Genomic Landscape, Clinical Features and Outcomes of Non-Small Cell Lung Cancer Patients Harboring BRAF Alterations of Distinct Functional Classes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Strategies
2.2. Study Population
2.3. Statistical Analysis
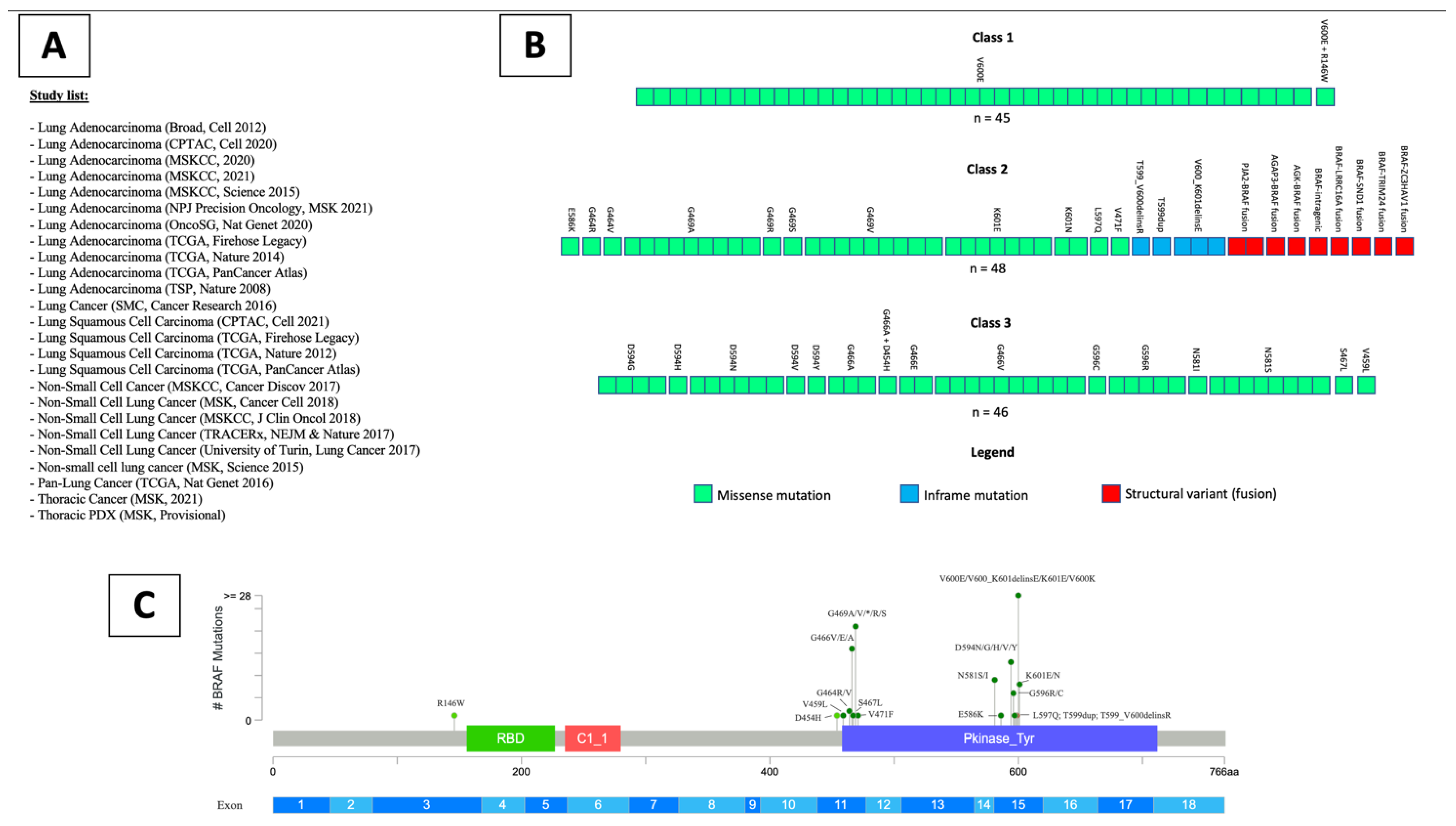
3. Results
3.1. Systematic Review
3.2. Study Population
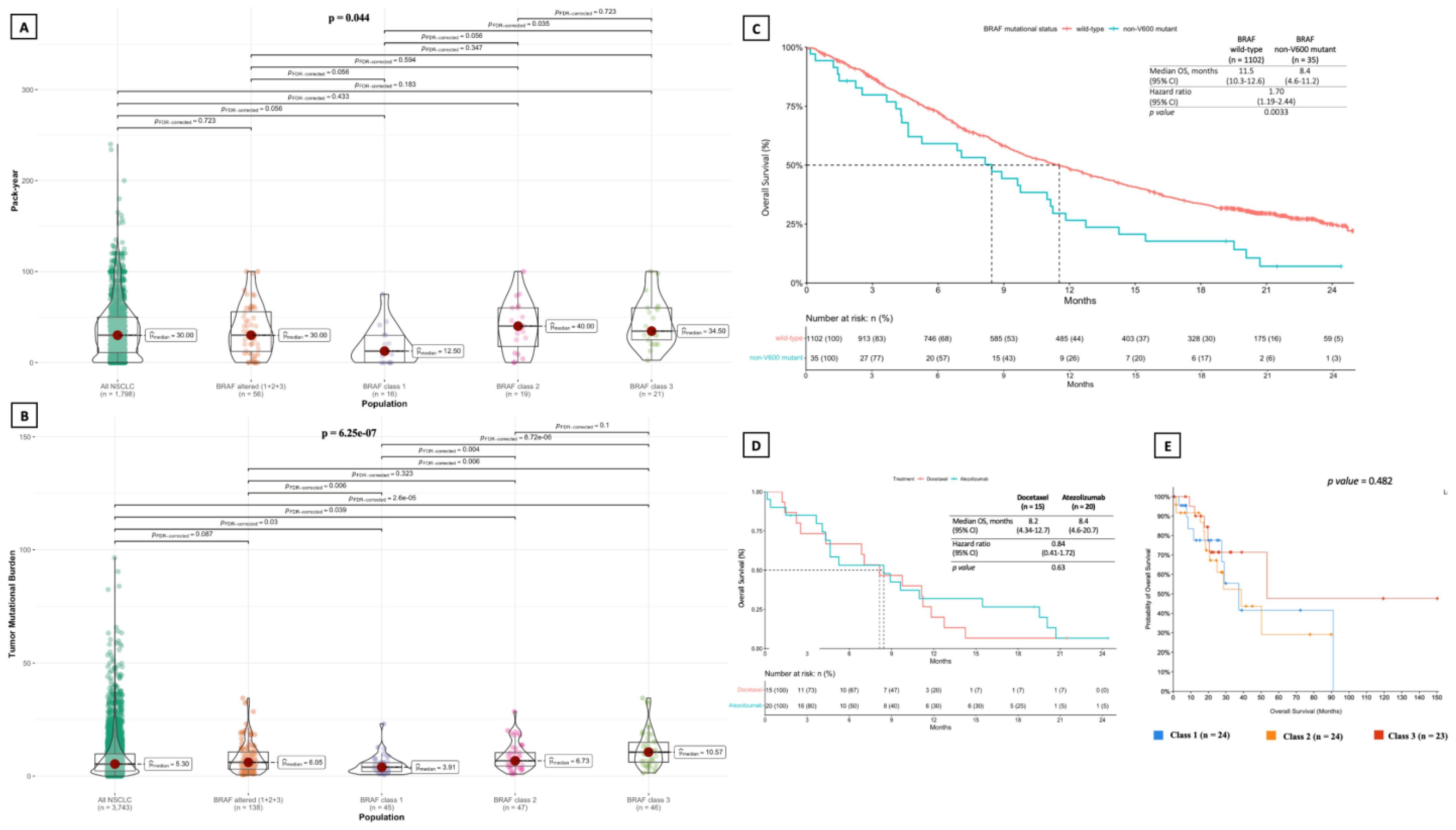
3.2.1. Clinical and Molecular Features and Survival Outcomes
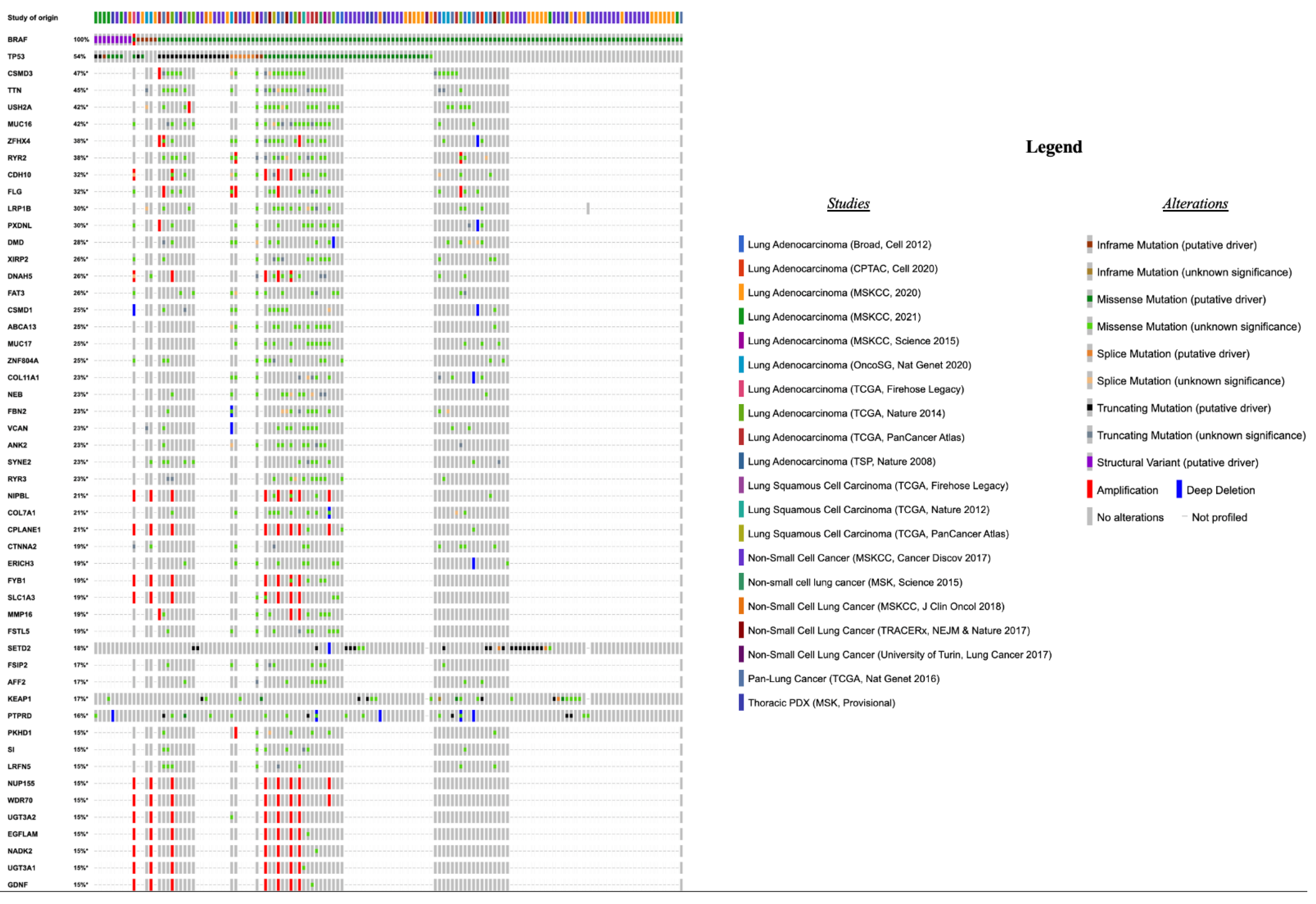
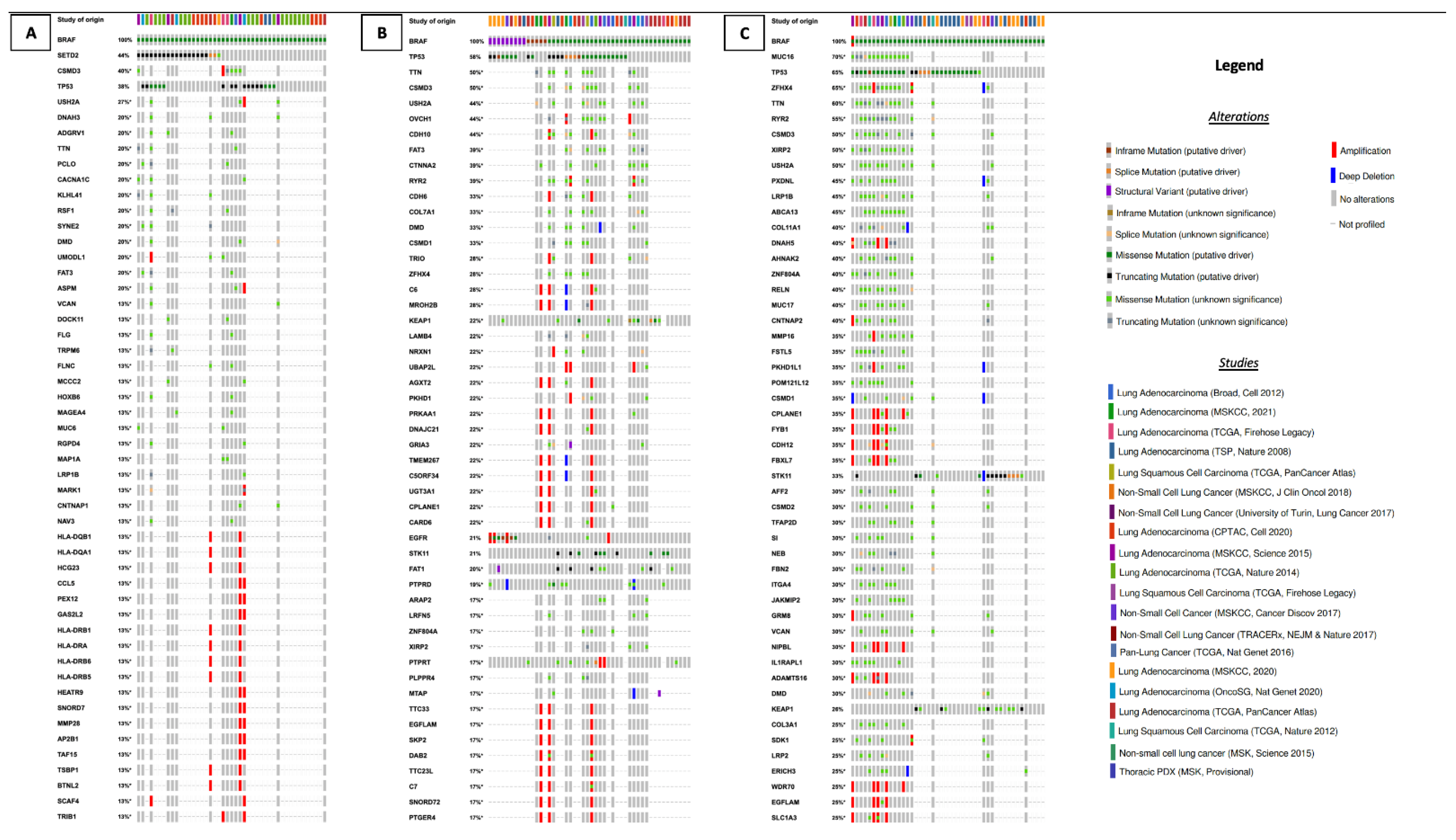
3.2.2. Concurrent Molecular Alterations
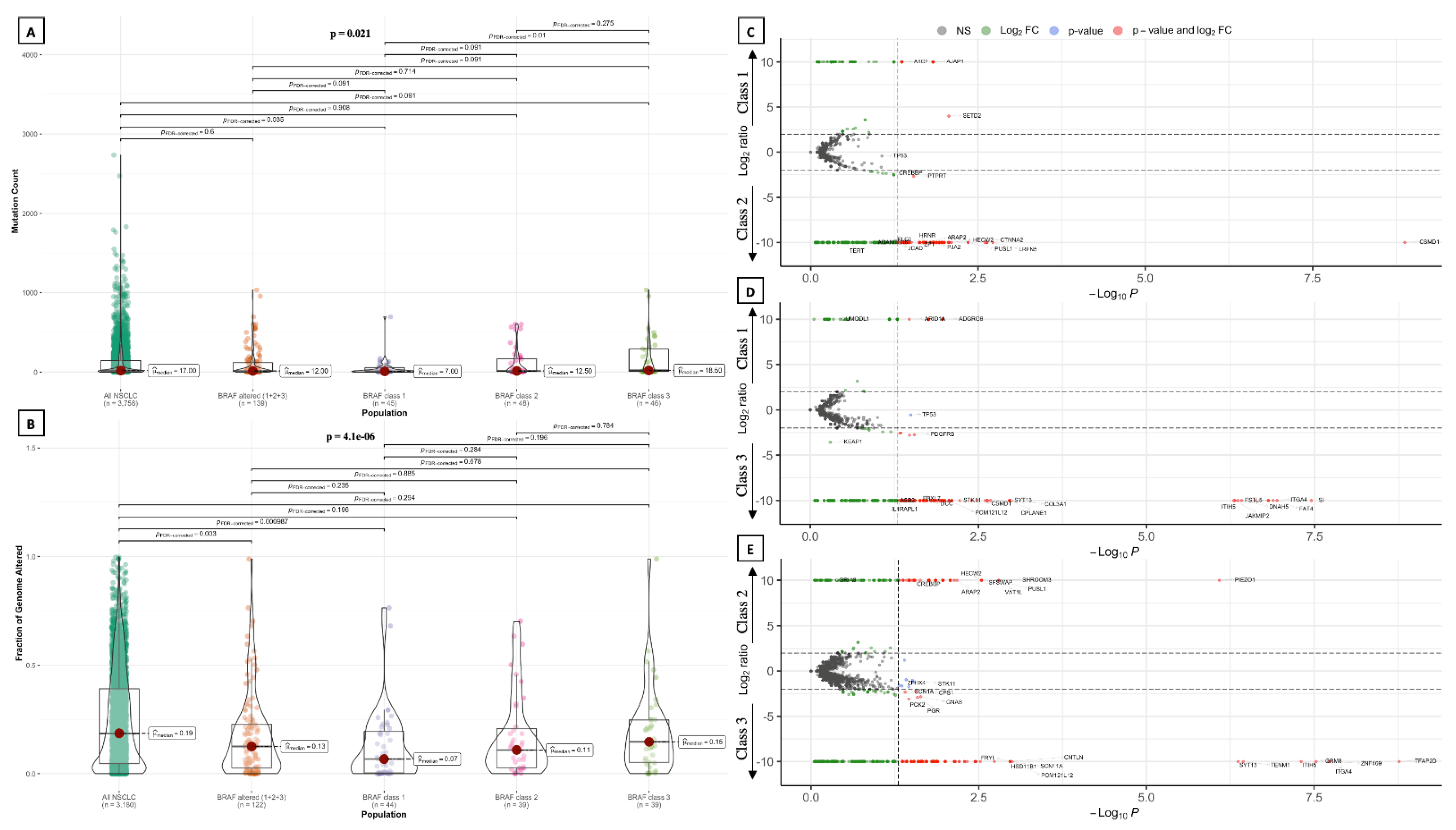
3.2.3. Tumor Mutational Burden, Mutation Count and Fraction of Genome Altered
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 26 January 2022).
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Andrini, E.; Sisi, M.; Rizzo, A.; Parisi, C.; Di Federico, A.; Gelsomino, F.; Ardizzoni, A. Beyond EGFR, ALK and ROS1: Current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit. Rev. Oncol. Hematol. 2020, 156, 103119. [Google Scholar] [CrossRef]
- Paik, P.K.; Arcila, M.E.; Fara, M.; Sima, C.S.; Miller, V.A.; Kris, M.G.; Ladanyi, M.; Riely, G.J. Clinical Characteristics of Patients with Lung Adenocarcinomas Harboring BRAF Mutations. J. Clin. Oncol. 2011, 29, 2046–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonetti, A.; Facchinetti, F.; Rossi, G.; Minari, R.; Conti, A.; Friboulet, L.; Tiseo, M.; Planchard, D. BRAF in non-small cell lung cancer (NSCLC): Pickaxing another brick in the wall. Cancer Treat. Rev. 2018, 66, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Litvak, A.M.; Paik, P.K.; Woo, K.M.; Sima, C.S.; Hellmann, M.D.; Arcila, M.E.; Ladanyi, M.; Rudin, C.M.; Kris, M.G.; Riely, G.J. Clinical Characteristics and Course of 63 Patients with BRAF Mutant Lung Cancers. J. Thorac. Oncol. 2014, 9, 1669–1674. [Google Scholar] [CrossRef] [Green Version]
- Dankner, M.; Rose, A.A.N.; Rajkumar, S.; Siegel, P.M.; Watson, I.R. Classifying BRAF alterations in cancer: New rational therapeutic strategies for actionable mutations. Oncogene 2018, 37, 3183–3199. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Hashemi, S.M.S.; Mazieres, J.; Kim, T.M.; Quoix, E.; Souquet, P.-J.; Barlesi, F.; Baik, C.; et al. Phase 2 Study of Dabrafenib Plus Trametinib in Patients with BRAF V600E-Mutant Metastatic NSCLC: Updated 5-Year Survival Rates and Genomic Analysis. J. Thorac. Oncol. 2022, 17, 103–115. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Souquet, P.-J.; Quoix, E.; Baik, C.S.; Barlesi, F.; Kim, T.M.; Mazieres, J.; Novello, S.; et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial. Lancet Oncol. 2016, 17, 984–993. [Google Scholar] [CrossRef] [Green Version]
