Clinical Implication of Circulating Tumor Cells Expressing Epithelial Mesenchymal Transition (EMT) and Cancer Stem Cell (CSC) Markers and Their Perspective in HCC: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Data Sources
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. EMT-CTCs Phenotype
3.2.1. Types of EMT Markers on CTCs in HCC
3.2.2. Association of EMT-CTC Subtypes with Clinicopathological Factors in Preoperative Studies
3.2.3. Association of EMT-CTC Subtypes with Clinicopathological Factors in Longitudinal and Postoperative Studies
3.2.4. Pooled Data from All EMT-CTC Subtype Analysis Reporting on Prognostic Factors for Relapse after the Curative Resection and Meta-Analysis Results
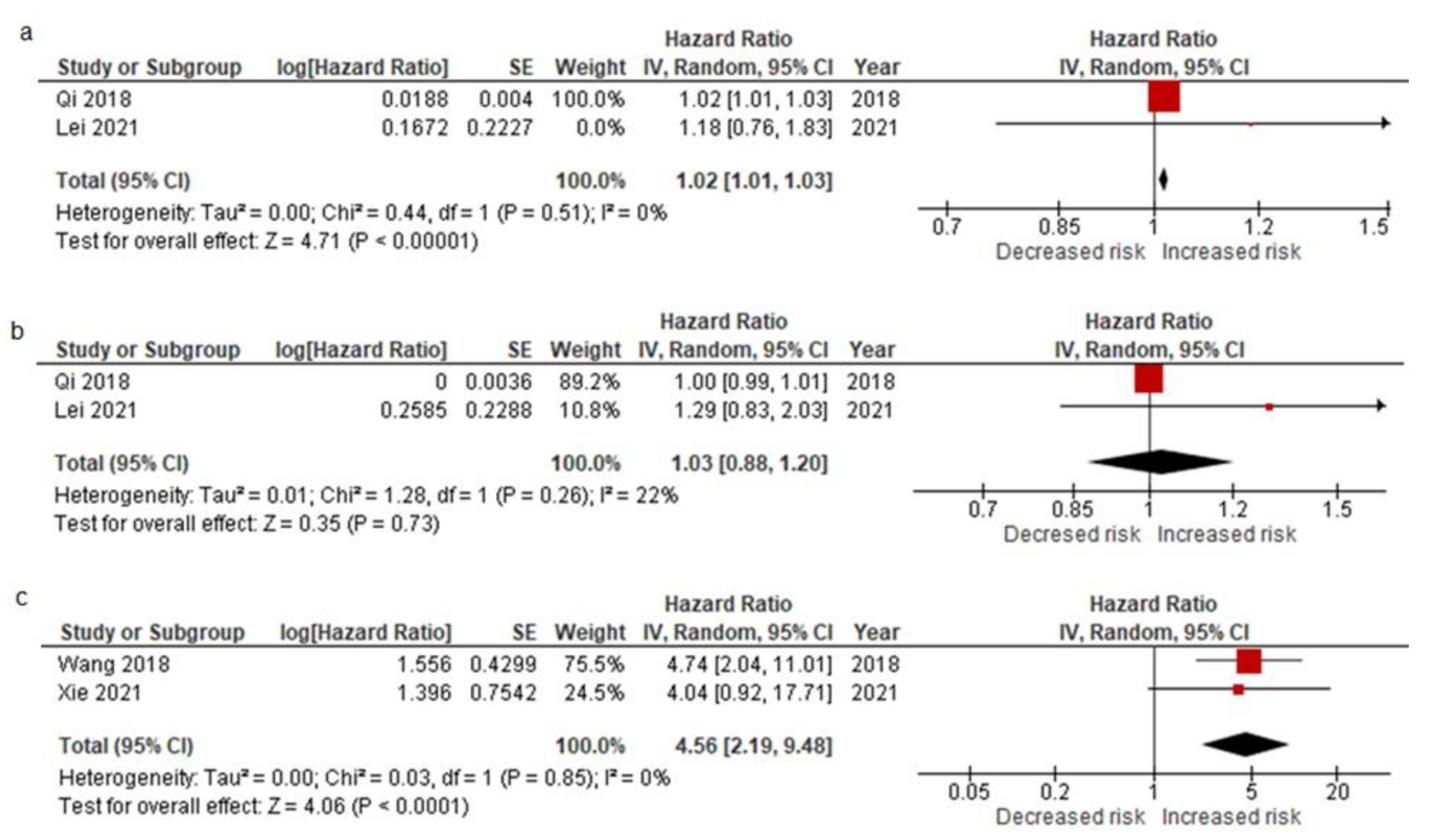
| Study | Preoperative Analysis | Postoperative Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Schulze, 2013 [20] | Court, 2018 [23] | Ou, 2018 [24] | Qi, 2018 [25] | Chen, 2019 [28] | Bai, 2020 [29] | Qi, 2020 [30] | Lei, 2021 [31] | Wang, 2018 [26] | Xie, 2021 [32] | ||
| Epithelial-CTCs | ||||||||||||
| TFS | Median | pos | 10 | |||||||||
| neg | 8 | |||||||||||
| p-value | 0.6745 | |||||||||||
| ER | HR | 1.000 | 1.295 | |||||||||
| 95% CI | 0.993–1.007 | 0.827–2.026 | ||||||||||
| p-value | 0.970 | 0.258 | ||||||||||
| OS | Median | pos | 15.3 | |||||||||
| neg | 24.9 | |||||||||||
| p-value | 0.017 | |||||||||||
| TTR RFS | HR | 1.446 | ||||||||||
| 95% CI | 0.667–3.133 | |||||||||||
| p-value | 0.006 | |||||||||||
| Means/SD | pos | 11.32 ± 2.83 | ||||||||||
| neg | 12.7 ± 3.1 | |||||||||||
| p-value | 0.523 | |||||||||||
| Hybrid-CTCs | ||||||||||||
| TFS | Median | pos | 6 | 7 | ||||||||
| neg | 7 | 24.5 | ||||||||||
| p-value | 0.692 | 0.003 | ||||||||||
| ER | HR | 1.068 | 2.935 | |||||||||
| 95% CI | 0.577–1.976 | 1.306–6.594 | ||||||||||
| p-value | 0.835 | 0.009 | ||||||||||
| TTR RFS | HR | 2.368 | ||||||||||
| 95% CI | 0.808–6.937 | |||||||||||
| p-value | 0.006 | |||||||||||
| Median | pos | 14 | ||||||||||
| neg | NR | |||||||||||
| p-value | 0.006 | |||||||||||
| Means/SD | pos | 12.14 ± 2.29 | ||||||||||
| neg | 10.82 ± 4.42 | |||||||||||
| p-value | 0.638” | |||||||||||
| Mesenchymal-CTCs | ||||||||||||
| PFS TFS | HR | 2.16 * | ||||||||||
| 95% CI | 1.38–4.42 | |||||||||||
| p-value | 0.002 | |||||||||||
| Median | pos | 5 | 5 | |||||||||
| neg | 13.3 | 17 | ||||||||||
| p-value | 0.009 | <0.0001 | ||||||||||
| ER | HR | 1.019 1.019 * | 1.182 | 4.740 3.453 * | 4.039 | |||||||
| 95% CI | 1.010–1.027 1.006–1.032 * | 0.764–1.83 | 2.041–11.01 1.393–8.559 * | 0.921–17.703 | ||||||||
| p-value | <0.001 0.003 * | 0.452 | <0.001 | 0.064 | ||||||||
| OS | HR | 2.21 * | ||||||||||
| 95% CI | 1.38–3.56 | |||||||||||
| p-value | 0.001 | |||||||||||
| TTR RFS | HR | 3.14 | 4.546 | |||||||||
| 95% CI | 1.50–6.57 | 2.203–9.381 | ||||||||||
| p-value | 0.002 | 0.006 | ||||||||||
| Median | pos | 6.4 | ||||||||||
| neg | NR | |||||||||||
| p-value | <0.006 | |||||||||||
| Means/ SD | pos | 9.21 ± 3.16 | ||||||||||
| neg | 13.8 ± 2.6 | |||||||||||
| p-value | 0.654 | |||||||||||
3.3. CCSCs Phenotype
3.3.1. Types of CSC Markers on CTCs in HCC
3.3.2. The Clinical Significance of CCSCs Subtype Associated with Clinicopathological Factors, Metastasis, and Recurrence
