Exploring Cost-Effectiveness of the Comprehensive Geriatric Assessment in Geriatric Oncology: A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.1.1. Data Sources
2.1.2. Search Terms
- -
- (comprehensive geriatric assessment OR (comprehensive geriatric assessment AND cancer) OR (comprehensive geriatric assessment AND oncology) OR (geriatric assessment AND cancer) OR (geriatric assessment AND oncology) OR (geriatric evaluation management) OR (geriatric evaluation management AND cancer) OR (geriatric evaluation management AND oncology) OR (geriatric co-management)) OR (geriatric co-management AND cancer) OR (geriatric co-management AND oncology) OR (geriatric comanagement) OR (geriatric comanagement AND cancer) OR (geriatric comanagement AND oncology) OR (geriatric intervention AND cancer) OR (geriatric intervention AND oncology)) AND (cost-effectiveness OR cost OR expenditure OR cost-utility OR utility-analysis);
- -
- (([comprehensive geriatric assessment] AND [cancer]) OR [geriatric comanagement AND cancer] OR [geriatric comanagement AND oncology]) (([comprehensive geriatric assessment AND oncology] OR [geriatric assessment AND cancer] OR [geriatric assessment AND oncology] OR [geriatric evaluation management AND cancer] OR [geriatric evaluation management AND oncology] OR [geriatric co-management AND cancer] OR [geriatric co-management AND oncology] OR [geriatric intervention AND cancer] OR [geriatric intervention AND oncology]) AND (length of stay OR LOS) OR (readmission) OR (falls) OR (complications) OR (emergency department);
- -
- ((comprehensive geriatric assessment) OR (geriatric assessment) OR (geriatric comanagement) OR (geriatric co-management) OR (geriatric evaluation management) OR (geriatric intervention)) AND toxicity).
2.1.3. Study Eligibility Criteria
- Editorials, protocols, score creation studies, ongoing registered trials or completed trials without available results;
- Studies without a specific focus on older adults (i.e., age < 60 years or no data about old age participants);
- Studies without a cancer population or without a reported percentage of cancer patients in the study cohort;
- Studies without measures of the cost-effectiveness or cost-sensitive measures;
- Nursing home patients/patients receiving home care;
- Studies enrolling less than 35 patients.
2.1.4. Analysis of Studies
3. Results
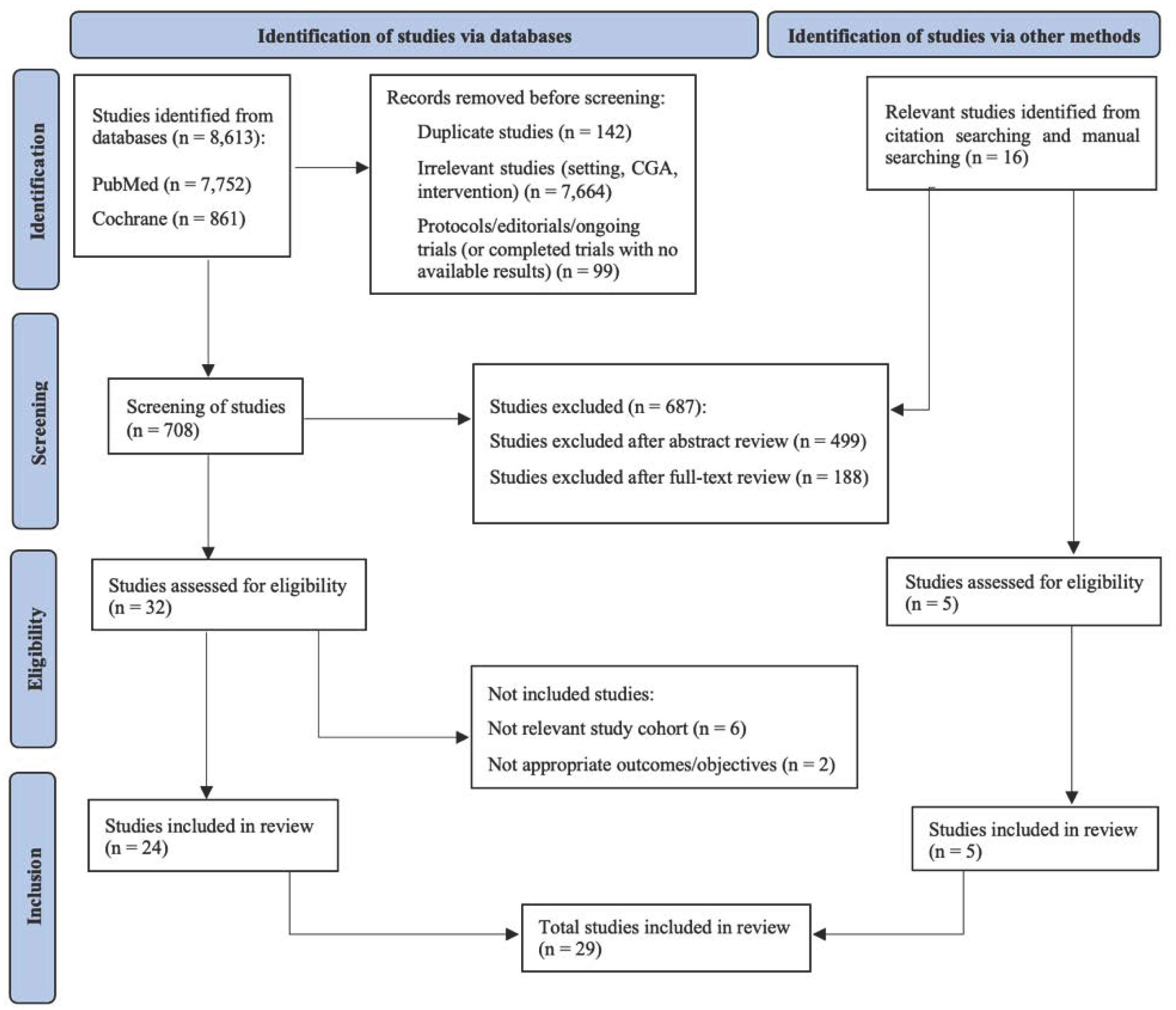
3.1. Identification of Relevant Studies
3.2. Studies Assessing the Effectiveness and Costs of CGA
Surgical and Medical Setting
3.3. Studies Reporting the Outcomes of CGA Interventions Other Than Treatment Toxicity
3.3.1. Surgical Setting
3.3.2. Medical Setting
3.4. Studies Reporting Outcomes of CGA Interventions: Treatment Toxicity and Other Complications
Medical Setting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parker, S.G.; McCue, P.; Phelps, K.; McCleod, A.; Arora, S.; Nockels, K.; Kennedy, S.; Roberts, H.; Conroy, S. What is comprehensive geriatric assessment (CGA)? An umbrella review. Age Ageing 2018, 47, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Fusco, D.; Ferrini, A.; Pasqualetti, G.; Giannotti, C.; Cesari, M.; Laudisio, A.; Ballestrero, A.; Scabini, S.; Odetti, P.R.; Colloca, G.F. Comprehensive geriatric assessment in older adults with cancer: Recommendations by the Italian Society of Geriatrics and Gerontology (SIGG). Eur. J. Clin. Investig. 2021, 51, e13347. [Google Scholar] [CrossRef] [PubMed]
- Sarrió, R.G.; Rebollo, M.A.; Garrido, M.M.; Guillén-Ponce, C.; Blanco, R.; Flores, E.G.; Saldaña, J. General recommendations paper on the management of older patients with cancer: The SEOM geriatric oncology task force’s position statement. Clin. Transl. Oncol. 2018, 20, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Stuck, A.E.; Siu, A.L.; Wieland, D. Impacts of geriatric evaluation and management programs on defined outcomes: Overview of the evidence. J. Am. Geriatr. Soc. 1991, 39, 8S–16S. [Google Scholar] [CrossRef]
- Azam, F.; Latif, M.F.; Farooq, A.; Tirmazy, S.H.; AlShahrani, S.; Bashir, S.; Bukhari, N. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep. Oncol. 2019, 12, 728–736. [Google Scholar] [CrossRef]
- Buccheri, G.; Ferrigno, D.; Tamburini, M. Karnofsky and ECOG performance status scoring in lung cancer: A prospective, longitudinal study of 536 patients from a single institution. Eur. J. Cancer 1996, 32, 1135–1141. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–656. [Google Scholar] [CrossRef]
- Baronner, A.; MacKenzie, A. Using geriatric assessment strategies to lead end-of-life care discussions. Curr. Oncol. Rep. 2017, 19, 75. [Google Scholar] [CrossRef]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 2011, 29, 3457. [Google Scholar] [CrossRef]
- Repetto, L.; Fratino, L.; Audisio, R.A.; Venturino, A.; Gianni, W.; Vercelli, M.; Parodi, S.; Dal Lago, D.; Gioia, F.; Monfardini, S. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J. Clin. Oncol. 2002, 20, 494–502. [Google Scholar] [CrossRef]
- Lin, Y.; Song, Y.; Xu, Y.; Yao, R.; Zhou, X.-T.; Wang, C.-J.; Huang, X.; Wang, X.-F.; Cao, X.; Sun, Q. Application Value of Abbreviated Comprehensive Geriatric Assessment in Elderly Female Breast Cancer Patients. Acta Acad. Med. Sin. 2021, 43, 395–401. [Google Scholar]
- Kirkhus, L.; Benth, J.Š.; Rostoft, S.; Grønberg, B.H.; Hjermstad, M.J.; Selbæk, G.; Wyller, T.B.; Harneshaug, M.; Jordhøy, M.S. Geriatric assessment is superior to oncologists’ clinical judgement in identifying frailty. Br. J. Cancer 2017, 117, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, C.L.; Kim, H.; Soto-Perez-de-Celis, E.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Cabrera Chien, L.; Charles, K.; et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults With Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, e214158. [Google Scholar] [CrossRef] [PubMed]
