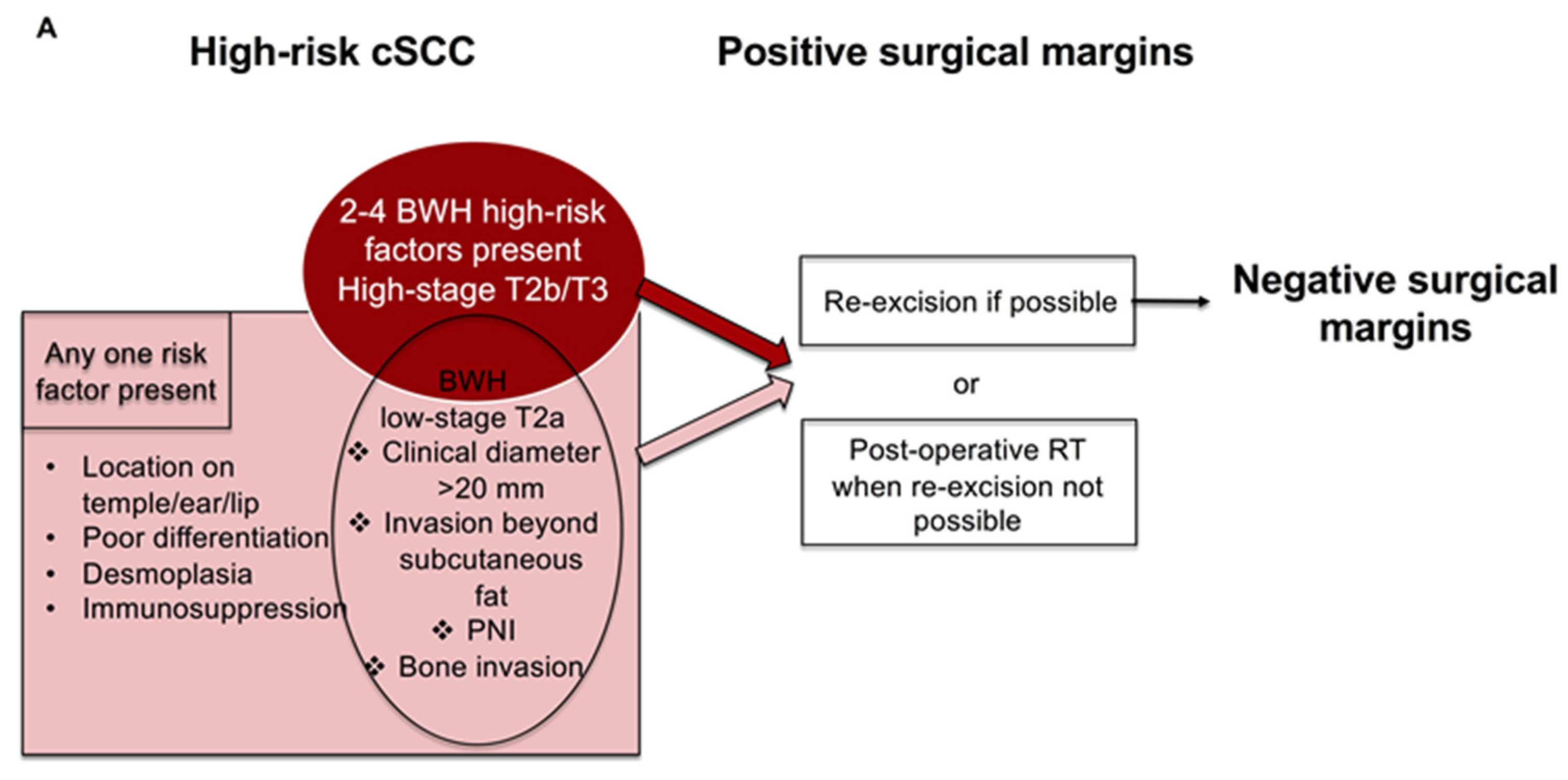
5. Post-Operative RT for High-Risk cSCC with Residual Disease after Surgery
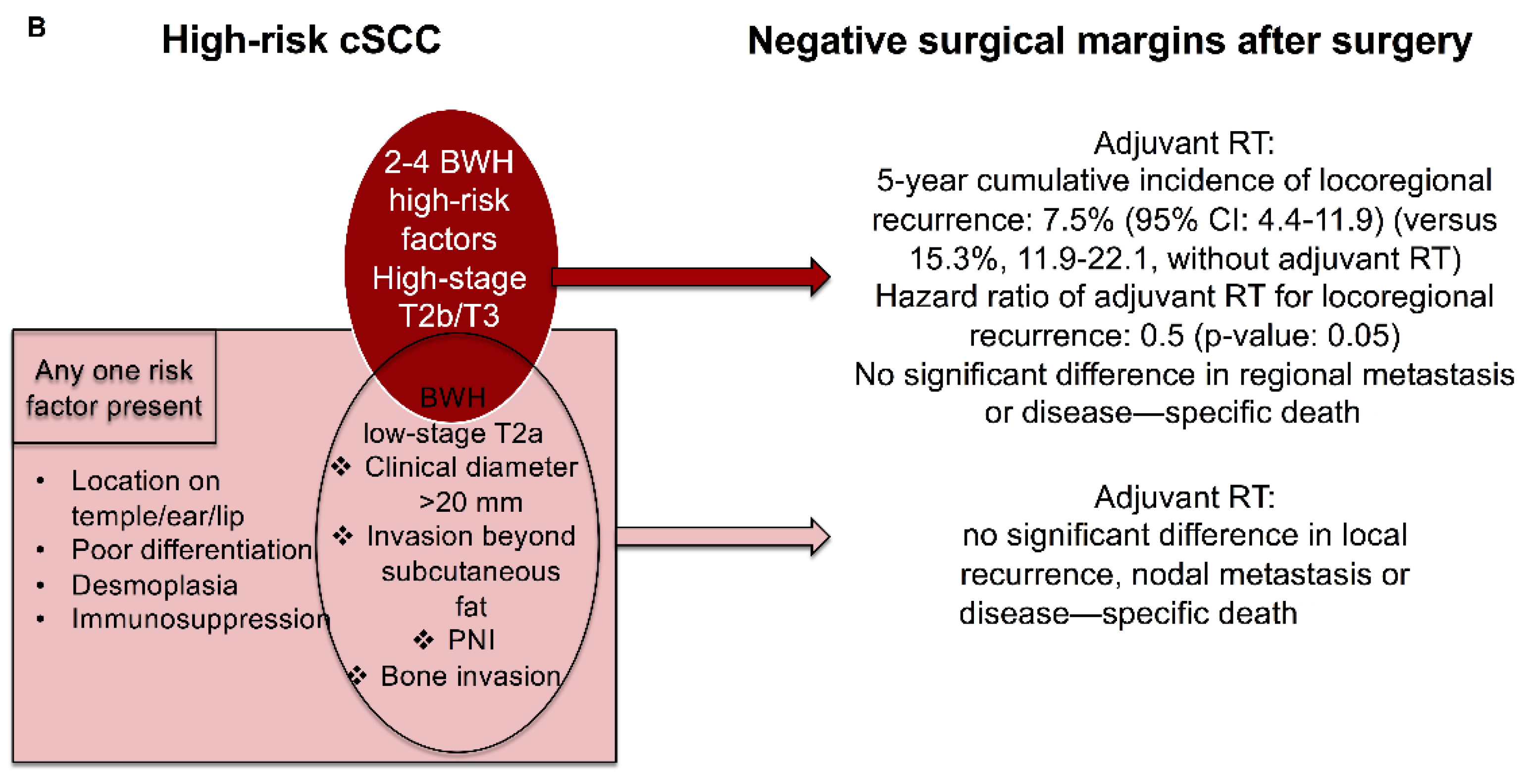
6. Adjuvant Treatment for High-Risk cSCC with Negative Surgical Margins
7. Referral of Patients with High-Risk cSCC to Multidisciplinary Tumor Board
8. Follow-Up of Patients with High-Risk cSCC
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef] [Green Version]
- Dessinioti, C.; Pitoulias, M.; Stratigos, A.J. Epidemiology of advanced cutaneous squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2021, 36, 39–50. [Google Scholar] [CrossRef]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Karia, P.S.; Besaw, R.; Schmults, C.D. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs the Brigham and Women’s Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2019, 155, 819–825. [Google Scholar] [CrossRef]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Breuninger, H.; Black, B.; Rassner, G. Microstaging of squamous cell carcinomas. Am J Clin Pathol. 1990, 94, 624–627. [Google Scholar] [CrossRef]