- Dankner, M.; Lajoie, M.; Moldoveanu, D.; Nguyen, T.-T.; Savage, P.; Rajkumar, S.; Huang, X.; Lvova, M.; Protopopov, A.; Vuzman, D.; et al. Dual MAPK Inhibition Is an Effective Therapeutic Strategy for a Subset of Class II BRAF Mutant Melanomas. Clin. Cancer Res. 2018, 24, 6483–6494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Negrao, M.V.; Raymond, V.M.; Lanman, R.B.; Robichaux, J.P.; He, J.; Nilsson, M.B.; Ng, P.K.S.; Amador, B.E.; Roarty, E.B.; Nagy, R.J.; et al. Molecular Landscape of BRAF-Mutant NSCLC Reveals an Association between Clonality and Driver Mutations and Identifies Targetable Non-V600 Driver Mutations. J. Thorac. Oncol. 2020, 15, 1611–1623. [Google Scholar] [CrossRef]
- Schirripa, M.; Biason, P.; Lonardi, S.; Pella, N.; Pino, M.S.; Urbano, F.; Antoniotti, C.; Cremolini, C.; Corallo, S.; Pietrantonio, F.; et al. Class 1, 2, and 3 BRAF-Mutated Metastatic Colorectal Cancer: A Detailed Clinical, Pathologic, and Molecular Characterization. Clin. Cancer Res. 2019, 25, 3954–3961. [Google Scholar] [CrossRef] [Green Version]
- Lokhandwala, P.M.; Tseng, L.-H.; Rodriguez, E.; Zheng, G.; Pallavajjalla, A.; Gocke, C.D.; Eshleman, J.R.; Lin, M.-T. Clinical mutational profiling and categorization of BRAF mutations in melanomas using next generation sequencing. BMC Cancer 2019, 19, 665. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, H.; Ding, H.; Qian, J.; Lizaso, A.; Lin, J.; Han-Zhang, H.; Xiang, J.; Li, Y.; Zhu, H. The association between BRAF mutation class and clinical features in BRAF-mutant Chinese non-small cell lung cancer patients. J. Transl. Med. 2019, 17, 298. [Google Scholar] [CrossRef] [Green Version]
- Bracht, J.W.P.; Karachaliou, N.; Bivona, T.; Lanman, R.B.; Faull, I.; Nagy, R.J.; Drozdowskyj, A.; Berenguer, J.; Fernandez-Bruno, M.; Molina-Vila, M.A.; et al. BRAF Mutations Classes I, II, and III in NSCLC Patients Included in the SLLIP Trial: The Need for a New Pre-Clinical Treatment Rationale. Cancers 2019, 11, 1381. [Google Scholar] [CrossRef] [Green Version]
- Owsley, J.; Stein, M.K.; Porter, J.; In, G.K.; Salem, M.; O’Day, S.; Elliott, A.; Poorman, K.; Gibney, G.; VanderWalde, A. Prevalence of class I–III BRAF mutations among 114,662 cancer patients in a large genomic database. Exp. Biol. Med. 2021, 246, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, H.; Ida, C.M.; Halling, K.C.; Kipp, B.R.; Geiersbach, K.; Rumilla, K.M.; Gupta, S.; Lin, M.-T.; Zheng, G. Assessment of RAS Dependency for BRAF Alterations Using Cancer Genomic Databases. JAMA Netw. Open 2021, 4, e2035479. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Yaeger, R.; Rodrik-Outmezguine, V.S.; Tao, A.; Torres, N.M.; Chang, M.T.; Drosten, M.; Zhao, H.; Cecchi, F.; Hembrough, T.; et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017, 548, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Kotani, D.; Mondaca, S.; Parikh, A.R.; Bando, H.; Van Seventer, E.E.; Taniguchi, H.; Zhao, H.; Thant, C.N.; de Stanchina, E.; et al. Response to Anti-EGFR Therapy in Patients with BRAF non-V600–Mutant Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 7089–7097. [Google Scholar] [CrossRef] [Green Version]