3.3.3. Pooled Data from CCSC Subtype Analysis Reporting on Prognostic Factors for Relapse after the Curative Resection
4. Discussion
Expert Opinion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Parikh, N.D.; Fidelman, N.; Owen, D. Frontiers of therapy for hepatocellular carcinoma. Abdom. Radiol. 2021, 46, 3648–3659. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D. PD-L1, TMB, and other potential predictors of response to immunotherapy for hepatocellular carcinoma: How can they assist drug clinical trials? Expert Opin. Investig. Drugs 2022, 31, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A. Invasion patterns and metastatic patterns of hepatocellular carcinoma. In Tumors and Tumor-Like Lesions of the Hepatobiliary Tract: General and Surgical Pathology; Zimmermann, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 91–119. [Google Scholar]
- Carissimi, F.; Barbaglia, M.N.; Salmi, L.; Ciulli, C.; Roccamatisi, L.; Cordaro, G.; Mallela, V.R.; Minisini, R.; Leone, B.E.; Donadon, M.; et al. Finding the seed of recurrence: Hepatocellular carcinoma circulating tumor cells and their potential to drive the surgical treatment. World J. Gastrointest. Surg. 2021, 13, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Buscail, E.; Chiche, L.; Laurent, C.; Vendrely, V.; Denost, Q.; Denis, J.; Thumerel, M.; Lacorte, J.M.; Bedel, A.; Moreau-Gaudry, F.; et al. Tumor-proximal liquid biopsy to improve diagnostic and prognostic performances of circulating tumor cells. Mol. Oncol. 2019, 13, 1811–1826. [Google Scholar] [CrossRef]
- Wan, C.; Zhou, B. Research progress on circulating tumor cells of hepatocellular carcinoma. J. Interv. Med. 2021, 4, 181–183. [Google Scholar] [CrossRef]
- Zhang, Q.; Rong, Y.; Yi, K.; Huang, L.; Chen, M.; Wang, F. Circulating tumor cells in hepatocellular carcinoma: Single-cell based analysis, preclinical models, and clinical applications. Theranostics 2020, 10, 12060–12071. [Google Scholar] [CrossRef]
- Giannelli, G.; Koudelkova, P.; Dituri, F.; Mikulits, W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J. Hepatol. 2016, 65, 798–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Li, J.; Cadilha, B.L.; Markota, A.; Voigt, C.; Huang, Z.; Lin, P.P.; Wang, D.D.; Dai, J.; Kranz, G.; et al. Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of metastasis. Sci. Adv. 2019, 5, eaav4275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnoletto, C.; Corrà, F.; Minotti, L.; Baldassari, F.; Crudele, F.; Cook, W.J.J.; Di Leva, G.; d’Adamo, A.P.; Gasparini, P.; Volinia, S. Heterogeneity in circulating tumor cells: The relevance of the stem-cell subset. Cancers 2019, 11, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayoun, T.; Faugeroux, V.; Oulhen, M.; Aberlenc, A.; Pawlikowska, P.; Farace, F. CTC-derived models: A window into the seeding capacity of circulating tumor cells (CTCs). Cells 2019, 8, 1145. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Tiede, B.; Massagué, J.; Kang, Y. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res. 2007, 17, 3–14. [Google Scholar] [CrossRef]