- Karia, P.S.; Jambusaria-Pahlajani, A.; Harrington, D.P.; Murphy, G.F.; Qureshi, A.A.; Schmults, C.D. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014, 32, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Rowe, D.E.; Carroll, R.J.; Day, C.L., Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef]
- AJCC Cancer Staging Manual, 8th ed.; Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) Springer: Basel, Switzerland, 2017. [Google Scholar]
- Union for International Cancer Control. TNM Classification of Malignant Tumours, 8th ed.; Brierley, J.D., Gospodarowicz, M.K., Wittekind, C.H., Eds.; John Wiley and Sons: Oxford, UK, 2017. [Google Scholar]
- Jambusaria-Pahlajani, A.; Kanetsky, P.A.; Karia, P.S.; Hwang, W.-T.; Gelfand, J.; Whalen, F.M.; Elenitsas, R.; Xu, X.; Schmults, C.D. Evaluation of AJCC Tumor Staging for Cutaneous Squamous Cell Carcinoma and a Proposed Alternative Tumor Staging System. JAMA Dermatol. 2013, 149, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Schmults, C.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Baumann, B.C.; Bordeaux, J.; Chen, P.; Chin, R.; Contreras, C.M.; et al. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Squamous Cell Skin Cancer. 2022; Version 2.2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf (accessed on 1 June 2022).