- Dagogo-Jack, I.; Martinez, P.; Yeap, B.Y.; Ambrogio, C.; Ferris, L.A.; Lydon, C.; Nguyen, T.; Jessop, N.A.; Iafrate, A.J.; Johnson, B.E.; et al. Impact of BRAF Mutation Class on Disease Characteristics and Clinical Outcomes in BRAF-mutant Lung Cancer. Clin. Cancer Res. 2019, 25, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Osumi, H.; Shinozaki, E.; Wakatsuki, T.; Suenaga, M.; Ichimura, T.; Ogura, M.; Takahari, D.; Ooki, A.; Suzuki, T.; Ota, Y.; et al. Non-V600E BRAF mutations and EGFR signaling pathway in colorectal cancer. Int. J. Cancer 2019, 145, 2488–2495. [Google Scholar] [CrossRef]
- Sheikine, Y.; Pavlick, D.; Klempner, S.J.; Trabucco, S.E.; Chung, J.H.; Rosenzweig, M.; Wang, K.; Velcheti, V.; Frampton, G.M.; Peled, N.; et al. BRAF in Lung Cancers: Analysis of Patient Cases Reveals Recurrent BRAF Mutations, Fusions, Kinase Duplications, and Concurrent Alterations. JCO Precis. Oncol. 2018, 2, PO.17.00172. [Google Scholar] [CrossRef]
- Lobo-Martins, S.; Pais, H.L.; Soares-de-Almeida, L.; Costa, L.; Mansinho, A.; Teixeira de Sousa, R. BRAF L597K mutation: An opportunity to treat. Dermatol. Online J. 2021, 27. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, M.; Wang, W.; Miao, Z.; Hou, P.; Chen, X.; Xu, F.; Zhu, G.; Sun, X.; Li, Y.; et al. Association of the T1799A BRAF mutation with tumor extrathyroidal invasion, higher peripheral platelet counts, and over-expression of platelet-derived growth factor-B in papillary thyroid cancer. Endocr. Relat. Cancer 2008, 15, 183–190. [Google Scholar] [CrossRef]
- Mayank, M.; Kaur, N.; Singh, N. Structural insights and influence of V599 mutations on the overall dynamics of BRAF protein against its kinase domains. Integr. Biol. 2018, 10, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Cañadas-Garre, M.; Fernandez-Escamilla, A.M.; Fernandez-Ballester, G.; Becerra-Massare, P.; García-Calvente, C.; Ramos, J.L.; Llamas-Elvira, J.M. Novel BRAFI599Ins Mutation Identified in a Follicular Variant of Papillary Thyroid Carcinoma: A Molecular Modeling Approach. Endocr. Pract. 2014, 20, e75–e79. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Spies, N.C.; Krysiak, K.; McMichael, J.F.; Coffman, A.C.; Danos, A.M.; Ainscough, B.J.; Ramirez, C.A.; Rieke, D.T.; Kujan, L.; et al. CIViC is a community knowledgebase for expert crowdsourcing the clinical interpretation of variants in cancer. Nat. Genet. 2017, 49, 170–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imielinski, M.; Berger, A.H.; Hammerman, P.S.; Hernandez, B.; Pugh, T.J.; Hodis, E.; Cho, J.; Suh, J.; Capelletti, M.; Sivachenko, A.; et al. Mapping the Hallmarks of Lung Adenocarcinoma with Massively Parallel Sequencing. Cell 2012, 150, 1107–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillette, M.A.; Satpathy, S.; Cao, S.; Dhanasekaran, S.M.; Vasaikar, S.V.; Krug, K.; Petralia, F.; Li, Y.; Liang, W.