PRIM2 Promotes Cell Cycle and Tumor Progression in p53-Mutant Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Analysis for Differential Gene Expression and Mutation
2.2. Gene Expression Omnibus
2.3. Survival Prognostic Analysis
2.4. Gene Set Enrichment Analysis
2.5. Correlation Analysis
2.6. Immunohistochemistry
2.7. Cell Culture
2.8. Real-Time PCR
2.9. Western Blot
2.10. Cell Growth Assays and Cell Senescence Assays
2.11. Statistics
3. Results
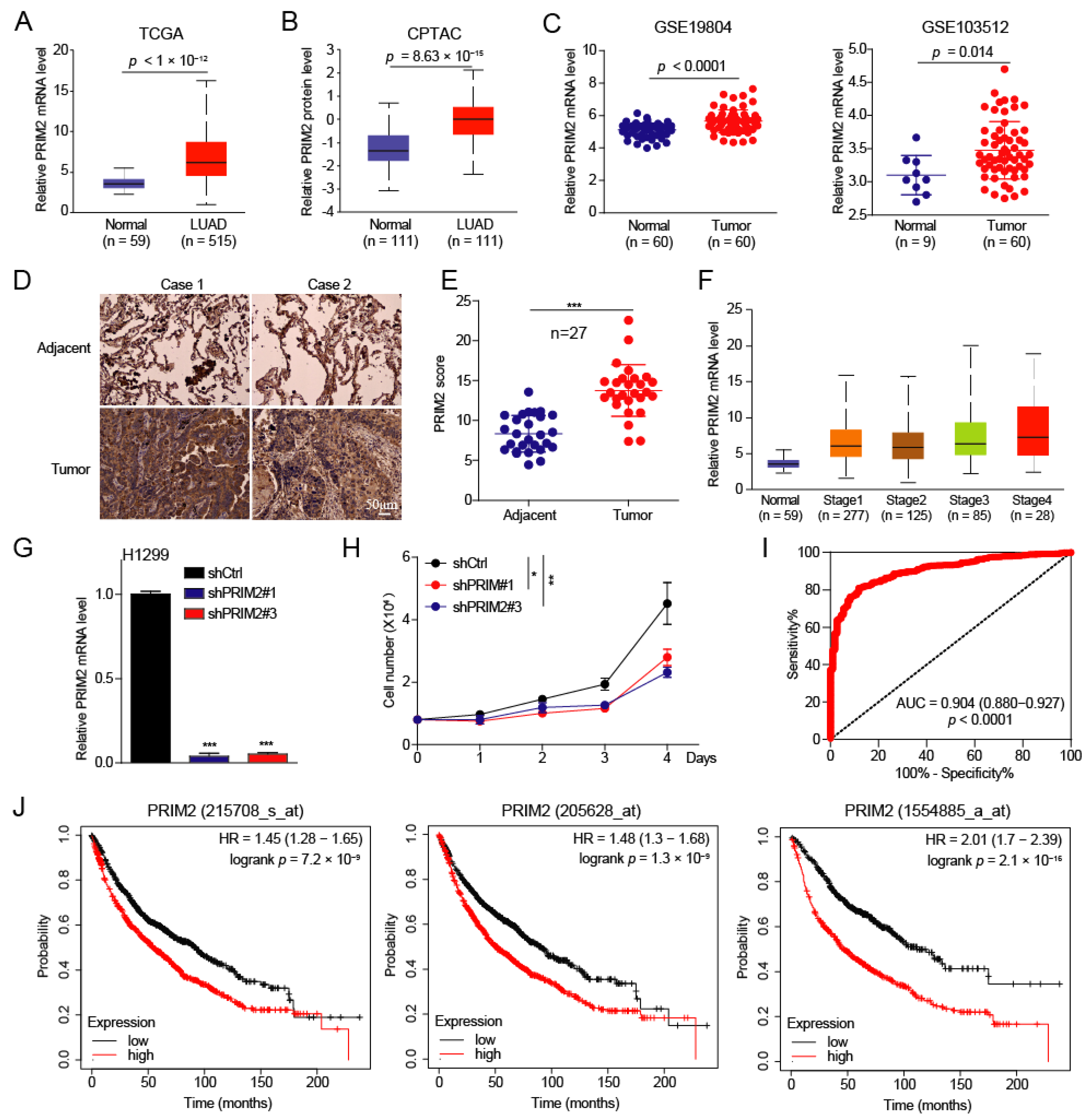
3.1. PRIM2 Is Upregulated in Lung Cancer with Poor Prognosis
3.2. PRIM2 Promotes Cell Cycle, DNA Replication, and Mismatch Repair through PCNA in Lung Cancer
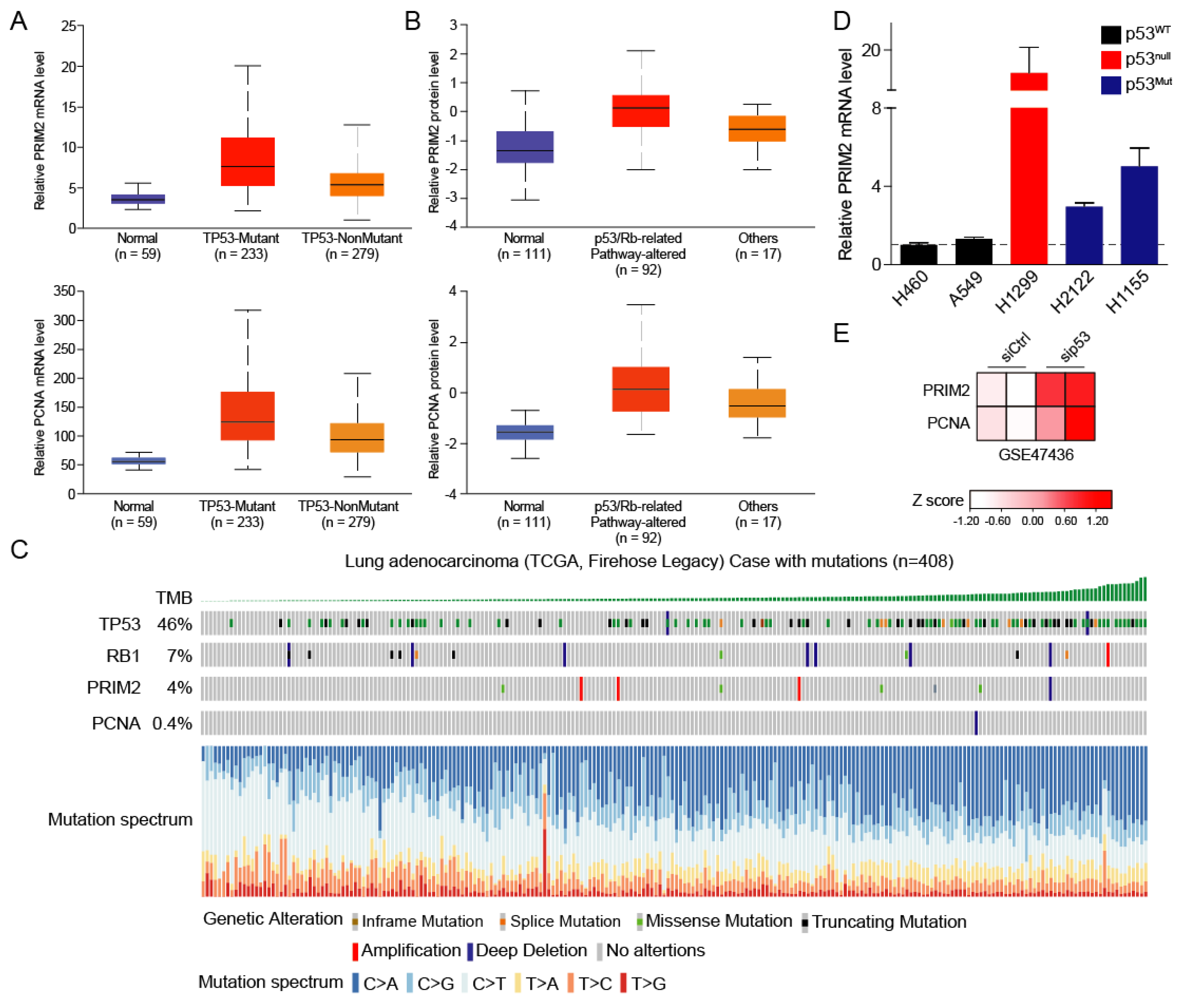
3.3. High PRIM2 Expression Is Dependent on Mutation of p53
3.4. The p53/RB Pathway–PRIM2–PCNA Axis Promotes Cell Growth by Regulating Cell Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre, A.; Ganti, A.K. Lung cancer—A global perspective. J. Surg. Oncol. 2017, 115, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Wadowska, K.; Bil-Lula, I. Genetic Markers in Lung Cancer Diagnosis: A Review. Int. J. Mol. Sci. 2020, 21, 4569. [Google Scholar] [CrossRef]
- Salomaa, E.R. Does the early detection of lung carcinoma improve prognosis? The Turku Study. Cancer 2000, 89, 2387–2391. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Mogi, A.; Kuwano, H. TP53 mutations in nonsmall cell lung cancer. J. Biomed. Biotechnol. 2011, 2011, 583929. [Google Scholar] [CrossRef] [Green Version]
- Hubers, A.J.; Prinsen, C.F.; Sozzi, G.; Witte, B.I.; Thunnissen, E. Molecular sputum analysis for the diagnosis of lung cancer. Br. J. Cancer 2013, 109, 530–537. [Google Scholar] [CrossRef]
- Jiao, X.D.; Qin, B.D.; You, P.; Cai, J.; Zang, Y.S. The prognostic value of TP53 and its correlation with EGFR mutation in advanced non-small cell lung cancer, an analysis based on cBioPortal data base. Lung Cancer 2018, 123, 70–75. [Google Scholar] [CrossRef]