- Keohane, S.; Botting, J.; Budny, P.; Dolan, O.; Fife, K.; Harwood, C.; Mallipeddi, R.; Marsden, J.; Motley, R.; Newlands, C.; et al. British Association of Dermatologists guidelines for the management of people with cutaneous squamous cell carcinoma 2020*. Br. J. Dermatol. 2020, 184, 401–414. [Google Scholar] [CrossRef]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Platsidaki, E.; Stratigos, A.J. A Sensitivity Meta-Analysis of Disease-Specific Death in Localized Cutaneous Squamous Cell Carcinoma. Dermatology 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, J.N.; Hajder, E.; van der Holt, B.; Bakker, M.A.D.; Hovius, S.E.; Mureau, M.A. The Effect of Differentiation Grade of Cutaneous Squamous Cell Carcinoma on Excision Margins, Local Recurrence, Metastasis, and Patient Survival. Ann. Plast. Surg. 2015, 75, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H.I.; Cooper, P.H.; Wanebo, H.J. Prognostic and therapeutic use of microstaging of cutaneous squamous cell carcinoma of the trunk and extremities. Cancer 1985, 56, 1099–1105. [Google Scholar] [CrossRef]
- Kyrgidis, A.; Tzellos, T.G.; Kechagias, N.; Patrikidou, A.; Xirou, P.; Kitikidou, K.; Bourlidou, E.; Vahtsevanos, K.; Antoniades, K. Cutaneous squamous cell carcinoma (SCC) of the head and neck: Risk factors of overall and recurrence-free survival. Eur. J. Cancer 2010, 46, 1563–1572. [Google Scholar] [CrossRef]
- Eigentler, T.K.; Leiter, U.; Häfner, H.-M.; Garbe, C.; Röcken, M.; Breuninger, H. Survival of Patients with Cutaneous Squamous Cell Carcinoma: Results of a Prospective Cohort Study. J. Investig. Dermatol. 2017, 137, 2309–2315. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, E.S.; Koyfman, S.A.; Kass, J.; Schmults, C.D. Surgery and Salvage Limited-Field Irradiation for Control of Cutaneous Squamous Cell Carcinoma with Microscopic Residual Disease. JAMA Dermatol. 2019, 155, 1193–1195. [Google Scholar] [CrossRef]
- Clayman, G.L.; Lee, J.J.; Holsinger, F.C.; Zhou, X.; Duvic, M.; El-Naggar, A.K.; Prieto, V.G.; Altamirano, E.; Tucker, S.L.; Strom, S.S.; et al. Mortality Risk from Squamous Cell Skin Cancer. J. Clin. Oncol. 2005, 23, 759–765. [Google Scholar] [CrossRef]
- Conde-Ferreirós, A.; Corchete, L.A.; Jaka, A.; Santos-Briz, Á.; Fuente, M.J.; Posada, R.; Pons, L.; Podlipnik, S.; Pujol, R.M.; Román-Curto, C.; et al. Patterns of incidental perineural invasion and prognosis in cutaneous squamous cell carcinoma: A multicenter, retrospective cohort study. J. Am. Acad. Dermatol. 2020, 84, 1708–1712. [Google Scholar] [CrossRef]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Tschetter, A.J.; Campoli, M.R.; Zitelli, J.A.; Brodland, D.G. Long-term clinical outcomes of patients with invasive cutaneous squamous cell carcinoma treated with Mohs micrographic surgery: A 5-year, multicenter, prospective cohort study. J. Am. Acad. Dermatol. 2019, 82, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.W.; Feeley, K.; Suvarna, S.K. Audit of clinical and histological prognostic factors in primary invasive squamous cell carcinoma of the skin: Assessment in a minimum 5 year follow-up study after conventional excisional surgery. Br J Plast Surg. 2002, 55, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.; Yao, C.M.K.L.; Amit, M.; Gajera, M.; Luo, X.; Treistman, R.; Khanna, A.; Aashiq, M.; Nagarajan, P.; Bell, D.; et al. Association of Immunosuppression With Outcomes of Patients With Cutaneous Squamous Cell Carcinoma of the Head and Neck. JAMA Otolaryngol. Neck Surg. 2020, 146, 128–135. [Google Scholar] [CrossRef]