-W.; Reva, B.; et al. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 2020, 182, 200–225.e35. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yang, H.; Teo, A.S.M.; Amer, L.B.; Sherbaf, F.G.; Tan, C.Q.; Alvarez, J.J.S.; Lu, B.; Lim, J.Q.; Takano, A.; et al. Genomic landscape of lung adenocarcinoma in East Asians. Nat. Genet. 2020, 52, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550, Erratum in Nature 2014, 514, 262. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Getz, G.; Wheeler, D.A.; Mardis, E.R.; McLellan, M.D.; Cibulskis, K.; Sougnez, C.; Greulich, H.; Muzny, D.M.; Morgan, M.B.; et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008, 455, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Um, S.-W.; Joung, J.-G.; Lee, H.; Kim, H.; Kim, K.-T.; Park, J.; Hayes, D.N.; Park, W.-Y. Molecular Evolution Patterns in Metastatic Lymph Nodes Reflect the Differential Treatment Response of Advanced Primary Lung Cancer. Cancer Res. 2016, 76, 6568–6576. [Google Scholar] [CrossRef] [Green Version]
- Satpathy, S.; Krug, K.; Jean Beltran, P.M.; Savage, S.R.; Petralia, F.; Kumar-Sinha, C.; Dou, Y.; Reva, B.; Kane, M.H.; Avanessian, S.C.; et al. A proteogenomic portrait of lung squamous cell carcinoma. Cell 2021, 184, 4348–4371.e40. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012, 489, 519–525, Erratum in Nature 2012, 491, 288. [Google Scholar] [CrossRef] [PubMed]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, E.J.; Kim, H.R.; Arcila, M.E.; Barron, D.; Chakravarty, D.; Gao, J.; Chang, M.T.; Ni, A.; Kundra, R.; Jonsson, P.; et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov. 2017, 7, 596–609. [Google Scholar] [CrossRef] [Green Version]
- Hellmann, M.D.; Nathanson, T.; Rizvi, H.; Creelan, B.C.; Sanchez-Vega, F.; Ahuja, A.; Ni, A.; Novik, J.B.; Mangarin, L.M.B.; Abu-Akeel, M.; et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018, 33, 843–852.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, H.; Sanchez-Vega, F.; La, K.; Chatila, W.; Jonsson, P.; Halpenny, D.; Plodkowski, A.; Long, N.; Sauter, J.L.; Rekhtman, N.; et al. Molecular Determinants of Response to Anti–Programmed Cell Death (PD)-1 and Anti–Programmed Death-Ligand 1 (PD-L1) Blockade in Patients with Non–Small-Cell Lung Cancer Profiled with Targeted Next-Generation Sequencing. J. Clin. Oncol. 2018, 36, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Wilson, G.A.; McGranahan, N.; Birkbak, N.J.; Watkins, T.B.K.; Veeriah, S.; Shafi, S.; Johnson, D.H.; Mitter, R.; Rosenthal, R.; et al. Tracking the Evolution of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef] [Green Version]