- Rostoft, S.; O’Donovan, A.; Soubeyran, P.; Alibhai, S.M.H.; Hamaker, M.E. Geriatric Assessment and Management in Cancer. J. Clin. Oncol. 2021, 39, 2058–2067. [Google Scholar] [CrossRef]
- Buske, C.; Hutchings, M.; Ladetto, M.; Goede, V.; Mey, U.; Soubeyran, P.; Spina, M.; Stauder, R.; Trněný, M.; Wedding, U. ESMO Consensus Conference on malignant lymphoma: General perspectives and recommendations for the clinical management of the elderly patient with malignant lymphoma. Ann. Oncol. 2018, 29, 544–562. [Google Scholar] [CrossRef]
- Montroni, I.; Ugolini, G.; Saur, N.M.; Spinelli, A.; Rostoft, S.; Millan, M.; Wolthuis, A.; Daniels, I.R.; Hompes, R.; Penna, M. Personalized management of elderly patients with rectal cancer: Expert recommendations of the European Society of Surgical Oncology, European Society of Coloproctology, International Society of Geriatric Oncology, and American College of Surgeons Commission on Cancer. Eur. J. Surg. Oncol. 2018, 44, 1685–1702. [Google Scholar]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J. Clin. Oncol. 2018, 36, 2326. [Google Scholar] [CrossRef]
- Dotan, E.; Walter, L.C.; Browner, I.S.; Clifton, K.; Cohen, H.J.; Extermann, M.; Gross, C.; Gupta, S.; Hollis, G.; Hubbard, J. NCCN Guidelines® Insights: Older Adult Oncology, Version 1.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 1006–1019. [Google Scholar] [CrossRef]
- Mohanty, S.; Rosenthal, R.A.; Russell, M.M.; Neuman, M.D.; Ko, C.Y.; Esnaola, N.F. Optimal perioperative management of the geriatric patient: A best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J. Am. Coll. Surg. 2016, 222, 930–947. [Google Scholar] [CrossRef]
- Chow, W.B.; Rosenthal, R.; Merkow, R.; Ko, C.; Esnaola, N. Optimal preoperative assessment of the geriatric surgical patient: A best practices guideline from the American College of Surgeons National Surgical Quality Improvement Pro-gram and the American Geriatrics Society. J. Am. Coll. Surg. 2012, 215, 453–466. [Google Scholar] [CrossRef]
- Decoster, L.; Van Puyvelde, K.; Mohile, S.; Wedding, U.; Basso, U.; Colloca, G.; Rostoft, S.; Overcash, J.; Wildiers, H.; Steer, C. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations. Ann. Oncol. 2015, 26, 288–300. [Google Scholar] [CrossRef]
- Extermann, M.; Aapro, M.; Bernabei, R.; Cohen, H.J.; Droz, J.-P.; Lichtman, S.; Mor, V.; Monfardini, S.; Repetto, L.; Sørbye, L. Use of comprehensive geriatric assessment in older cancer patients:: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit. Rev. Oncol. Hematol. 2005, 55, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Chaïbi, P.; Magné, N.; Breton, S.; Chebib, A.; Watson, S.; Duron, J.-J.; Hannoun, L.; Lefranc, J.-P.; Piette, F.; Menegaux, F. Influence of geriatric consultation with comprehensive geriatric assessment on final therapeutic decision in elderly cancer patients. Crit. Rev. Oncol. Hematol. 2011, 79, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Girre, V.; Falcou, M.-C.; Gisselbrecht, M.; Gridel, G.; Mosseri, V.; Bouleuc, C.; Poinsot, R.; Vedrine, L.; Ollivier, L.; Garabige, V. Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Marenco, D.; Marinello, R.; Berruti, A.; Gaspari, F.; Stasi, M.; Rosato, R.; Bertetto, O.; Molaschi, M.; Ciccone, G. Multidimensional geriatric assessment in treatment decision in elderly cancer patients: 6-year experience in an outpatient geriatric oncology service. Crit. Rev. Oncol. Hematol. 2008, 68, 157–164. [Google Scholar] [CrossRef]
- Caillet, P.; Laurent, M.; Bastuji-Garin, S.; Liuu, E.; Culine, S.; Lagrange, J.-L.; Canoui-Poitrine, F.; Paillaud, E. Optimal management of elderly cancer patients: Usefulness of the Comprehensive Geriatric Assessment. Clin. Interv. Aging 2014, 9, 1645. [Google Scholar]
- Massa, E.; Madeddu, C.; Astara, G.; Pisano, M.; Spiga, C.; Tanca, F.M.; Sanna, E.; Puddu, I.; Patteri, E.; Lamonica, G. An attempt to correlate a “Multidimensional Geriatric Assessment”(MGA), treatment assignment and clinical outcome in elderly cancer patients: Results of a phase II open study. Crit. Rev. Oncol. Hematol. 2008, 66, 75–83. [Google Scholar] [CrossRef]
- DuMontier, C.; Sedrak, M.S.; Soo, W.K.; Kenis, C.; Williams, G.R.; Haase, K.; Harneshaug, M.; Mian, H.; Loh, K.P.; Rostoft, S. Arti Hurria and the progress in integrating the geriatric assessment into oncology: Young International Society of Geriatric Oncology review paper. J. Geriatr. Oncol. 2020, 11, 203–211. [Google Scholar] [CrossRef]
- Van Walree, I.C.; van Soolingen, N.J.; Hamaker, M.E.; Smorenburg, C.H.; Louwers, J.A.; van Huis-Tanja, L.H. Treatment decision-making in elderly women with ovarian cancer: An age-based comparison. Int. J. Gynecol. Cancer 2019, 29, 158–165. [Google Scholar] [CrossRef]
- Mohile, S.G.; Epstein, R.M.; Hurria, A.; Heckler, C.E.; Canin, B.; Culakova, E.; Duberstein, P.; Gilmore, N.; Xu, H.; Plumb, S. Communication with older patients with cancer using geriatric assessment: A cluster-randomized clinical trial from the National Cancer Institute Community Oncology Research Program. JAMA Oncol. 2020, 6, 196–204. [Google Scholar] [CrossRef]
- Schiphorst, A.H.; Ten Bokkel Huinink, D.; Breumelhof, R.; Burgmans, J.P.; Pronk, A.; Hamaker, M.E. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur. J. Cancer Care 2016, 25, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, M.E.; Te Molder, M.; Thielen, N.; van Munster, B.C.; Schiphorst, A.H.; van Huis, L.H. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients—A systematic review. J. Geriatr. Oncol. 2018, 9, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Dunham, L.; Chang, Y.; Deal, A.M.; Pergolotti, M.; Lund, J.L.; Guerard, E.; Kenzik, K.; Muss, H.B.; Sanoff, H.K. Geriatric assessment predicts hospitalization frequency and long-term care use in older adult cancer survivors. J. Oncol. Pract. 2019, 15, e399–e409. [Google Scholar] [CrossRef] [PubMed]