- Toss, A.; Mu, Z.; Fernandez, S.; Cristofanilli, M. CTC enumeration and characterization: Moving toward personalized medicine. Ann. Transl. Med. 2014, 2, 108. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Li, Y.-M.; Xu, S.-C.; Li, J.; Han, K.-Q.; Pi, H.-F.; Zheng, L.; Zuo, G.-H.; Huang, X.-B.; Li, H.-Y.; Zhao, H.-Z.; et al. Epithelial–mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013, 4, e831. [Google Scholar] [CrossRef]
- Schulze, K.; Gasch, C.; Staufer, K.; Nashan, B.; Lohse, A.W.; Pantel, K.; Riethdorf, S.; Wege, H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int. J. Cancer 2013, 133, 2165–2171. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Hu, B.-S.; Li, Z.-L.; He, X.; Li, Y.; Lu, L.-G. An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hepatol. Int. 2016, 10, 640–646. [Google Scholar] [CrossRef]
- Chen, J.; Cao, S.-W.; Cai, Z.; Zheng, L.; Wang, Q. Epithelial-mesenchymal transition phenotypes of circulating tumor cells correlate with the clinical stages and cancer metastasis in hepatocellular carcinoma patients. Cancer Biomark. 2017, 20, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Court, C.M.; Hou, S.; Winograd, P.; Segel, N.H.; Li, Q.W.; Zhu, Y.; Sadeghi, S.; Finn, R.S.; Ganapathy, E.; Song, M.; et al. A novel multimarker assay for the phenotypic profiling of circulating tumor cells in hepatocellular carcinoma. Liver Transplant. 2018, 24, 946–960. [Google Scholar] [CrossRef]
- Ou, H.; Huang, Y.; Xiang, L.; Chen, Z.; Fang, Y.; Lin, Y.; Cui, Z.; Yu, S.; Li, X.; Yang, D. Circulating tumor cell phenotype indicates poor survival and recurrence after surgery for hepatocellular carcinoma. Am. J. Dig. Dis. 2018, 63, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-N.; Xiang, B.-D.; Wu, F.-X.; Ye, J.-Z.; Zhong, J.-H.; Wang, Y.-Y.; Chen, Y.-Y.; Chen, Z.-S.; Ma, L.; Chen, J.; et al. Circulating tumor cells undergoing EMT provide a metric for diagnosis and prognosis of patients with hepatocellular carcinoma. Cancer Res. 2018, 78, 4731–4744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Luo, L.; Cheng, Y.; He, G.; Peng, B.; Gao, Y.; Jiang, Z.-S.; Pan, M.X. Correlation between postoperative early recurrence of hepatocellular carcinoma and mesenchymal circulating tumor cells in peripheral blood. J. Gastrointest. Surg. 2018, 22, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.-C.; Luo, Z.-C.; Gao, Y.-X.; Li, Y.; Peng, Q.; Gao, Y. Twist expression in circulating hepatocellular carcinoma cells predicts metastasis and prognoses. BioMed Res. Int. 2018, 2018, 3789613. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Li, W.; Yang, R.; Zhang, X.; Ye, Y.; Yu, J.; Ye, L.; Tang, W. Circulating tumor cells undergoing EMT are poorly correlated with clinical stages or predictive of recurrence in hepatocellular carcinoma. Sci. Rep. 2019, 9, 7084. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Mai, R.; Ye, J.; Chen, J.; Qi, L.; Tang, J.; Wei, M.; Zhang, L.; Chen, Z.; Tang, Z.; et al. Circulating tumor cells and CXCR4 in the prognosis of hepatocellular carcinoma. Transl. Cancer Res. 2020, 9, 1384–1394. [Google Scholar] [CrossRef]