- Vavalà, T.; Monica, V.; Lo Iacono, M.; Mele, T.; Busso, S.; Righi, L.; Papotti, M.; Scagliotti, G.V.; Novello, S. Precision medicine in age-specific non-small-cell-lung-cancer patients: Integrating biomolecular results into clinical practice—A new approach to improve personalized translational research. Lung Cancer 2017, 107, 84–90. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Kuziora, M.; Quinn, K.J.; Helman, E.; Ye, J.; Liu, F.; Scheuring, U.; Peters, S.; Rizvi, N.A.; Brohawn, P.Z.; et al. A Blood-based Assay for Assessment of Tumor Mutational Burden in First-line Metastatic NSCLC Treatment: Results from the MYSTIC Study. Clin. Cancer Res. 2021, 27, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Dziadziusko, R.; Peters, S.; Gadgeel, S.; Mathisen, M.S.; Shagan, S.M.; Felip, E.; Morabito, A.; Cheema, P.; Cobo Dols, M.; Andric, Z.; et al. Atezolizumab (atezo) vs. platinum-based chemo in bloodbasedtumour mutational burden-positive (bTMB+) patients(pts) with first-line (1L) advanced/metastatic (m)NSCLC: Results of the Blood First Assay Screening Trial (BFAST)phase III cohort C. Ann. Oncol. 2021, 32, S949–S1039. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Pak, T.; Mondaca, S.; Flynn, J.R.; Montecalvo, J.; Rekhtman, N.; Halpenny, D.; Plodkowski, A.J.; Wu, S.L.; Kris, M.G.; et al. Immune biomarkers and response to checkpoint inhibition of BRAFV600 and BRAF non-V600 altered lung cancers. Br. J. Cancer 2022, 126, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Dudnik, E.; Peled, N.; Nechushtan, H.; Wollner, M.; Onn, A.; Agbarya, A.; Moskovitz, M.; Keren, S.; Popovits-Hadari, N.; Urban, D.; et al. BRAF Mutant Lung Cancer: Programmed Death Ligand 1 Expression, Tumor Mutational Burden, Microsatellite Instability Status, and Response to Immune Check-Point Inhibitors. J. Thorac. Oncol. 2018, 13, 1128–1137. [Google Scholar] [CrossRef] [Green Version]
- Rihawi, K.; Giannarelli, D.; Galetta, D.; Delmonte, A.; Giavarra, M.; Turci, D.; Garassino, M.; Tiseo, M.; Barbieri, F.; Panni, S.; et al. BRAF Mutant NSCLC and Immune Checkpoint Inhibitors: Results From a Real-World Experience. J. Thorac. Oncol. 2019, 14, e57–e59. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, Y.; Shi, C.; Zhou, X.; Xu, K.; Jiao, D.; Sun, Z.; Han, X. A novel immune classification reveals distinct immune escape mechanism and genomic alterations: Implications for immunotherapy in hepatocellular carcinoma. J. Transl. Med. 2021, 19, 5. [Google Scholar] [CrossRef]
- Jones, G.D.; Brandt, W.S.; Shen, R.; Sanchez-Vega, F.; Tan, K.S.; Martin, A.; Zhou, J.; Berger, M.; Solit, D.B.; Schultz, N.; et al. A Genomic-Pathologic Annotated Risk Model to Predict Recurrence in Early-Stage Lung Adenocarcinoma. JAMA Surg. 2021, 156, e205601. [Google Scholar] [CrossRef]