- Applegate, W.B.; Burns, R. Geriatric medicine. JAMA 1996, 275, 1812–1813. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, G.A.; Bullock, A.F.; Greenley, S.L.; Lind, M.J.; Johnson, M.J.; Pearson, M. Implementation of geriatric assessment in oncology settings: A systematic realist review. J. Geriatr. Oncol. 2021, 12, 22–33. [Google Scholar] [CrossRef]
- Balducci, L.; Extermann, M. Cancer and aging: An evolving panorama. Hematol. Oncol. Clin. North Am. 2000, 14, 1–16. [Google Scholar] [CrossRef]
- Cepeda, O.A.; Gammack, J.K. Cancer in older men: A gender-based review. Aging Male 2006, 9, 149–158. [Google Scholar] [CrossRef]
- Cope, D.G. Cancer and the aging population. In An Evidence-Based Approach to the Treatment and Care of the Older Adult with Cancer; Oncology Nursing Society: Pittsburgh, PA, USA, 2006; pp. 1–11. [Google Scholar]
- Gladman, J.R.; Conroy, S.P.; Ranhoff, A.H.; Gordon, A.L. New horizons in the implementation and research of comprehensive geriatric assessment: Knowing, doing and the ‘know-do’gap. Age Ageing 2016, 45, 194–200. [Google Scholar] [CrossRef]
- Puts, M.; Hardt, J.; Monette, J.; Girre, V.; Springall, E.; Alibhai, S. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J. Natl. Cancer Inst. 2012, 104, 1134–1164. [Google Scholar] [CrossRef]
- Wieland, D. The effectiveness and costs of comprehensive geriatric evaluation and management. Crit. Rev. Oncol. Hematol. 2003, 48, 227–237. [Google Scholar] [CrossRef]
- Fox, M.T.; Persaud, M.; Maimets, I.; O’Brien, K.; Brooks, D.; Tregunno, D.; Schraa, E. Effectiveness of acute geriatric unit care using acute care for elders components: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2012, 60, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Baztán, J.J.; Suárez-García, F.M.; López-Arrieta, J.; Rodríguez-Mañas, L.; Rodríguez-Artalejo, F. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: Meta-analysis. BMJ 2009, 338, b50. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.; Gardner, M.; Tsiachristas, A.; Langhorne, P.; Burke, O.; Harwood, R.H.; Conroy, S.P.; Kircher, T.; Somme, D.; Saltvedt, I. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst. Rev. 2017, 2017, CD006211. [Google Scholar] [CrossRef] [PubMed]
- Schapira, M.; Outumuro, M.B.; Giber, F.; Pino, C.; Mattiussi, M.; Montero-Odasso, M.; Boietti, B.; Saimovici, J.; Gallo, C.; Hornstein, L. Geriatric co-management and interdisciplinary transitional care reduced hospital readmissions in frail older patients in Argentina: Results from a randomized controlled trial. Aging Clin. Exp. Res. 2022, 34, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Khadaroo, R.G.; Padwal, R.S.; Wagg, A.S.; Clement, F.; Warkentin, L.M.; Holroyd-Leduc, J. Optimizing senior’s surgical care-Elder-friendly Approaches to the Surgical Environment (EASE) study: Rationale and objectives. BMC Health Serv. Res. 2015, 15, 338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hofmeister, M.; Khadaroo, R.G.; Holroyd-Leduc, J.; Padwal, R.; Wagg, A.; Warkentin, L.; Clement, F. Cost-effectiveness Analysis of the Elder-Friendly Approaches to the Surgical Environment (EASE) intervention for emergency abdominal surgical care of adults aged 65 years and older. JAMA Netw. Open 2020, 3, e202034. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Qian, C.L.; Knight, H.P.; Ferrone, C.R.; Kunitake, H.; Fernandez-del Castillo, C.; Lanuti, M.; Qadan, M.; Ricciardi, R.; Lillemoe, K.D.; Kaslow-Zieve, E.R. Randomized trial of a perioperative geriatric intervention for older adults with cancer. J. Clin. Oncol. 2020, 38, 12012. [Google Scholar] [CrossRef]
- Puts, M.T.; Hsu, T.; Mariano, C.; Monette, J.; Brennenstuhl, S.; Pitters, E.; Ray, J.; Wan-Chow-Wah, D.; Kozlowski, N.; Krzyzanowska, M. Clinical and Cost-effectiveness of a Comprehensive geriatric assessment and management for Canadian elders with Cancer—The 5C study: A study protocol for a randomised controlled phase III trial. BMJ Open 2019, 9, e024485. [Google Scholar] [CrossRef]
- Puts, M.; Alqurini, N.; Strohschein, F.; Monette, J.; Wan-Chow-Wah, D.; Koneru, R.; Szumacher, E.; Mehta, R.; Mariano, C.J.; Li, A. Comprehensive Geriatric Assessment and Management for Canadian Elders with Cancer: The 5C Study; American Society of Clinical Oncology (ASCO): Alexandria, VA, USA, 2021. [Google Scholar]
- Aizawa, K.-i.; Kanai, T.; Saikawa, Y.; Takabayashi, T.; Kawano, Y.; Miyazawa, N.; Yamamoto, T. A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery. Surg. Today 2002, 32, 310–314. [Google Scholar] [CrossRef]
- Berkel, A.E.; Bongers, B.C.; Kotte, H.; Weltevreden, P.; de Jongh, F.H.; Eijsvogel, M.M.; Wymenga, A.M.; Bigirwamungu-Bargeman, M.; van der Palen, J.; van Det, M.J. Effects of Community-based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery With High Risk for Postoperative Complications: Results of a Randomized Clinical Trial. Ann. Surg. 2021, 275, e299–e306. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.R.; Heflin, M.T.; Whitson, H.E.; Dalton, T.O.; Lidsky, M.E.; Liu, P.; Poer, C.M.; Sloane, R.; Thacker, J.K.; White, H.K. Association of integrated care coordination with postsurgical outcomes in high-risk older adults: The Perioperative Optimization of Senior Health (POSH) initiative. JAMA Surg. 2018, 153, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Slaets, J.; Kauffmann, R.; Duivenvoorden, H.; Pelemans, W.; Schudel, W. A randomized trial of geriatric liaison intervention in elderly medical inpatients. Psychosom. Med. 1997, 59, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, S.S.; Matzen, L.-E.; Brink, C.; Bliucukiene, R.; Kasch, S.; Schytte, T.; Kristiansen, C.; Hansen, O. Impact of comprehensive geriatric assessment on quality of life, overall survival, and unplanned admission in patients with non-small cell lung cancer treated with stereotactic body radiotherapy. J. Geriatr. Oncol. 2018, 9, 575–582. [Google Scholar] [CrossRef]