- Qi, L.-N.; Ma, L.; Chen, Y.-Y.; Chen, Z.-S.; Zhong, J.-H.; Gong, W.-F.; Lu, Y.; Xiang, B.-D.; Li, L.-Q. Outcomes of anatomical versus non-anatomical resection for hepatocellular carcinoma according to circulating tumour-cell status. Ann. Med. 2020, 52, 21–31. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, X.; Sun, H.; Fu, Y.; Tian, Y.; Yang, L.; Wang, J.; Xia, F. Association of preoperative NANOG-positive circulating tumor cell levels with recurrence of hepatocellular carcinoma. Front. Oncol. 2021, 11, 730. [Google Scholar] [CrossRef]
- Xie, Y.-L.; Yang, Z.; Feng, X.; Yang, Q.; Ye, L.-S.; Li, X.-B.; Tang, H.; Zhang, Y.-C.; Liu, W.; Zhang, T.; et al. Association of phenotypic transformation of circulating tumor cells and early recurrence in patients with hepatocellular carcinoma following liver transplantation. Asian J. Surg. 2021, 45, 435–440. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, W.; Zhang, J.; Hu, J.; Qi, L.; Xiang, B. Circulating tumor cells undergoing the epithelial–mesenchymal transition: Influence on prognosis in cytokeratin 19-positive hepatocellular carcinoma. OncoTargets Ther. 2021, 14, 1543–1552. [Google Scholar] [CrossRef]
- Fan, S.T.; Yang, Z.F.; Ho, D.W.Y.; Ng, M.N.P.; Yu, W.C.; Wong, J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: A prospective study. Ann. Surg. 2011, 254, 569–576. [Google Scholar] [CrossRef]
- Liu, S.; Li, N.; Yu, X.; Xiao, X.; Cheng, K.; Hu, J.; Wang, J.; Zhang, D.; Cheng, S.; Liu, S. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology 2013, 144, 1031–1041.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Sun, Y.-F.; Shen, M.-N.; Ma, X.-L.; Wu, J.; Zhang, C.-Y.; Zhou, Y.; Xu, Y.; Hu, B.; Zhang, M.; et al. Circulating tumor cells with stem-like phenotypes for diagnosis, prognosis, and therapeutic response evaluation in hepatocellular carcinoma. Clin. Cancer Res. 2018, 24, 2203–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, S.; Kim, T.H.; Smith, K.J.; Delaney, R.; Park, G.-S.; Guo, H.; Lin, E.; Plegue, T.; Kuo, N.; Steffes, J.; et al. New labyrinth microfluidic device detects circulating tumor cells expressing cancer stem cell marker and circulating tumor microemboli in hepatocellular carcinoma. Sci. Rep. 2019, 9, 18575. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, Y.; Liu, Q.; Zhou, K.; Zhao, W.; Liu, S.; Yang, J.; Jiang, Y.; Sui, G. Rapid detection of hepatocellular carcinoma metastasis using reverse transcription loop-mediated isothermal amplification. Talanta 2019, 208, 120402. [Google Scholar] [CrossRef]
- Chan, M.Y.; She, W.H.; Dai, W.C.; Tsang, S.H.Y.; Chok, K.S.H.; Chan, A.C.Y.; Fung, J.; Lo, C.M.; Cheung, T.T. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy- an analysis of 1,182 patients in Hong Kong. Transl. Gastroenterol. Hepatol. 2019, 4, 52. [Google Scholar] [CrossRef]
- Chen, H.-L.; Chen, Y.-H.; Du, L.; Song, Y.-P.; Zhu, B. Elevated serum alpha-fetoprotein levels are associated with poor prognosis of hepatocellular carcinoma after surgical resection: A systematic review and meta-analysis. Arab J. Gastroenterol. 2021, 22, 12–22. [Google Scholar] [CrossRef]
- Li, T.; Qin, L.X.; Gong, X.; Zhou, J.; Sun, H.C.; Qiu, S.J.; Ye, Q.H.; Wang, L.; Fan, J. Hepatitis B virus surface antigen-negative and hepatitis C virus antibody-negative hepatocellular carcinoma: Clinical characteristics, outcome, and risk factors for early and late intrahepatic recurrence after resection. Cancer 2013, 119, 126–135. [Google Scholar] [CrossRef]