- Mehta, K.R.; Nakao, K.; Zuraek, M.B.; Ruan, D.T.; Bergsland, E.K.; Venook, A.P.; Moore, D.H.; Tokuyasu, T.A.; Jain, A.N.; Warren, R.S.; et al. Fractional Genomic Alteration Detected by Array-Based Comparative Genomic Hybridization Independently Predicts Survival after Hepatic Resection for Metastatic Colorectal Cancer. Clin. Cancer Res. 2005, 11, 1791–1797. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, G.; Atiq, M.; Nandakumar, S.; Mazzu, Y.Z.; Armenia, J.; Yoshikawa, Y.; Khan, N.; Lee, G.-S.M.; Mucci, L.; Kantoff, P.W. Abstract 2534: A comparative analysis of fraction genome altered vs tumor mutational count in prostate cancer. Cancer Res. 2019, 79, 2534. [Google Scholar] [CrossRef]
- Chakraborty, G.; Ghosh, A.; Nandakumar, S.; Armenia, J.; Mazzu, Y.Z.; Atiq, M.O.; Lee, G.-S.M.; Mucci, L.A.; Merghoub, T.; Wolchok, J.D.; et al. Fraction genome altered (FGA) to regulate both cell autonomous and non-cell autonomous functions in prostate cancer and its effect on prostate cancer aggressiveness. J. Clin. Oncol. 2020, 38, 347. [Google Scholar] [CrossRef]
- Marinelli, D.; Mazzotta, M.; Scalera, S.; Terrenato, I.; Sperati, F.; D’Ambrosio, L.; Pallocca, M.; Corleone, G.; Krasniqi, E.; Pizzuti, L.; et al. KEAP1-driven co-mutations in lung adenocarcinoma unresponsive to immunotherapy despite high tumor mutational burden. Ann. Oncol. 2020, 31, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Lopes, G.; Kowalski, D.M.; Kasahara, K.; Wu, Y.-L.; Castro, G.; Turna, H.Z.; Cristescu, R.; Aurora-Garg, D.; Loboda, A.; et al. Abstract CT084: Relationship between STK11 and KEAP1 mutational status and efficacy in KEYNOTE-042: Pembrolizumab monotherapy versus platinum-based chemotherapy as first-line therapy for PD-L1-positive advanced NSCLC. Cancer Res. 2020, 80, CT084. [Google Scholar] [CrossRef]
- Di Federico, A.; De Giglio, A.; Parisi, C.; Gelsomino, F. STK11/LKB1 and KEAP1 mutations in non-small cell lung cancer: Prognostic rather than predictive? Eur. J. Cancer 2021, 157, 108–113. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, Y.; Huang, L.; Ou, W.; Wu, J.; Li, S.; Xu, J.; Feng, J.; Liu, B. TP53 mutation is associated with a poor clinical outcome for non-small cell lung cancer: Evidence from a meta-analysis. Mol. Clin. Oncol. 2016, 5, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Tsao, M.-S.; Aviel-Ronen, S.; Ding, K.; Lau, D.; Liu, N.; Sakurada, A.; Whitehead, M.; Zhu, C.-Q.; Livingston, R.; Johnson, D.H.; et al. Prognostic and Predictive Importance of p53 and RAS for Adjuvant Chemotherapy in Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2007, 25, 5240–5247. [Google Scholar] [CrossRef]
- Custodio, A.B.; González-Larriba, J.L.; Bobokova, J.; Calles, A.; Álvarez, R.; Cuadrado, E.; Manzano, A.; Díaz-Rubio, E. Prognostic and Predictive Markers of Benefit from Adjuvant Chemotherapy in Early-Stage Non-small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 891–910. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.-J.; Kim, D.-S.; Park, W.-J.; Lee, H.; Choi, I.-J.; Park, J.-Y.; Lee, J.-H. Mutation of the TERT promoter leads to poor prognosis of patients with non-small cell lung cancer. Oncol. Lett. 2017, 14, 1609–1614. [Google Scholar] [CrossRef] [Green Version]