- Signal, V.; Jackson, C.; Signal, L.; Hardie, C.; Holst, K.; McLaughlin, M.; Steele, C.; Sarfati, D. Improving management of comorbidity in patients with colorectal cancer using comprehensive medical assessment: A pilot study. BMC Cancer 2020, 20, 50. [Google Scholar] [CrossRef]
- Soo, W.-K.; King, M.; Pope, A.; Parente, P.; Darzins, P.; Davis, I.D. Integrated geriatric assessment and treatment (integerate) in older people with cancer planned for systemic anticancer therapy. J. Clin. Oncol. 2020, 38, 12011. [Google Scholar] [CrossRef]
- Ho, M.F.; Dai, D.L.K.; Lee, J.F.Y. A randomized controlled clinical trial to assess the impact of enhanced geriatric input on elderly patients undergoing colorectal cancer surgery. Surg. Pract. 2017, 21, 4. [Google Scholar]
- Mak, T.; Dai, D.L.W. A pilot study of enhanced geriatric input in management of elderly patients undergoing colorectal cancer surgery: LTP072. Colorectal Dis. 2014, 16, 56. [Google Scholar]
- Li, D.; Sun, C.-L.; Kim, H.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Chien, L.; Charles, K.; Fernandes Dos Santos Hughes, S. Geriatric assessment-driven intervention (GAIN) on chemotherapy toxicity in older adults with cancer: A randomized controlled trial. J. Clin. Oncol. 2020, 38, 12010. [Google Scholar] [CrossRef]
- Mohile, S.G.; Mohamed, M.R.; Xu, H.; Culakova, E.; Loh, K.P.; Magnuson, A.; Flannery, M.A.; Obrecht, S.; Gilmore, N.; Ramsdale, E. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): A cluster-randomised study. Lancet 2021, 398, 1894–1904. [Google Scholar] [CrossRef]
- Hempenius, L.; Slaets, J.P.; van Asselt, D.; de Bock, G.H.; Wiggers, T.; van Leeuwen, B.L. Outcomes of a geriatric liaison intervention to prevent the development of postoperative delirium in frail elderly cancer patients: Report on a multicentre, randomized, controlled trial. PLoS ONE 2013, 8, e64834. [Google Scholar] [CrossRef] [PubMed]
- Ommundsen, N.; Wyller, T.; Nesbakken, A.; Bakka, A.; Jordhøy, M.; Skovlund, E.; Rostoft, S. Preoperative geriatric assessment and tailored interventions in frail older patients with colorectal cancer: A randomized controlled trial. Colorectal Dis. 2018, 20, 16–25. [Google Scholar] [CrossRef] [PubMed]
- DuMontier, C.; Uno, H.; Hshieh, T.; Zhou, G.; Chen, R.; Magnavita, E.S.; Mozessohn, L.; Javedan, H.; Stone, R.M.; Soiffer, R.J. Randomized controlled trial of geriatric consultation versus standard care in older adults with hematologic malignancies. Haematologica 2022, 107, 1172. [Google Scholar] [CrossRef] [PubMed]
- Corre, R.; Greillier, L.; Le Caër, H.; Audigier-Valette, C.; Baize, N.; Bérard, H.; Falchero, L.; Monnet, I.; Dansin, E.; Vergnenegre, A. Use of a comprehensive geriatric assessment for the management of elderly patients with advanced non–small-cell lung cancer: The phase III randomized ESOGIA-GFPC-GECP 08-02 study. J. Clin. Oncol. 2016, 34, 1476–1483. [Google Scholar] [CrossRef]
- Lund, C.M.; Vistisen, K.K.; Olsen, A.P.; Bardal, P.; Schultz, M.; Dolin, T.G.; Rønholt, F.; Johansen, J.S.; Nielsen, D.L. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: A randomised trial (GERICO). Br. J. Cancer 2021, 124, 1949–1958. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Blanc-Bisson, C.; Doussau, A.; Germain, C.; Blanc, J.-F.; Dauba, J.; Lahmar, C.; Terrebonne, E.; Lecaille, C.; Ceccaldi, J. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: A two-year randomized controlled trial. PLoS ONE 2014, 9, e108687. [Google Scholar] [CrossRef]
- Nadaraja, S.; Matzen, L.-E.; Jørgensen, T.L.; Dysager, L.; Knudsen, A.Ø.; Jeppesen, S.S.; Möller, S.; Herrstedt, J. The impact of comprehensive geriatric assessment for optimal treatment of older patients with cancer: A randomized parallel-group clinical trial. J. Geriatr. Oncol. 2020, 11, 488–495. [Google Scholar] [CrossRef]
- Nipp, R.D.; Temel, B.; Fuh, C.-X.; Kay, P.; Landay, S.; Lage, D.; Franco-Garcia, E.; Scott, E.; Stevens, E.; O’Malley, T. Pilot randomized trial of a transdisciplinary geriatric and palliative care intervention for older adults with cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 591–598. [Google Scholar] [CrossRef]
- Ørum, M.; Eriksen, S.V.; Gregersen, M.; Jensen, A.R.; Jensen, K.; Meldgaard, P.; Nordsmark, M.; Damsgaard, E.M. The impact of a tailored follow-up intervention on comprehensive geriatric assessment in older patients with cancer-a randomised controlled trial. J. Geriatr. Oncol. 2021, 12, 41–48. [Google Scholar] [CrossRef]
- Nipp, R.D.; Qian, C.L.; Knight, H.P.; Ferrone, C.R.; Kunitake, H.; Fernandez-del Castillo, C.; Lanuti, M.; Qadan, M.; Ricciardi, R.; Lillemoe, K.D. Effects of a perioperative geriatric intervention for older adults with Cancer: A randomized clinical trial. J. Geriatr. Oncol. 2022, 13, 410–415. [Google Scholar] [CrossRef]
- Puts, M.T.; Sattar, S.; Kulik, M.; MacDonald, M.E.; McWatters, K.; Lee, K.; Brennenstuhl, S.; Jang, R.; Amir, E.; Krzyzanowska, M.K. A randomized phase II trial of geriatric assessment and management for older cancer patients. Supportive Care Cancer 2018, 26, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, T.; Babic-Illman, G.; Ross, P.; Maisey, N.; Hughes, S.; Fields, P.; Martin, F.; Wang, Y.; Harari, D. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br. J. Cancer 2015, 112, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Santabalbina, F.J.; Llabata-Broseta, J.; Belenguer-Varea, Á.; Álvarez-Martínez, D.; Cuesta-Peredo, D.; Avellana-Zaragoza, J.A. A daily multidisciplinary assessment of older adults undergoing elective colorectal cancer surgery is associated with reduced delirium and geriatric syndromes. J. Geriatr. Oncol. 2019, 10, 298–303. [Google Scholar] [CrossRef]