- Tokez, S.; Venables, Z.C.; Hollestein, L.M.; Qi, H.; Bramer, E.M.; Rentroia-Pacheco, B.; Bos, R.R.V.D.; Rous, B.; Leigh, I.M.; Nijsten, T.; et al. Risk factors for metastatic cutaneous squamous cell carcinoma: Refinement and replication based on 2 nationwide nested case-control studies. J. Am. Acad. Dermatol. 2022, 87, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Tokez, S.; Wakkee, M.; Kan, W.; Venables, Z.C.; Mooyaart, A.L.; Louwman, M.; Nijsten, T.; Hollestein, L.M. Cumulative incidence and disease-specific survival of metastatic cutaneous squamous cell carcinoma: A nationwide cancer registry study. J. Am. Acad. Dermatol. 2022, 86, 331–338. [Google Scholar] [CrossRef]
- Wysong, A.; Newman, J.G.; Covington, K.R.; Kurley, S.; Ibrahim, S.F.; Farberg, A.S.; Bar, A.; Cleaver, N.J.; Somani, A.-K.; Panther, D.; et al. Validation of a 40-gene expression profile test to predict metastatic risk in localized high-risk cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2020, 84, 361–369. [Google Scholar] [CrossRef]
- Ibrahim, S.F.; Kasprzak, J.M.; Hall, M.A.; Fitzgerald, A.L.; Siegel, J.J.; Kurley, S.J.; Covington, K.R.; Goldberg, M.S.; Farberg, A.S.; Trotter, S.C.; et al. Enhanced metastatic risk assessment in cutaneous squamous cell carcinoma with the 40-gene expression profile test. Future Oncol. 2021, 18, 833–847. [Google Scholar] [CrossRef]
- Tokez, S.; Koekelkoren, F.H.J.; de Jong, R.J.B.; Grünhagen, D.J.; Mooyaart, A.L.; Nijsten, T.; van der Lugt, A.; Wakkee, M. Assessment of the Diagnostic Accuracy of Baseline Clinical Examination and Ultrasonographic Imaging for the Detection of Lymph Node Metastasis in Patients With High-risk Cutaneous Squamous Cell Carcinoma of the Head and Neck. JAMA Dermatol. 2022, 158, 151. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Karia, P.S.; Morgan, F.C.; Schmults, C.D. The positive impact of radiologic imaging on high-stage cutaneous squamous cell carcinoma management. J. Am. Acad. Dermatol. 2016, 76, 217–225. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Moore, B.A.; Schmalbach, C.E. Utility of head and neck cutaneous squamous cell carcinoma sentinel node biopsy: A systematic review. Otolaryngol. 2014, 150, 180–187. [Google Scholar] [CrossRef]
- Kwon, S.; Dong, Z.M.; Wu, P.C. Sentinel lymph node biopsy for high-risk cutaneous squamous cell carcinoma: Clinical experience and review of literature. World J. Surg. Oncol. 2011, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarrete-Dechent, C.; Veness, M.J.; Droppelmann, N.; Uribe, P. High-risk cutaneous squamous cell carcinoma and the emerging role of sentinel lymph node biopsy: A literature review. J. Am. Acad. Dermatol. 2015, 73, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.R.; Brewer, J.D.; Bordeaux, J.S.; Baum, C.L. Staging for cutaneous squamous cell carcinoma as a predictor of sentinel lymph node biopsy results: Meta-analysis of American Joint Committee on Cancer criteria and a proposed alternative system. JAMA Dermatol. 2014, 150, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur. J. Cancer 2020, 128, 83–102. [Google Scholar] [CrossRef] [Green Version]
- Dessinioti, C.; Stratigos, A. Overview of guideline recommendations for the management of high-risk and advanced cutaneous squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2021, 36, 11–18. [Google Scholar] [CrossRef]
- Moehrle, M.; Breuninger, H.; Rocken, M. A confusing world: What to call histology of three-dimensional tumour margins? J Eur Acad Dermatol Venereol. 2007, 25, 591–595. [Google Scholar] [CrossRef]
- Loser, C.R.; Rompel, R.; Mohrle, M.; Hafner, H.M.; Kunte, C.; Hassel, J.; Hohenleutner, U.; Podda, M.; Sebastian, G.; Hafner, J.; et al. S1 guideline: Microscopically controlled surgery (MCS). J. Dtsch. Dermatol. Ges. 2015, 13, 942–951. [Google Scholar] [CrossRef]
- Mohrle, M.; Breuninger, H. The Muffin technique--an alternative to Mohs’ micrographic surgery. J. Dtsch. Dermatol. Ges. 2006, 4, 1080–1084. [Google Scholar]