- Vinagre, J.; Almeida, A.; Pópulo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.; Sagar, S.; Liu, X.; Lee, H.-R.; Grunkemeyer, J.A.; Grandgenett, P.M.; Caffrey, T.; O’Connell, K.A.; Swanson, B.; Marcos-Silva, L.; et al. Isoforms of MUC16 activate oncogenic signaling through EGF receptors to enhance the progression of pancreatic cancer. Mol. Ther. 2021, 29, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhao, B.; Liu, M.; Wu, L.; Li, Y.; Zhai, Y.; Shen, X. Pan-cancer analysis of SETD2 mutation and its association with the efficacy of immunotherapy. npj Precis. Oncol. 2021, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-H.; Jang, S.J.; Kim, J.; Sohn, I.; Lee, J.-Y.; Cho, E.J.; Chun, S.-M.; Sung, C.O. Spontaneous mutations in the single TTN gene represent high tumor mutation burden. npj Genomic Med. 2020, 5, 33. [Google Scholar] [CrossRef]
- Lu, N.; Liu, J.; Xu, M.; Liang, J.; Wang, Y.; Wu, Z.; Xing, Y.; Diao, F. CSMD3 is Associated with Tumor Mutation Burden and Immune Infiltration in Ovarian Cancer Patients. Int. J. Gen. Med. 2021, Volume 14, 7647–7657. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Yao, W.; Liu, X.; Yang, Y.; Ma, B.; Xue, D. USH2A Mutation is Associated with Tumor Mutation Burden and Antitumor Immunity in Patients with Colon Adenocarcinoma. Front. Genet. 2021, 12, 762160. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Jiao, D.; Guo, C.; Wang, L.; Li, Z.; Sun, Z.; Zhao, Y.; Han, X. Association of RYR2 Mutation with Tumor Mutation Burden, Prognosis, and Antitumor Immunity in Patients with Esophageal Adenocarcinoma. Front. Genet. 2021, 12, 669694. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Andrini, E.; Sisi, M.; Federico, A.D.; Ricciuti, B. Targeting DNA damage response and repair genes to enhance anticancer immunotherapy: Rationale and clinical implication. Futur. Oncol. 2020, 16, 1751–1766. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Total (n = 236) | Class 1 (n = 45) | Class 2 (n = 48) | Class 3 (n = 46) | Undefined Class (n = 97) | p Value |
|---|---|---|---|---|---|---|
| Age, Mean (SD) | 66.0 (9.4) | 66.6 (10.6) | 68.5 (9.6) | 65.7 (8.8) | 64.8 (8.9) | 0.243 |
| Sex, n (%) | ||||||
| Female | 118 (51.5) | 30 (68.2) | 22 (47.8) | 27 (58.7) | 39 (41.9) | 0.023 |
| Male | 111 (48.5) | 14 (31.8) | 24 (52.2) | 19 (41.3) | 54 (58.1) | |
| Histology, n (%) | ||||||
| Adenocarcinoma | 205 (86.9) | 45 (100.0) | 43 (89.6) | 41 (89.1) | 76 (78.4) | 0.005 |
| Squamous | 23 (9.7) | 0 (0) | 2 (4.2) | 3 (6.5) | 18 (18.6) | |