- Shahrokni, A.; Tin, A.L.; Sarraf, S.; Alexander, K.; Sun, S.; Kim, S.J.; McMillan, S.; Yulico, H.; Amirnia, F.; Downey, R.J. Association of geriatric comanagement and 90-day postoperative mortality among patients aged 75 years and older with cancer. JAMA Netw. Open 2020, 3, e209265. [Google Scholar] [CrossRef]
- Souwer, E.; Bastiaannet, E.; de Bruijn, S.; Breugom, A.; van den Bos, F.; Portielje, J.; Dekker, J. Comprehensive multidisciplinary care program for elderly colorectal cancer patients:“From prehabilitation to independence”. Eur. J. Surg. Oncol. 2018, 44, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Van der Vlies, E.; Smits, A.B.; Los, M.; van Hengel, M.; Bos, W.J.W.; Dijksman, L.M.; van Dongen, E.P.; Noordzij, P.G. Implementation of a preoperative multidisciplinary team approach for frail colorectal cancer patients: Influence on patient selection, prehabilitation and outcome. J. Geriatr. Oncol. 2020, 11, 1237–1243. [Google Scholar] [CrossRef]
- Indrakusuma, R.; Dunker, M.; Peetoom, J.; Schreurs, W. Evaluation of preoperative geriatric assessment of elderly patients with colorectal carcinoma. A retrospective study. Eur. J. Surg. Oncol. (EJSO) 2015, 41, 21–27. [Google Scholar] [CrossRef]
- Janssen, T.; Steyerberg, E.; Langenberg, J.; de Lepper, C.v.H.; Wielders, D.; Seerden, T.; de Lange, D.; Wijsman, J.; Ho, G.; Gobardhan, P. Multimodal prehabilitation to reduce the incidence of delirium and other adverse events in elderly patients undergoing elective major abdominal surgery: An uncontrolled before-and-after study. PLoS ONE 2019, 14, e0218152. [Google Scholar]
- Koh, F.H.; Loh, C.H.; Tan, W.J.; Ho, L.M.; Yen, D.; Chua, J.M.; Kok, S.S.; Sivarajah, S.S.; Chew, M.H.; Foo, F.J. Structured presurgery prehabilitation for aged patients undergoing elective surgery significantly improves surgical outcomes and reduces cost: A nonrandomized sequential comparative prospective cohort study. Nutr. Clin. Pract. 2022, 37, 645–653. [Google Scholar] [CrossRef]
- Shipway, D.; Koizia, L.; Winterkorn, N.; Fertleman, M.; Ziprin, P.; Moorthy, K. Embedded geriatric surgical liaison is associated with reduced inpatient length of stay in older patients admitted for gastrointestinal surgery. Future Healthc. J. 2018, 5, 108–116. [Google Scholar] [CrossRef]
- Magnuson, A.; Lemelman, T.; Pandya, C.; Goodman, M.; Noel, M.; Tejani, M.; Doughtery, D.; Dale, W.; Hurria, A.; Janelsins, M. Geriatric assessment with management intervention in older adults with cancer: A randomized pilot study. Supportive Care Cancer 2018, 26, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Ramsdale, E.; Lemelman, T.; Loh, K.P.; Flannery, M.; Kehoe, L.; Mullaney, T.; Wells, M.; Gilmore, N.; Plumb, S.; Mohile, S. Geriatric assessment-driven polypharmacy discussions between oncologists, older patients, and their caregivers. J. Geriatr. Oncol. 2018, 9, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, C.; Leguelinel-Blache, G.; Roux-Marson, C.; Jamet, C.; Martin-Allier, A.; Kinowski, J.-M.; Le Guillou, C.; Richard, H.; Antoine, V. Impact of a pharmacist and geriatrician medication review on drug-related problems in older outpatients with cancer. J. Geriatr. Oncol. 2021, 12, 57–63. [Google Scholar] [CrossRef]
- Rao, A.V.; Hsieh, F.; Feussner, J.R.; Cohen, H.J. Geriatric evaluation and management units in the care of the frail elderly cancer patient. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.; Alibhai, S.M.; Brennenstuhl, S.; Kulik, M.; MacDonald, M.E.; McWatters, K.; Lee, K.; Jang, R.; Amir, E.; Krzyzanowska, M.K. Health status, emergency department visits, and oncologists’ feedback: An analysis of secondary endpoints from a randomized phase II geriatric assessment trial. J. Geriatr. Oncol. 2019, 10, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Regueme, S.C.; Echeverria, I.; Monéger, N.; Durrieu, J.; Becerro-Hallard, M.; Duc, S.; Lafargue, A.; Mertens, C.; Laksir, H.; Ceccaldi, J. Protein intake, weight loss, dietary intervention, and worsening of quality of life in older patients during chemotherapy for cancer. Supportive Care Cancer 2021, 29, 687–696. [Google Scholar] [CrossRef]
- Eamer, G.; Saravana-Bawan, B.; van der Westhuizen, B.; Chambers, T.; Ohinmaa, A.; Khadaroo, R.G. Economic evaluations of comprehensive geriatric assessment in surgical patients: A systematic review. J. Surg. Res. 2017, 218, 9–17. [Google Scholar] [CrossRef]
- Soto-Perez-de-Celis, E.; Aapro, M.; Muss, H. ASCO 2020: The geriatric assessment comes of age. Oncologist 2020, 25, 909. [Google Scholar] [CrossRef]
- Shahrokni, A.; Kim, S.J.; Bosl, G.J.; Korc-Grodzicki, B. How We Care for an Older Patient With Cancer. J. Oncol. Pract. 2017, 13, 95–102. [Google Scholar] [CrossRef]
- Hamaker, M.; Seynaeve, C.; Wymenga, A.; van Tinteren, H.; Nortier, J.W.; Maartense, E.; de Graaf, H.; de Jongh, F.; Braun, J.J.; Los, M. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: Results from the OMEGA study of the Dutch breast cancer trialists’ group. Breast 2014, 23, 81–87. [Google Scholar] [CrossRef]
- Rapoport, J.; Teres, D.; Zhao, Y.; Lemeshow, S. Length of stay data as a guide to hospital economic performance for ICU patients. Med. Care 2003, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Leidl, R.; Stratmann, D. Economic Evaluation is Essential in Healthcare for the Elderly. Drugs Aging 1998, 13, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Mohile, S.; Gajra, A.; Klepin, H.; Muss, H.; Chapman, A.; Feng, T.; Smith, D.; Sun, C.-L.; De Glas, N. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J. Clin. Oncol. 2016, 34, 2366. [Google Scholar] [CrossRef]