- Lansbury, L.; Bath-Hextall, F.; Perkins, W.; Stanton, W.; Leonardi-Bee, J. Interventions for non-metastatic squamous cell carcinoma of the skin: Systematic review and pooled analysis of observational studies. BMJ 2013, 347, f6153. [Google Scholar] [CrossRef] [Green Version]
- Krausz, A.E.; Ji-Xu, A.; Smile, T.; Koyfman, S.; Schmults, C.D.; Ruiz, E.S. A Systematic Review of Primary, Adjuvant, and Salvage Radiation Therapy for Cutaneous Squamous Cell Carcinoma. Dermatol. Surg. 2021, 47, 587–592. [Google Scholar] [CrossRef]
- Cañueto, J.; Jaka, A.; Sánchez, L.A.C.; Pérez, A.M.G.; García-Castro, R.; Fuente, M.; Membrive, I.; March, Á.; Mañes, A.; Posada, R.; et al. Postoperative radiotherapy provides better local control and long-term outcome in selective cases of cutaneous squamous cell carcinoma with perineural invasion. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Revelles-Peñas, L.; Revilla-Nebreda, D.; Becerril, S.; Corchete, L.; Domínguez-Rullán, I.; Martins-Lopes, M.; Arias-Rodríguez, P.; Rodríguez-Guitiérrez, A.; Pérez-Romansanta, L.; Román-Curto, C.; et al. Outcome of cutaneous squamous cell carcinoma with microscopic residual disease after surgery and usefulness of postoperative radiotherapy: A retrospective cohort study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lehrer, E.J.; Wirth, P.J.; Khesroh, E.A.; Brewer, J.D.; Billingsley, E.M.; Zaorsky, N.G.; Lam, C. Adjuvant radiotherapy may not significantly change outcomes in high-risk cutaneous squamous cell carcinomas with clear surgical margins: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021, 86, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Li, J.N.; Matsumoto, M.; Pineider, J.; Nijhawan, R.I.; Srivastava, D. Factors predicting outcomes of patients with high-risk squamous cell carcinoma treated with Mohs micrographic surgery. J. Am. Acad. Dermatol. 2021, 85, 588–595. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Koyfman, S.A.; Que, S.K.T.; Kass, J.; Schmults, C.D. Evaluation of the utility of localized adjuvant radiation for node-negative primary cutaneous squamous cell carcinoma with clear histologic margins. J. Am. Acad. Dermatol. 2020, 82, 420–429. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Kus, K.J.; Smile, T.D.; Murad, F.; Zhou, G.; Ilori, E.O.; Schoenfeld, J.D.; Margalit, D.N.; Tishler, R.B.; Vidimos, A.T.; et al. Adjuvant radiation following clear margin resection of high T-stage cutaneous squamous cell carcinoma halves the risk of local and locoregional recurrence: A dual-center retrospective study. J. Am. Acad. Dermatol. 2022, 87, 87–94. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wijaya, W.; Liang, Z.; Chen, J. Efficacy and prognostic factors of adjuvant radiotherapy for cutaneous squamous cell carcinoma: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1777–1787. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Heath, C.H.; Deep, N.L.; Nabell, L.; Carroll, W.R.; Desmond, R.; Clemons, L.; Spencer, S.; Magnuson, J.S.; Rosenthal, E.L. Phase 1 Study of Erlotinib Plus Radiation Therapy in Patients with Advanced Cutaneous Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2013, 85, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Brewster, A.M.; Lee, J.J.; Clayman, G.L.; Clifford, J.L.; Reyes, M.J.T.N.; Zhou, X.; Sabichi, A.L.; Strom, S.S.; Collins, R.; Meyers, C.A.; et al. Randomized Trial of Adjuvant 13-cis-Retinoic Acid and Interferon Alfa for Patients with Aggressive Skin Squamous Cell Carcinoma. J. Clin. Oncol. 2007, 25, 1974–1978. [Google Scholar] [CrossRef]