| Non-Small Cell Lung Cancer NOS | 8 (3.4) | 0 (0) | 3 (6.2) | 2 (4.3) | 3 (3.1) | |
| Geographical origin, n (%) | ||||||
| Caucasian | 65 (73.9) | 5 (45.5) | 13 (65.0) | 12 (100.0) | 35 (77.8) | 0.059 |
| Asian | 17 (19.3) | 5 (45.5) | 6 (30.0) | 0 (0) | 6 (13.3) | |
| African | 6 (6.8) | 1 (9.1) | 1 (5.0) | 0 (0) | 4 (8.9) | |
| Smoking habit, n (%) | ||||||
| Yes | 146 (83.4) | 21 (56.8) | 31 (93.9) | 36 (92.3) | 58 (87.9) | <0.001 |
| No | 29 (16.6) | 16 (43.2) | 2 (6.1) | 3 (7.7) | 8 (12.1) |
| Clinical Characteristics | Total (n = 35) | Class 2 (n = 12) | Class 3 (n = 10) | Undefined Class (n = 13) | p Value |
|---|---|---|---|---|---|
| Age, Mean (SD) | 64.1 (9.2) | 65.1 (7.5) | 65.4 (5.7) | 62.1 (12.5) | 0.415 |
| Sex, n (%) | |||||
| Female | 14 (40.0) | 6 (50.0) | 3 (30.0) | 5 (38.5) | 0.581 |
| Male | 21 (60.0) | 6 (50.0) | 7 (70.0) | 8 (61.5) | |
| Histology, n (%) | |||||
| Adenocarcinoma | 26 (74.3) | 10 (83.3) | 9 (90.0) | 7 (53.8) | 0.091 |
| Squamous | 9 (25.7) | 2 (16.7) | 1 (10.0) | 6 (46.2) | |
| Geographical origin, n (%) | |||||
| Caucasian | 27 (77.1) | 9 (75.0) | 7 (70.0) | 11 (84.6) | 0.669 |
| Asian | 6 (17.1) | 2 (16.7) | 2 (20.0) | 2 (15.4) | |
| Other | 2 (5.7) | 1 (8.3) | 1 (10.0) | 0 (0) | |
| Smoking habit, n (%) | |||||
| Yes | 33 (94.3) | 12 (100) | 9 (90.0) | 12 (92.3) | 0.431 |
| No | 2 (5.7) | 0 (0) | 1 (10.0) | 1 (7.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Federico, A.; De Giglio, A.; Gelsomino, F.; De Biase, D.; Giunchi, F.; Palladini, A.; Sperandi, F.; Melotti, B.; Ardizzoni, A. Genomic Landscape, Clinical Features and Outcomes of Non-Small Cell Lung Cancer Patients Harboring BRAF Alterations of Distinct Functional Classes. Cancers 2022, 14, 3472. https://doi.org/10.3390/cancers14143472
Di Federico A, De Giglio A, Gelsomino F, De Biase D, Giunchi F, Palladini A, Sperandi F, Melotti B, Ardizzoni A. Genomic Landscape, Clinical Features and Outcomes of Non-Small Cell Lung Cancer Patients Harboring BRAF Alterations of Distinct Functional Classes. Cancers. 2022; 14(14):3472. https://doi.org/10.3390/cancers14143472
Chicago/Turabian StyleDi Federico, Alessandro, Andrea De Giglio, Francesco Gelsomino, Dario De Biase, Francesca Giunchi, Arianna Palladini, Francesca Sperandi, Barbara Melotti, and Andrea Ardizzoni. 2022. "Genomic Landscape, Clinical Features and Outcomes of Non-Small Cell Lung Cancer Patients Harboring BRAF Alterations of Distinct Functional Classes" Cancers 14, no. 14: 3472. https://doi.org/10.3390/cancers14143472
APA StyleDi Federico, A., De Giglio, A., Gelsomino, F., De Biase, D., Giunchi, F., Palladini, A., Sperandi, F., Melotti, B., & Ardizzoni, A. (2022). Genomic Landscape, Clinical Features and Outcomes of Non-Small Cell Lung Cancer Patients Harboring BRAF Alterations of Distinct Functional Classes. Cancers, 14(14), 3472. https://doi.org/10.3390/cancers14143472