- Giannini, E.G.; Marenco, S.; Borgonovo, G.; Savarino, V.; Farinati, F.; Del Poggio, P.; Rapaccini, G.L.; Anna Di Nolfo, M.; Benvegnù, L.; Zoli, M.; et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology 2012, 56, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-L.; Yang, Y.-F.; Yuan, C.-H.; Chen, H.; Wang, F.-B. Circulating tumor cells for predicting the prognostic of patients with hepatocellular carcinoma: A meta analysis. Cell. Physiol. Biochem. 2015, 37, 629–640. [Google Scholar] [CrossRef]
- Chen, V.L.; Xu, D.; Wicha, M.S.; Lok, A.S.; Parikh, N.D. Utility of liquid biopsy analysis in detection of hepatocellular carcinoma, determination of prognosis, and disease monitoring: A systematic review. Clin. Gastroenterol. Hepatol. 2020, 18, 2879–2902.e9. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Ou, Y.; Shen, Y.; Li, S.; Sun, Z. Clinical value of circulating tumor cells for the diagnosis and prognosis of hepatocellular carcinoma (HCC): A systematic review and meta-analysis. Medicine 2020, 99, e22242. [Google Scholar] [CrossRef]
- Knights, A.J.; Funnell, A.P.W.; Crossley, M.; Pearson, R.C.M. Holding tight: Cell junctions and cancer spread. Trends Cancer Res. 2012, 8, 61–69. [Google Scholar]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- McDonald, D.M.; Baluk, P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002, 62, 5381–5385. [Google Scholar]
- Leonard, F.; Godin, B. Unraveling bio-social hierarchy in cancer collective invasion: Symbiosis between leaders and followers. Transl. Cancer Res. 2017, 6, S1084–S1087. [Google Scholar] [CrossRef]
- Celià-Terrassa, T.; Meca-Cortés, O.; Mateo, F.; de Paz, A.M.; Rubio, N.; Arnal-Estapé, A.; Ell, B.J.; Bermudo, R.; Díaz, A.; Guerra-Rebollo, M.; et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J. Clin. Investig. 2012, 122, 1849–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banyard, J.; Bielenberg, D.R. The role of EMT and MET in cancer dissemination. Connect. Tissue Res. 2015, 56, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paizal, J.P.; Au, S.H.; Bakal, C. Squeezing through the microcirculation: Survival adaptations of circulating tumour cells to seed metastasis. Br. J. Cancer 2021, 124, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Wang, X.; Lu, C.; Ma, W.; Wang, Y.; Xia, G.; Wang, C.; Yang, G. Cancer stem-like cells with hybrid epithelial/mesenchymal phenotype leading the collective invasion. Cancer Sci. 2020, 111, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Lecharpentier, A.; Vielh, P.; Perez-Moreno, P.; Planchard, D.; Soria, J.C.; Farace, F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br. J. Cancer 2011, 105, 1338–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, A.J.; Marengo, M.S.; Oltean, S.; Kemeny, G.; Bitting, R.L.; Turnbull, J.D.; Herold, C.I.; Marcom, P.K.; George, D.J.; Garcia-Blanco, M.A. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 2011, 9, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Francescangeli, F.; Magri, V.; De Angelis, M.L.; De Renzi, G.; Gandini, O.; Zeuner, A.; Gazzaniga, P.; Nicolazzo, C. Sequential isolation and characterization of single CTCs and large CTC clusters in metastatic colorectal cancer patients. Cancers 2021, 13, 6362. [Google Scholar] [CrossRef]