- Goyal, U.; Prabhakar, N.K.; Davuluri, R.; Morrison, C.M.; Yi, S.K. Role of Concurrent Systemic Therapy with Adjuvant Radiation Therapy for Locally Advanced Cutaneous Head and Neck Squamous Cell Carcinoma. Cureus 2017, 9, e1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, J.; Schneider, C.J.; Hockstein, N.; Hanlon, A.L.; Silberg, J.; Strasser, J.; Mauer, E.A.; Dzeda, M.; Witt, R.; Raben, A. Combination of post-operative radiotherapy and cetuximab for high-risk cutaneous squamous cell cancer of the head and neck: A propensity score analysis. Oral Oncol. 2018, 78, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Porceddu, S.V.; Bressel, M.; Poulsen, M.G.; Stoneley, A.; Veness, M.J.; Kenny, L.M.; Wratten, C.; Corry, J.; Cooper, S.; Fogarty, G.B.; et al. Postoperative Concurrent Chemoradiotherapy Versus Postoperative Radiotherapy in High-Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck: The Randomized Phase III TROG 05.01 Trial. J. Clin. Oncol. 2018, 36, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Study of Adjuvant Cemiplimab Versus Placebo after Surgery and Radiation Therapy in Patients with High Risk Cutaneous Squamous Cell Carcinoma. ClinicalTrials.gov Identifier: NCT03969004. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03969004?cond=high-risk+cutaneous+squamous+cell+carcinoma&draw=2&rank=2 (accessed on 2 June 2022).
- The Addition of Pembrolizumab to Postoperative Radiotherapy in Cutaneous Squamous Cell Cancer of the Head and Neck. ClinicalTrials.gov Identifier: NCT03057613. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03057613?cond=high-risk+cutaneous+squamous+cell+carcinoma&draw=2&rank=6 (accessed on 2 June 2022).
- Venables, Z.C.; Autier, P.; Nijsten, T.; Wong, K.F.; Langan, S.M.; Rous, B.; Broggio, J.; Harwood, C.; Henson, K.; Proby, C.M.; et al. Nationwide Incidence of Metastatic Cutaneous Squamous Cell Carcinoma in England. JAMA Dermatol. 2019, 155, 298–306. [Google Scholar] [CrossRef]
- Maher, J.M.; Schmults, C.D.; Murad, F.; Karia, P.S.; Benson, C.B.; Ruiz, E.S. Detection of subclinical disease with baseline and surveillance imaging in high-risk cutaneous squamous cell carcinomas. J. Am. Acad. Dermatol. 2019, 82, 920–926. [Google Scholar] [CrossRef]
| High-Risk Factors | European 2020 [1] | US NCCN 2022 [12] | UK BAD 2020 [13] | ||
|---|---|---|---|---|---|
| High Risk for Recurrence (Local or Metastatic) | High-Risk Factor for Local Recurrence, Metastasis, or Disease-Specific Death | Very High Risk for Local Recurrence, Metastasis, or Disease-Specific Death | High Risk for Local Recurrence, Nodal Metastasis, Or Disease-Specific Death | Very High Risk for Local Recurrence, Nodal Metastasis, Or Disease-Specific Death | |
| Tumor-Related High-Risk Factors | |||||
| Tumor diameter | >20 mm | Trunk, extremities >2 cm–≤4 cm | >4 cm any location | >20–40 mm | >40 mm |
| Localization | On temple/ear/lip | Head, neck, hands, feet, pretibial, and anogenital (any size) | On ear/lip | - | |
| Thickness | Thickness > 6 mm or Invasion beyond subcutaneous fat | - | >6 mm or Invasion beyond subcutaneous fat | Thickness > 4–6 mm | Thickness > 6 mm |
| Invasion | Invasion into subcutaneous fat | Invasion beyond subcutaneous fat | |||
| Differentiation | Poor grade differentiation | - | Poor grade differentiation | Poor grade differentiation | - |
| Histological feature | Desmoplasia | Acandtholytic (adenoid), adenosquamous, or metaplastic (carcinosarcomatous) | Desmoplasia | Lymphovascular invasion | High-grade histological subtype—adenosquamous, desmoplastic, spindle/sarcomatoid/metaplastic |
| Perineural invasion (PNI) | Histological/symptomatic/radiological PNI | Yes | PNI of a nerve lying deeper than the dermis or measuring ≥ 0.1 mm | Perineural invasion—dermal only; nerve diameter < 0.1 mm | Perineural invasion present in named nerve; nerve ≥0.1 mm; or nerve beyond dermis |