- Extermann, M.; Boler, I.; Reich, R.R.; Lyman, G.H.; Brown, R.H.; DeFelice, J.; Levine, R.M.; Lubiner, E.T.; Reyes, P.; Schreiber III, F.J. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012, 118, 3377–3386. [Google Scholar] [CrossRef]
- Liou, S.; Stephens, J.; Carpiuc, K.; Feng, W.; Botteman, M.; Hay, J. Economic burden of haematological adverse effects in cancer patients. Clin. Drug Investig. 2007, 27, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Jupiter, D.; Chavez-MacGregor, M.; de Oliveira, C.; Kaul, S. High-Cost Hospitalizations Among Elderly Patients With Cancer. J. Oncol. Pract. 2019, 15, e447–e457. [Google Scholar] [CrossRef] [PubMed]
- Burchardi, H.; Schneider, H. Economic aspects of severe sepsis. Pharmacoeconomics 2004, 22, 793–813. [Google Scholar] [CrossRef]
- Williams, M.D.; Braun, L.A.; Cooper, L.M.; Johnston, J.; Weiss, R.V.; Qualy, R.L.; Linde-Zwirble, W. Hospitalized cancer patients with severe sepsis: Analysis of incidence, mortality, and associated costs of care. Crit. Care 2004, 8, R291–R298. [Google Scholar] [CrossRef]
- Leslie, D.L.; Marcantonio, E.R.; Zhang, Y.; Leo-Summers, L.; Inouye, S.K. One-year health care costs associated with delirium in the elderly population. Arch. Intern. Med. 2008, 168, 27–32. [Google Scholar] [CrossRef]
- Stuck, A.E.; Iliffe, S. Comprehensive geriatric assessment for older adults. BMJ 2011, 343, d6799. [Google Scholar] [CrossRef]
- Goodwin, J.S.; Satish, S.; Anderson, E.T.; Nattinger, A.B.; Freeman, J.L. Effect of nurse case management on the treatment of older women with breast cancer. J. Am. Geriatr. Soc. 2003, 51, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Overcash, J.; Momeyer, M.A. Comprehensive geriatric assessment and caring for the older person with cancer. Semin. Oncol. Nurs. 2017, 33, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Overcash, J. Integrating geriatrics into oncology ambulatory care clinics. Clin. J. Oncol. Nurs. 2015, 19, E80–E86. [Google Scholar] [CrossRef] [PubMed]
- Kocman, D.; Regen, E.; Phelps, K.; Martin, G.; Parker, S.; Gilbert, T.; Conroy, S. Can comprehensive geriatric assessment be delivered without the need for geriatricians? A formative evaluation in two perioperative surgical settings. Age Ageing 2019, 48, 644–649. [Google Scholar] [CrossRef]
| Proponents | Target Population | Recommendations |
|---|---|---|
| The International Society of Geriatric Oncology (SIOG) [21,22] | Cancer patients aged 70 or older |
|
| The European Society for Medical Oncology (ESMO) [15] | Patients aged 70 or older with diffuse large B-cell lymphoma (DLBCL) |
|
| The American Society of Clinical Oncology (ASCO) [17] | Cancer patients aged 65 or older receiving chemotherapy |
|
| The National Comprehensive Cancer Network (NCCN), U.S.A. [18] | Older cancer patients |
|
| The Italian Society of Geriatrics and Gerontology (SIGG) [2] | Cancer patients aged 65 or older |
|
| European Society of Surgical Oncology (ESSO) [16] | Patients aged 70 or older with rectal cancer |
|
| American College of Surgeons (ACS) (and the American Geriatrics Society—AGS) [19,20] | Older cancer patients undergoing oncological surgery |
|
| Authors | Participants | Type of Study | CGA and Geriatric Interventions | Effectiveness Measures Suitable for Cost-Effectiveness | Main Effect of CGA and Geriatric Interventions | Cost-Effectiveness Propensity |
|---|---|---|---|---|---|---|
| Nipp et al., 2022 [72] | 98 patients (per-protocol) ≥65 years Type of cancer: GI [pancreatic, gastric/oesophageal, CRC, hepatobiliary cancer]
| RCT | Random assignment to PERI-OP or usual care. Patients assigned to PERI-OP met with a geriatrician preoperatively and postoperatively. Geriatricians communicated findings to surgical/oncology teams or (after surgery) to inpatient team. | Postoperative LoS Postoperative ICU use 90-day hospital readmissions | In per-protocol analyses, PERI-OP patients had shorter postoperative LoS (5.90 vs. 8.21 days, p = 0.024), with non-significantly lower rates of postoperative ICU use and 90-day readmissions. | Positive Neutral Neutral |
| Complications | In per-protocol analyses, differences in CD complication rates between PERI-OP and usual care group (6.7% vs. 20.6%, p = 0.137) were non-significant. | Neutral | ||||
| Koh et al., 2021 [81] | 81 patients ≥70 years Type of cancer: CRC (elective surgery)
Female PEERS: 43%; non-PEERS: 48%. | Before-and-after | A structured multidisciplinary prehabilitation program prior to surgery: PEERS. The program included CGA, nutrition supplementation, resistance training, optimisation of cardiac risk for operation, optimisation of the discharge process to avoid institutionalisation. The control group did not benefit of PEERS program. | QoL LoS | PEERS group had significant improvement in median EQ-5D (0.70 pre-surgery to 0.80 6-months’ post-surgery, p = 0.01). After multivariate analysis, average LoS in PEERS group was 6.8 days shorter (p = 0.018) after adjusting for modality of surgery and complications, representing a cost saving of USD 11,838.80 per patient. | Positive |
| Surgical complications Anthropometric and functional characteristics 30-days’ morbidity rate | Rate of CD grade 3+ complications were similar between groups. No significant improvement of anthropometric and functional characteristics before and after PEERS. Both groups had similar 30-days’ morbidity rates (8.6% vs. 17.4%, p = 0.26). | Neutral | ||||
| Shahrokni et al., 2020 [76] | 1892 patients ≥75 years Mean age: 81.5 years Female: 50% Type of cancer: different malignant neoplasms (and different surgical procedures).