- Rostami, P.; Kashaninejad, N.; Moshksayan, K.; Saidi, M.S.; Firoozabadi, B.; Nguyen, N.-T. Novel approaches in cancer management with circulating tumor cell clusters. J. Sci. Adv. Mater. Devices 2019, 4, 1–18. [Google Scholar] [CrossRef]
- Krawczyk, N.; Meier-Stiegen, F.; Banys, M.; Neubauer, H.; Ruckhaeberle, E.; Fehm, T. Expression of stem cell and epithelial-mesenchymal transition markers in circulating tumor cells of breast cancer patients. BioMed Res. Int. 2014, 2014, 415721. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, W.; Hanna, D.L.; Yang, D.; Okazaki, S.; Berger, M.D.; Miyamoto, Y.; Suenaga, M.; Schirripa, M.; El-Khoueiry, A.; et al. Clinical relevance of EMT and stem-like gene expression in circulating tumor cells of metastatic colorectal cancer patients. Pharm. J. 2018, 18, 29–34. [Google Scholar] [CrossRef]
- Liao, T.-T.; Yang, M.-H. Hybrid epithelial/mesenchymal state in cancer metastasis: Clinical significance and regulatory mechanisms. Cells 2020, 9, 623. [Google Scholar] [CrossRef] [Green Version]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-mesenchymal transition (EMT) as a therapeutic target. Cells Tissues Organs 2022, 211, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Imrali, A.; Heeschen, C. Circulating cancer stem cells: The importance to select. Chin. J. Cancer Res. 2015, 27, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Onoda, H.; Fushiya, N.; Koike, K.; Nishino, H.; Tajiri, H. Staging systems for hepatocellular carcinoma: Current status and future perspectives. World J. Hepatol. 2015, 7, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Lee, J.-M.; Sirlin, C.B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 2014, 273, 30–50. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S. The role of liver biopsy in hepatocellular carcinoma. Gastroenterol. Hepatol. 2016, 12, 628–630. [Google Scholar]
- Barbier, L.; Muscari, F.; Le Guellec, S.; Pariente, A.; Otal, P.; Suc, B. Liver resection after downstaging hepatocellular carcinoma with sorafenib. Int. J. Hepatol. 2011, 2011, 791013. [Google Scholar] [CrossRef] [Green Version]
- Williet, N.; Dubreuil, O.; Boussaha, T.; Trouilloud, I.; Landi, B.; Housset, M.; Botti, M.; Rougier, P.; Belghiti, J.; Taieb, J. Neoadjuvant sorafenib combined with gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma. J. Hepatol. 2011, 17, 2255–2258. [Google Scholar] [CrossRef]
- Tomonari, T.; Sato, Y.; Tanaka, H.; Tanaka, T.; Taniguchi, T.; Sogabe, M.; Okamoto, K.; Miyamoto, H.; Muguruma, N.; Saito, Y.; et al. Conversion therapy for unresectable hepatocellular carcinoma after lenvatinib: Three case reports. Medicine 2020, 99, e22782. [Google Scholar] [CrossRef]
- Ho, W.J.; Zhu, Q.; Durham, J.; Popovic, A.; Xavier, S.; Leatherman, J.; Mohan, A.; Mo, G.; Zhang, S.; Gross, N.; et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced HCC into resectable disease with enhanced antitumor immunity. Nat. Cancer 2021, 2, 891–903. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.C.; Guan, Y.; Ho, M.C.; Lu, S.; Spahn, J.; Hsu, C.H. Atezolizumab plus bevacizumab combination enables an unresectable hepatocellular carcinoma resectable and links immune exclusion and tumor dedifferentiation to acquired resistance. Exp. Hematol. Oncol. 2021, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.-L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef] [PubMed]