| Lymphatic or vascular involvement | - | - | Yes | - | - |
| Bone erosion/invasion | Bone erosion | - | - | - | Any bone invasion |
| Tumor on scar/chronic inflammation/RT | - | Site of prior RT or chronic inflammation | - | Tumor arising within scar or area of chronic inflammation | - |
| In-transit metastasis | - | - | - | - | In-transit metastasis |
| Borders | - | Poorly defined | - | - | - |
| Primary vs recurrent | - | Recurrent | - | - | - |
| Rapidly growing tumor | - | Yes | - | - | - |
| Neurologic symptoms | - | Yes | - | - | - |
| Patient-Related Risk Factors | |||||
| Immunosuppression | Yes | Yes | - | Iatrogenic IS or biological therapies, frailty and co-morbidities, HIV, HAART | As for high risk, especially SOTRs, hematological malignancies, such as CLL or myelofibrosis, other significant IS |
| Extrinsic Risk Factors | |||||
| Positive margins | Yes | - | - | One or more involved or close margin in a pT1 tumor. Close margins in a pT2 tumor. | One or more involved or close margin in a high-risk tumor. |
| Grade of recommendation | B (recommendation) | - | Category 2A (lower-level evidence, uniform NCCN consensus) | GPP (informal consensus) | GPP (informal consensus) |
| Risk Factor | Thompson, 2016 [14] | Dessinioti, 2022 [15] | ||||
|---|---|---|---|---|---|---|
| Local Recurrence | Nodal Metastasis | Disease-Specific Death | Disease-Specific Death in Localized cSCC at Presentation | |||
| Risk Ratio (95% CI) | Risk Ratio (95% CI) | Included Studies | Risk Ratio (95% CI) | Included Studies | Risk Ratio (95% CI) | |
| Poor differentiation | 2.66 (1.72–4.14) | 4.98 (3.30–7.49) | Brinkman et al. [16] Friedman et al. [17] Karia et al. [7] Kyrgidis et al. [18] | 5.65 (1.76–18.20) | Brinkman et al. [16] Eigentler et al. [19] Karia et al. [7] Ruiz et al. [20] | 3.72 (0.80–17.28) |
| Depth beyond fat | 7.61 (4.17–13.88) | 11.21 (3.59–34.97) | Clayman et al. [21] Friedman et al. [17] Karia et al. [7] Kyrgidis et al. [18] | 4.49 (2.05–9.82) | Conde-Ferreiros et al. [22] Karia et al. [7] Ruiz et al. [20] | 2.24 (0.34–14.75) |
| Diameter 20 mm or more | 3.22 (1.91–5.45) | 6.15 (3.56–10.65) | Karia et al. [7] | 19.10 (5.80–62.95) | Karia et al. [7] Ruiz et al. [20] | 4.57 (0.20–106.66) |
| PNI present | 4.30 (2.80–6.60) | 2.95 (2.31–3.75) | Clayman et al. [21] Kyrgidis et al. [18] Schmults et al. [23] | 4.06 (3.10–5.32) | Schmults et al. [23] Ruiz et al. [20] | 1.63 (0.21–12.88) |
| Thickness ≥ 6 mm | 7.13 (3.04–16.72) | 6.93 (4.02–11.94) | - | - | Conde-Ferreiros et al. [22] Eigentler et al. [19] | 2.44 (0.30–19.66) |
| Thickness (continuous) | - | - | - | - | Tschetter et al. [24] | 1.20 (1.00–1.44) |
| Thickness > 2 mm | 9.64 (1.30–71.52) | 10.76 (2.55–45.31) | - | - | - | - |
| Location ear | 1.28 (0.56–2.90) | 2.33 (1.67–3.23) | Griffiths et al. [25] Schmults et al. [23] | 4.67 (1.28–17.12) | Eigentler et al. [19] Griffiths et al. [25] Schmults et al. [23] | 1.71 (0.61–4.78) |
| Location lip | 1.28 (0.56–2.90) | 2.28 (1.54–3.37) | - | 4.55 (1.41–14.69) | - | - |
| Location head/neck | - | - | - | - | Schmults et al. [23] Ruiz et al. [20] | 0.98 (0.29–3.24) |
| Location temple | 3.20 (1.12–9.15) | 2.82 (1.72–4.63) | - | 1.80 (0.22–14.79) | - | - |
| Immunosuppression | 1.51 (0.81–2.81) | 1.59 (1.07–2.37) | Karia et al. [7] | 0.35 (0.05–2.58) | Eigentler et al. [19] Karia et al [7] Ruiz et al. [20] Tam et al. [26] | 1.85 (1.32–2.61) |
| Treatment for High-Risk cSCC | European 2020 [37] | US NCCN 2022 [12] | UK BAD 2020 [13] |
|---|---|---|---|