| Retrospective | Geriatric co-management of care with pre-operative (electronic Rapid Fitness Assessment) and postoperative evaluations. | LoS | Patients in the geriatric co-management group were older compared with surgical service group. The intervention group had longer operative time and longer LoS. | Negative |
| Adverse surgical events Discharge destination | CD adverse surgical outcomes within 30 days of surgical treatment did not differ between groups. A higher proportion of patients in the geriatric co-management group were discharged home with home supportive services (18.0% vs. 13.6%, p < 0.001). In fully adjusted model, geriatric co-management was significantly associated with reduced 90-day mortality. | Neutral Positive | ||||
| van der Vlies et al., 2020 [78] | 433 patients ≥70 years Median age: 80 years (MDT patients), 75 years (non-MDT patients). Female: 41% Type of cancer: (stadium I-IV) CRC (elective curative surgery)
| Retrospective | Intervention: extended preoperative CGA. MDT estimated the risk of a surgical procedure and when patients were considered eligible for surgery, a prehabilitation program was initiated based on comorbidity and frailty characteristics. Control group: no preoperative MDT approach. | LoS Readmission rate Unplanned ICU admission | Readmission rates were similar between groups and most frequently caused by an infectious complication. No significant results were found for LoS and unplanned ICU admissions. | Neutral |
| Severity of postoperative complications Discharge destination | Despite at increased risk, MDT patients did not suffer more postoperative CD III-V complications than non-MDT patients (14.9% vs. 12.4%; p = 0.48). Control group was discharged independently at home more often than MDT patients. | Neutral | ||||
| Janssen et al., 2019 [80] | 627 patients ≥70 years Mean age: 76 years Female: 36% Type of cancer: CRC (79.7%) [or patients with abdominal aortic aneurysm (20.3%)] (elective abdominal surgery)
| Before-and-after | Prehabilitation group received interventions to improve physical health, nutritional status, factors of frailty and preoperative anaemia prior to surgery. During the outpatient visit, a nurse practitioner and a physiotherapist re-evaluated patient global health, fitness and frailty. The control group was not pre-habilitated. | In-hospital LoS Hospital readmission rate Unplanned ICU admission ICU LoS | The prehabilitation group had a higher burden of comorbidities and was more physically and visually impaired at baseline. No effect of prehabilitation on LoS, readmissions, unplanned ICU admissions and LoS in ICU. | Neutral |
| Delirium Postoperative complications Institutionalisation rate | At adjusted logistic regression analysis, prehabilitation significantly reduced the incidence of delirium. No effect was observed for postoperative complications, institutionalisation and short-term mortality. | Positive Neutral Neutral | ||||
| Tarazona-Santabalbina et al., 2019 [75] | 310 patients ≥70 years Female: 63% Type of cancer: CRC (elective surgery)
| Retrospective | In GS group, the geriatrician performed a CGA and established a care plan, then applied and monitored by the geriatrician and multidisciplinary team. Control group was assessed daily by the General Surgery Service in accordance with the usual practice criteria. | LoS ICU admission Hospital readmissions | At baseline, patients in the GS group presented poorer clinical conditions than controls. LoS was similar in groups, but patients in the GS group stayed more frequently over ten days in hospital and were more frequently hospitalised and admitted to the ICU. No significant differences were observed between groups regarding readmissions and in-hospital and post-discharge mortality. | Neutral |
| Delirium Geriatric syndromes Number of perioperative complications | 54 patients experienced delirium (11.3% and 29.2% in GS and control group respectively, p < 0.001), and 49 patient experienced other geriatric syndromes (10.3%and 26.2% in the GS and control group respectively, p < 0.001). Serious complications were more frequent in the GS group (75.9% vs. 56.1%, p < 0.001). | Positive Positive Negative | ||||
| Ommundsen et al., 2018 [64] | 122 frail patients >65 years Mean age: 78.6 years Female: 52% Type of cancer: CRC (elective surgery)
| RCT | CGA followed by a tailored intervention or usual care. | LoS Reoperations Hospital readmissions | No differences in term of LoS between groups. No statistically significant differences between intervention and control group for reoperations (19% vs. 11%) or readmissions (16% vs. 6%). | Neutral |
| Severity of postoperative complications | In the secondary analyses, a statistically significant difference in favour of the intervention in terms of lower CD grade I–V complications (p = 0.05) was found. No statistically significant differences between intervention and control group for CD grade II–V complications (68% vs. 75%) or 30-day survival (4% vs. 5%). | Positive | ||||
| Shipway et al., 2018 [82] | 682 cancer patients (84% were cancer patients) ≥60 years Mean age: 72 years Type of cancer: resectable CRC, esophagogastric cancer (undergoing surgery).
| Before-and-after | Preoperative CGA and corresponding interventions, postoperative patient co-management by a geriatrician. Geriatrician involvement also in the definition of the postoperative discharge plan and in the implementation of a rehabilitation program. | LoS | Intervention was associated with a LoS significant reduction (by 3.1 days) for all surgical patients aged > 60 years, with esteemed cost savings of approximately £300,000/annum. In patients admitted electively for GI surgery, LoS reductions did not reach statistical significance, although a trend reduction was seen indicating possibly greater reduction with advancing age. | Positive |
| Medical complications | No statistically difference in term of medical complications (the reduction of LoS could reflect the prevalence of these). | Neutral | ||||
| Souwer et al., 2018 [77] | 86 patients ≥75 years Median age: 81 years Female: 51% Type of cancer: (stage I-III) CRC (elective surgery)
| Retrospective | Multidisciplinary pre- and rehabilitation (cohort 2014–2015): preoperative assessment with geriatric screening, subsequent CGA when indicated, rehabilitation care. Retrospectively identified historic control cohorts of patients operated at same centre (cohorts 2010–2011, 2012–2013). | LoS Readmission (within 30 days) | The number of patients with a prolonged LoS (>14 days) decreased from 27% in 2010–2011 to 13% in 2012–2013 and 6% in 2014–2015 (p = 0.001), with readmission rates of 3%, 8% and 8% respectively. | Positive Neutral |
| Postoperative complications Adjuvant CT | Severe complications and cardiac complications after surgery were significantly reduced. Number of surgical and pulmonary complications did not differ between the three cohorts. Six patients in 2010–2011, seven in 2012–2013 and 11 in the study cohort received adjuvant CT. | Positive | ||||
| Ho et al., 2017 [59] | 74 patients >70 years Type of cancer: CRC (undergoing curative surgery) | RCT | All patients are randomised to either conventional surgical care or enhanced geriatric input. | LoS | The median LoS was statistically significantly shorter in the intervention group when compared to control (7.1 ± 4.0 days vs. 14.0 ± 10.9 days, p < 0.0001). | Positive |
| Postoperative complications | Postoperative complications were significantly lower in the intervention group (16.2% vs. 54.1%, p < 0.001). | Positive | ||||
| Indrakusuma et al., 2015 [79] | 100 patients ≥70 years Type of cancer: CRC (elective resection). Cohort ISAR – (2008–2010): ISAR questionnaire was not used. Cohort ISAR + (2011–2013): ISAR questionnaire was used. Match-control comparison: 50 DOG patients are compared with 50 matched controls from cohort ISAR −. | Retrospective cohort and match-control study | Patients from cohort ISAR + with a positive ISAR score were referred to the geriatric specialists for DOG assessment. The assessment encompassed CGA and geriatric interventions (e.g., vitamin supplementation, dietary supplements, consult with cardiologist, transfusion, haloperidol prophylaxis). | LoS | LoS was only statistically significant shorter in the cohort (ISAR+ vs. ISAR-) comparison (but since 2011 the use of laparoscopic resection increased, preoperative workup improved, and a postoperative fast track program was implemented). | Neutral |