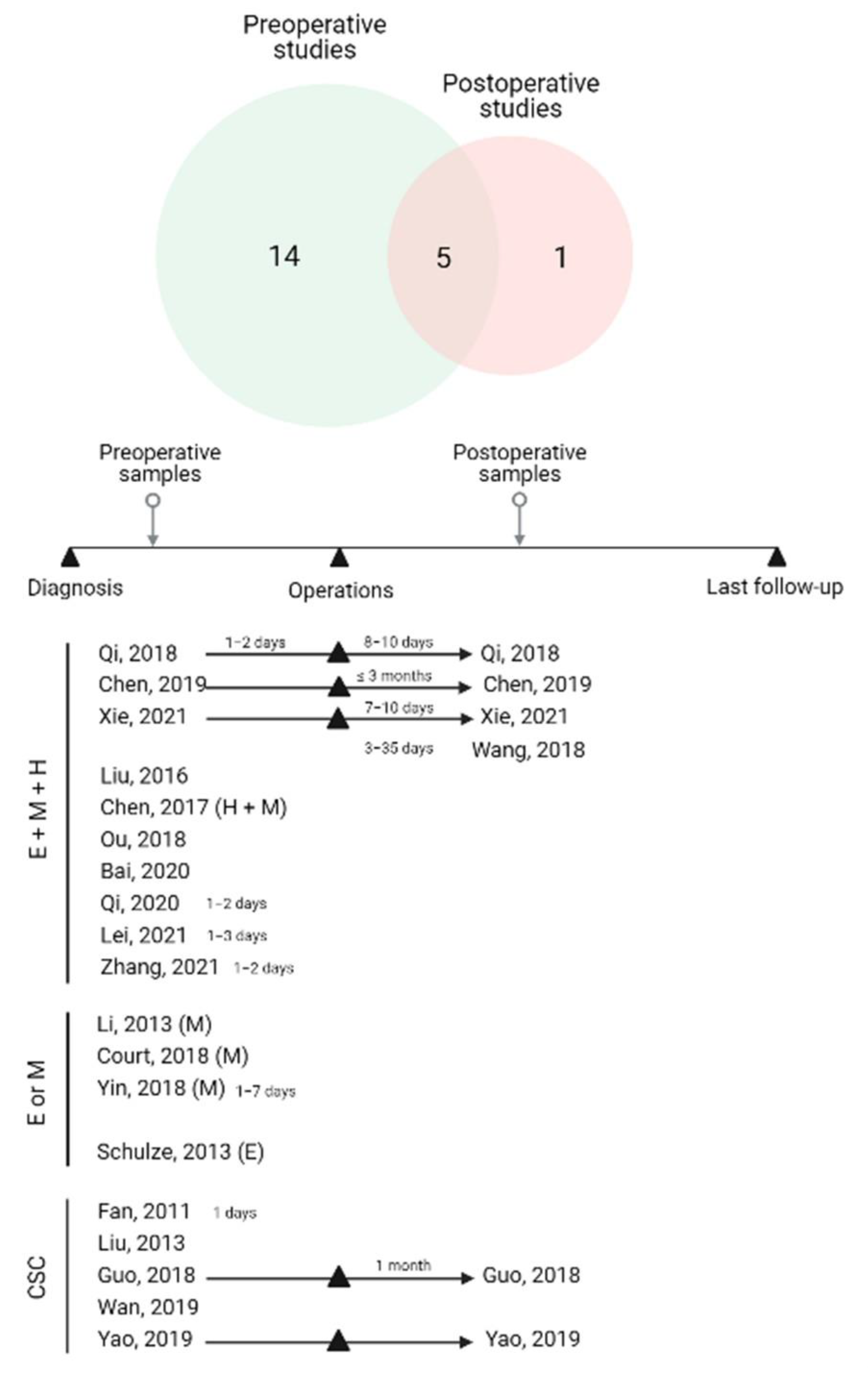
| Author Year | Country | HCC Cohort | Blood (mL) | Time Collection | Treatment | Enrichment Platform | Category Technique | Downstream Methods | EMT Marker | Clinical Significances | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al., 2013 [19] | China | 60 | 10 | Preoperative | - | DG-IM | Positive | IF | Twist, vimentin | PVTT, tumor size, TNM | - |
| Schulze et al., 2013 [20] | Germany | 59 | 7.5 | Preoperative | Any therapies | CellSearch | Positive | IF | EpCAM | BCLC, MaVI, MiVI, | OS |
| Liu et al., 2016 [21] | China | 33 | 5 | - | - | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, vimentin | Tumor number | MET |
| Chen et al., 2017 [22] | China | 99 | 5 | Preoperative | Surgical resection, radiochemical | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, vimentin | BCLC stages, metastasis | |
| Court et al., 2018 [23] | USA | 61 | 4 | Preoperative | Any therapies | NanoVelcro | Positive, MF-IC | IF | Vimentin | Tumor stage PVI | PFS, OS, TTR |
| Ou et al., 2018 [24] | China | 165 | 5 | Preoperative | Surgical resection | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, vimentin | Tumor number, TNM, BCLC | RFS |
| Qi et al., 2018 [25] | China | 112 | 5 | Preoperative, Postoperative | Surgical resection | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, vimentin | BCLC | MET, ER |
| Wang et al., 2018 [26] | China | 62 | 5 | Postoperative | Surgical resection | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19 twist, vimentin | - | ER |
| Yin et al., 2018 [27] | China | 80 | 5 | Preoperative | Surgical resection, TACE | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19 twist, vimentin | Tumor number, tumor size, PVTT, TNM | MET, RECUR |
| Chen et al., 2019 [28] | China | 143 | 5 | Preoperative, Postoperative | Surgical resection, ablation | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, vimentin | NS | TTR |
| Bai et al., 2020 [29] | China | 99 | 5 | Preoperative | Surgical resection | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, vimentin | BCLC, tumor size, PVTT | PFS |
| Qi et al., 2020 [30] | China | 136 | 5 | Preoperative | Surgical resection | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, vimentin | - | TFS, INR, EXR, RECUR |
| Lei et al., 2021 [31] | China | 160 | 15 | Preoperative | Surgical resection | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, bimentin | Tumor size, BCLC | ER |
| Xie et al., 2021 [32] | China | 56 | 5 | Preoperative, Postoperative | Liver transplant | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, vimentin | - | RECUR ER |
| Zhang et al., 2021 [33] | China | 105 | 5 | Preoperative | Surgical resection | CanPatrol | Negative, FT | FISH | EpCAM, CK8/18/19, twist, vimentin | CK19 | - |
| Author Year | Country | HCC Cohort | Blood (mL) | Time Collection | Treatment | Enrichment Platform | Category Technique | Downstream Methods | CCSC Marker | Clinical Significances | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fan et al., 2011 [34] | China | 82 | 10 | Preoperative | Surgical resection | DG-FACS | Positive | - | CD90, CD44 | - | INR, EXR, RFS, OS |