- Viktorsson, K.; De Petris, L.; Lewensohn, R. The role of p53 in treatment responses of lung cancer. Biochem. Biophys. Res. Commun. 2005, 331, 868–880. [Google Scholar] [CrossRef]
- Kaye, F.J. RB and cyclin dependent kinase pathways: Defining a distinction between RB and p16 loss in lung cancer. Oncogene 2002, 21, 6908–6914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederst, M.J.; Sequist, L.V.; Poirier, J.T.; Mermel, C.H.; Lockerman, E.L.; Garcia, A.R.; Katayama, R.; Costa, C.; Ross, K.N.; Moran, T.; et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat. Commun. 2015, 6, 6377. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, J.; Ke, K.; Chen, R.; Zuo, A.; Zhang, R.; Wan, W.; Xie, X.; Li, X.; Song, N.; et al. Polyphyllin I, a lethal partner of Palbociclib, suppresses non-small cell lung cancer through activation of p21/CDK2/Rb pathway in vitro and in vivo. Cell Cycle 2021, 20, 2494–2506. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Mu, R.; Liu, H.; Luo, S. Genetic variants of CHEK1, PRIM2 and CDK6 in the mitotic phase-related pathway are associated with nonsmall cell lung cancer survival. Int. J. Cancer 2021, 149, 1302–1312. [Google Scholar] [CrossRef]
- Yuan, B.; Liao, F.; Shi, Z.Z.; Ren, Y.; Deng, X.L.; Yang, T.T.; Li, D.Y.; Li, R.F.; Pu, D.D.; Wang, Y.J.; et al. Dihydroartemisinin Inhibits the Proliferation, Colony Formation and Induces Ferroptosis of Lung Cancer Cells by Inhibiting PRIM2/SLC7A11 Axis. OncoTargets Ther. 2020, 13, 10829–10840. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Chen, F.; Chandrashekar, D.S.; Varambally, S.; Creighton, C.J. Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers. Nat. Commun. 2019, 10, 5679. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, l1. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.P.; Tsai, M.H.; Lee, J.M.; Hsu, C.P.; Chen, P.C.; Lin, C.W.; Shih, J.Y.; Yang, P.C.; Hsiao, C.K.; Lai, L.C.; et al. Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2590–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer-Visser, J.; Cheng, W.Y.; Bauer-Mehren, A.; Maisel, D.; Lechner, K.; Andersson, E.; Dudley, J.T.; Milletti, F. Regulatory T-cell Genes Drive Altered Immune Microenvironment in Adult Solid Cancers and Allow for Immune Contextual Patient Subtyping. Cancer Epidemiol. Biomark. Prev. 2018, 27, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsubo, C.; Otomo, R.; Miyazaki, M.; Matsushima-Hibiya, Y.; Kohno, T.; Iwakawa, R.; Takeshita, F.; Okayama, H.; Ichikawa, H.; Saya, H.; et al. TSPAN2 is involved in cell invasion and motility during lung cancer progression. Cell Rep. 2014, 7, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangavel, C.; Boopathi, E.; Liu, Y.; Haber, A.; Ertel, A.; Bhardwaj, A.; Addya, S.; Williams, N.; Ciment, S.J.; Cotzia, P.; et al. RB Loss Promotes Prostate Cancer Metastasis. Cancer Res. 2017, 77, 982–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Győrffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, Y.; Sun, M.; Sun, H.; Xie, F.; Chang, H.; Wang, Y.; Song, J.; Lai, S.; Yang, C.; et al. FTO promotes colorectal cancer progression and chemotherapy resistance via demethylating G6PD/PARP1. Clin. Transl. Med. 2022, 12, e772. [Google Scholar] [CrossRef]
- Wang, J.; Chang, H.; Su, M.; Qiao, Y.; Sun, H.; Zhao, Y.; Zhang, S.; Shan, C. Identification of HGD and GSTZ1 as Biomarkers Involved Metabolic Reprogramming in Kidney Renal Clear Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 4583. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Y.; Sun, H.; Chang, H.; Zhao, H.; Zhang, S.; Shan, C. Decreased SLC27A5 Suppresses Lipid Synthesis and Tyrosine Metabolism to Activate the Cell Cycle in Hepatocellular Carcinoma. Biomedicines 2022, 10, 234. [Google Scholar] [CrossRef]