| Surgery | As first-line treatment: excision with histological control aiming at R0 excision (GOR: A) | Mohs or other forms of PDEMA (preferred for very high risk) Or standard excision with wider surgical margins and postoperative margin assessment (GOR: 2A) | Offer standard surgical excision as first-line treatment for resectable primary cSCC (GOR: Strong) |
| Standard excision with histological confirmation of peripheral and deep margins or MMS/MCS (GOR: B) | Consider MMS in selected cSCC after SSMDT (GOR: Weak) | ||
| Clinical safety margins | 6–10 mm (GOR: B) | Wider than 6 mm (GOR: 2A) | ≥6 mm for high risk ≥10 mm for very high risk (GOR: Strong) |
| Primary RT | Primary RT should be considered as an alternative to surgery for inoperable or difficult-to-operate tumors or in the absence of consent to surgical excision (GOR: B) | Primary RT +/− systemic therapy, as an alternative to surgery for non-surgical candidates (GOR: 2A) | Offer to selected people with cSCC as an option after MDT Offer when surgery is not feasible or would be challenging or likely to result in an unacceptable functional or aesthetic outcome (GOR: Strong) |
| - | - | Consider primary RT for locally recurrent cSCC (GOR: GPP) | |
| - | - | Consider conformal RT including the entire course of the involved nerve in people with cSCC with symptomatic PNI and/or radiologic evidence of PNI when surgery is inappropriate (GOR: Weak) | |
| Systemic Therapy | - | RT +/− systemic therapy for high-risk/very-high-risk cSCC, for non-surgical candidates. Discuss in multidisciplinary consultation, RT +/− systemic therapy for high-risk/very-high-risk cSCC with positive margins if re-excision not feasible. | - |
| Adjuvant Therapy | European 2020 [37] | US NCCN 2022 [12] | UK BAD 2020 [13] |
|---|---|---|---|
| Adjuvant radiotherapy | Post-operative RT should be considered after surgical excision for cSCC with positive margins and re-excision not possible | Recommend multidisciplinary consultation and consider adjuvant RT, for local, high-risk/very-high-risk cSCC with negative margins, if extensive perineural, larger, or named nerve involvement, or if other poor prognostic features. Noted that the outcome of adjuvant RT following resection of any cSCC with negative surgical margins is uncertain (GOR: 2A) | Offer adjuvant RT to people with incompletely excised cSCC, where further surgery is not possible and in those at high risk for local recurrence (PNI [multifocal, named nerve, and/or diameter of nerve >0.1 mm, below the dermis], immunosuppression or recurrent disease) (GOR: Strong) |
| - | Consider adjuvant RT for completely excised T3 tumors, with multiple high-risk factors including >6 mm thickness and invasion beyond subcutaneous fat (GOR: Weak) | ||
| Consider adjuvant RT for locally recurrent cSCC (GOR: GPP) | |||
| Do not offer post-operative RT for people with completely excised T1 or T2 cSCC and with microscopic, dermal only, nerve diameter < 0.1 mm PNI (GOR: Strong against) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessinioti, C.; Stratigos, A.J. Recent Advances in the Diagnosis and Management of High-Risk Cutaneous Squamous Cell Carcinoma. Cancers 2022, 14, 3556. https://doi.org/10.3390/cancers14143556
Dessinioti C, Stratigos AJ. Recent Advances in the Diagnosis and Management of High-Risk Cutaneous Squamous Cell Carcinoma. Cancers. 2022; 14(14):3556. https://doi.org/10.3390/cancers14143556
Chicago/Turabian StyleDessinioti, Clio, and Alexander J. Stratigos. 2022. "Recent Advances in the Diagnosis and Management of High-Risk Cutaneous Squamous Cell Carcinoma" Cancers 14, no. 14: 3556. https://doi.org/10.3390/cancers14143556
APA StyleDessinioti, C., & Stratigos, A. J. (2022). Recent Advances in the Diagnosis and Management of High-Risk Cutaneous Squamous Cell Carcinoma. Cancers, 14(14), 3556. https://doi.org/10.3390/cancers14143556