| Liu et al., 2013 [35] | China | 60 | - | - | - | FACS | Positive | - | ICAM | NS | DFS, OS |
| Guo et al., 2018 [36] | China | 130 | 5 | Preoperative, Postoperative | Surgical resection | RosetteSep | Negative | qRT-PCR | EpCAM, CD133, CD90, CK19 | - | TTR, RECUR |
| Wan et al., 2019 [37] | China | 42 | 10 | Preoperative | - | Labyrinth | Negative, MF | IF | CD44 | TNM | - |
| Yao et al., 2019 [38] | China | 10 | 10 | Preoperative, Postoperative | Surgical resection | RosetteSep | Negative, DG-IC | RT-LAMP | CD90, CD133 | Vascular invasion | MET |
| Lei et al., 2021 [31] | China | 160 | 15 | Preoperative | Surgical resection | CanPatrol | Negative, FT | FISH | Nanog | Tumor size, BCLC | ER |
| Study | Preoperative Analysis | |||||
|---|---|---|---|---|---|---|
| Outcomes | Fan, 2011 [34] | Liu, 2013 [35] | Guo, 2018 [36] | Lei, 2021 [31] | ||
| RFS DFS TTR | HR | 7.15 | 3.127 | |||
| 95% CI | 2.99–17.09 | 1.360–7.190 | ||||
| p-value | 0.0001 | 0.007 | ||||
| RR | 4.175 | |||||
| 95% CI | 2.143–8.133 | |||||
| p-value | <0.0001 | |||||
| Median | pos | 6 | ||||
| neg | 46.5 | |||||
| p-value | <0.0001 | |||||
| RR | 4.175 | |||||
| 95% CI | 2.143–8.133 | |||||
| p value | <0.0001 | |||||
| ER | HR | 2.33 * | ||||
| 95% CI | 1.476–3.679 | |||||
| p-value | 0.000282 | |||||
| OS | HR | 2.28 | ||||
| 95% CI | 0.95–7.82 | |||||
| p-value | 0.062 | |||||
| RR | 4.735 | |||||
| 95% CI | 1.709–13.12 | |||||
| p-value | 0.003 | |||||
| Median | pos | 30 | ||||
| neg | >57.1 | |||||
| p-value | 0.0005 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orrapin, S.; Udomruk, S.; Lapisatepun, W.; Moonmuang, S.; Phanphaisarn, A.; Phinyo, P.; Pruksakorn, D.; Chaiyawat, P. Clinical Implication of Circulating Tumor Cells Expressing Epithelial Mesenchymal Transition (EMT) and Cancer Stem Cell (CSC) Markers and Their Perspective in HCC: A Systematic Review. Cancers 2022, 14, 3373. https://doi.org/10.3390/cancers14143373
Orrapin S, Udomruk S, Lapisatepun W, Moonmuang S, Phanphaisarn A, Phinyo P, Pruksakorn D, Chaiyawat P. Clinical Implication of Circulating Tumor Cells Expressing Epithelial Mesenchymal Transition (EMT) and Cancer Stem Cell (CSC) Markers and Their Perspective in HCC: A Systematic Review. Cancers. 2022; 14(14):3373. https://doi.org/10.3390/cancers14143373
Chicago/Turabian StyleOrrapin, Santhasiri, Sasimol Udomruk, Worakitti Lapisatepun, Sutpirat Moonmuang, Areerak Phanphaisarn, Phichayut Phinyo, Dumnoensun Pruksakorn, and Parunya Chaiyawat. 2022. "Clinical Implication of Circulating Tumor Cells Expressing Epithelial Mesenchymal Transition (EMT) and Cancer Stem Cell (CSC) Markers and Their Perspective in HCC: A Systematic Review" Cancers 14, no. 14: 3373. https://doi.org/10.3390/cancers14143373
APA StyleOrrapin, S., Udomruk, S., Lapisatepun, W., Moonmuang, S., Phanphaisarn, A., Phinyo, P., Pruksakorn, D., & Chaiyawat, P. (2022). Clinical Implication of Circulating Tumor Cells Expressing Epithelial Mesenchymal Transition (EMT) and Cancer Stem Cell (CSC) Markers and Their Perspective in HCC: A Systematic Review. Cancers, 14(14), 3373. https://doi.org/10.3390/cancers14143373