- Li, P.; Wang, Q.; Wang, H. MicroRNA-204 inhibits the proliferation, migration and invasion of human lung cancer cells by targeting PCNA-1 and inhibits tumor growth in vivo. Int. J. Mol. Med. 2019, 43, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, T.; Huang, H.; Jiang, Y.; Yang, L.; Lin, Z.; He, H.; Liu, T.; Wu, B.; Chen, J.; et al. miR-363–3p inhibits tumor growth by targeting PCNA in lung adenocarcinoma. Oncotarget 2017, 8, 20133–20144. [Google Scholar] [CrossRef]


| Gene | Sequence (5′→3′) |
|---|---|
| PRIM2 | AATGCTTCCTACCCTCATTGC |
| AGCTCACTCTCCAACTTACTCTG | |
| PCNA | CCTGCTGGGATATTAGCTCCA |
| CAGCGGTAGGTGTCGAAGC | |
| ACTIN | GGAAATCGTGCGTGACAT |
| TGCCAATGGTGATGACCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Tang, T.; Jiang, Y.; He, T.; Qi, L.; Chang, H.; Qiao, Y.; Sun, M.; Shan, C.; Zhu, X.; et al. PRIM2 Promotes Cell Cycle and Tumor Progression in p53-Mutant Lung Cancer. Cancers 2022, 14, 3370. https://doi.org/10.3390/cancers14143370
Wang T, Tang T, Jiang Y, He T, Qi L, Chang H, Qiao Y, Sun M, Shan C, Zhu X, et al. PRIM2 Promotes Cell Cycle and Tumor Progression in p53-Mutant Lung Cancer. Cancers. 2022; 14(14):3370. https://doi.org/10.3390/cancers14143370
Chicago/Turabian StyleWang, Taoyuan, Tiansheng Tang, Youguo Jiang, Tao He, Luyu Qi, Hongkai Chang, Yaya Qiao, Mingming Sun, Changliang Shan, Xinyuan Zhu, and et al. 2022. "PRIM2 Promotes Cell Cycle and Tumor Progression in p53-Mutant Lung Cancer" Cancers 14, no. 14: 3370. https://doi.org/10.3390/cancers14143370
APA StyleWang, T., Tang, T., Jiang, Y., He, T., Qi, L., Chang, H., Qiao, Y., Sun, M., Shan, C., Zhu, X., Liu, J., & Wang, J. (2022). PRIM2 Promotes Cell Cycle and Tumor Progression in p53-Mutant Lung Cancer. Cancers, 14(14), 3370. https://doi.org/10.3390/cancers14143370