| PoD Postoperative complications | Compared with controls, DOG patients were older and underwent laparoscopic resection more often. Hearing and cognitive impairment were more prevalent among DOG patients, as history of delirium. Even if significantly more at risk for postoperative complications, DOG patients had comparable postoperative outcomes as controls in general/surgical and medical complications. DOG patients had similar outcomes in mortality and PoD compared to controls. | Positive | ||||
| Mak et al., 2014 [60] | 78 patients >70 years Type of cancer: CRC (surgical treatment)
| Prospective pilot study | Intervention group: perioperative assessment and active management of their pre-existing medical problems, nutritional status and social status were carried out. Patients were jointly managed perioperatively by the colorectal and geriatric teams with further input on discharge. Control group: standard care | LoS | The interventional group had shorter mean LoS (9.31 vs. 12.2 days; p < 0.0001). | Positive |
| Discharge destination | Discharge destination (i.e., home, nursing home or rehabilitation hospital) in both groups was not different. Older patients who received geriatric input had lower 30-day morbidity when compared with controls (15.4% vs. 20.4%; p < 0.01). | Neutral | ||||
| Hempenius et al., 2013 [63] | 260 frail patients ≥65 years Mean age: 77 years Female: 73% Type of cancer: solid cancers (elective surgery)
| RCT | Geriatric liaison intervention to prevent PoD: pre-operative CGA by a geriatric team, individual treatment plan, daily visits by a geriatric nurse during the hospital stay and advice on emerging medical problems. The intervention focused on best supportive care and the prevention of delirium. Control group: standard care (additional geriatric care was only provided at the request of the treating physician). | LoS QoL | Median LoS was 8 days in both groups. No significant difference between the groups in most aspects of the SF-36 scale to estimate QoL, although intervention group did report significantly less bodily pain at discharge than at admission compared with the usual-care group (OR: 0.49, 95% CI: 0.29–0.82). | Neutral Positive |
| Postoperative complications PoD incidence and severity Return to an independent preoperative living situation Care dependency | No significant difference between groups in number and type of complications. PoD occurred in 31 patients (11.9%). No significant difference in the incidence of PoD between the intervention and usual-care group as well as for severity of PoD. This was a significant difference in term of return to preoperative living situation and care in favour of the intervention group, as opposed to the care dependency. | Neutral Negative Positive |
| Authors | Participants | Type of Study | CGA and Geriatric Interventions | Effectiveness Measures Suitable for Cost-Effectiveness | Main Effect of CGA and Geriatric Interventions | Cost-Effectiveness Propensity |
|---|---|---|---|---|---|---|
| Lund et al., 2021 [67] | 142 frail patients ≥70 years Median age: 75 years Female: 43% Type of cancer: stage II–IV CRC [receiving adjuvant (58%) or first-line palliative/downstaging CT (42%)]
| RCT | CGA-based intervention: CGA at the start of CT, follow-up after 2 months or more frequently if needed. Interventions included medication changes (62%), nutritional therapy (51%), physiotherapy (39%). Control group: standard care, co-existing health problems assessed by oncologist or GP. | Hospitalisations QoL | Hospitalisation during CT occurred with equal frequency in both groups. QoL (EORTC QLQ-C30, EORTC QLQ ELD-14) was better in intervention patients, with decreased burden of illness (p = 0.048) and improved mobility (p = 0.008). | Neutral Positive |
| ADEs CT completion | Grade 3+ toxicity occurred in 39% of patients from the control arm and in 28% of patients from the CGA arm (p = 0.156). 20% in the intervention group and 30% in the control group discontinued CT due to toxicity (p = 0.173). More patients from the CGA arm completed the scheduled CT compared with controls (45% vs. 28%, p = 0.0366). | Neutral Positive | ||||
| Mohile et al., 2021 [62] | 718 frail patients ≥70 years Mean age: 77,2 years Female: 43% Type of cancer: incurable advanced (stage III-IV) solid cancer or lymphoma (starting a new treatment regimen with a high risk of toxic effects within 4 weeks) Frailty: at least one impaired CGA domain other than PP.
| RCT | Intervention (CGA and CGA-guided management integrated into oncology care): patients completed CGA, oncologists received a tailored CGA summary and management recommendations. Usual care: patients completed CGA, no CGA summary or management recommendations were provided to oncologists (only alerts for significantly impaired scores on depression and cognitive screening were sent). | ADEs PP Falls | A lower proportion of patients in intervention group had grade 3–5 ADEs compared with usual care group (51% vs. 71%), with a reduced risk of ADEs (p = 0.0001). Patients in the intervention group had fewer falls (12% vs. 21%) with lower risk of having new falls (p = 0.0035) and had more medications discontinued (p = 0.015), reducing PP. | Positive |
| Choukroun et al., 2021 [85] | 51 frail outpatients ≥75 years Mean age: 83,7 years Median age: 83 years Female: 57% Type of cancer: not-hematologic solid cancers [breast (27%), CRC (16%), metastatic cancer (42%)].
| Prospective observational study | Five-stage process (outpatient): preparation phase; face-to-face pharmaceutical consultation with the patient; CGA performed by an ergotherapist and a geriatrician (geriatric tools; physical examination); pharmaceutical medication analysis (PP, PIM, DRP and DDI); final multidisciplinary medication review by the clinical pharmacist and a geriatrician team (recommendations to reduce DRP and optimise prescriptions). After the medication review, the proposals for prescription modification were sent to GPs and oncologists. | PIM ADE risk PP | A significant decrease was observed in prevalence of PIM use and ADE risk. A not significant trend was observed for a lower number of medications. | Positive |
| DuMontier et al., 2020 [65] | 160 frail/prefrail patients [per-protocol intervention and control (n = 148)] ≥75 years Median age: 80,4 years Female: 45% Type of cancer: lymphoma, leukaemia, or multiple myeloma (transplant-ineligible patients). Randomization stratified by disease type.
| RCT | Prefrail and frail patients were randomised to standard oncologic care or standard care plus consultation with a geriatrician. Geriatrician provided individualised interventions, if indicated, he communicated with patient’s primary care provider and utilized referral systems (e.g., psychiatry). Follow-up was encouraged, but not required. Most common interventions fell within the comorbidity/PP domain (81%); followed by nutrition (54%); function/falls (48%); cognition (31%) and depression/mood (17%). | LoS ED visits Unplanned hospitalisations | Consultation did not significantly reduce the incidence of ED visits, hospitalisations (6 months follow-up), or days in hospital. | Neutral |
| Discussion on EoL goals | Consultation did improve the odds of having EoL goals of care discussions (OR = 3.12, 95% CI: 1.03–9.41). | Positive | ||||
| Li et al., 2020 [61] | 605 patients ≥65 years Mean age: 72 years Median age: 71 years Female: 59% Type of cancer: solid malignant neoplasm [GI (33.4%), breast (22.5%), lung (16%), GU (15%), gynaecologic (8.9%), other (4.1%)] (starting a new CT regimen). 71.4% of patients with stage IV cancer.
| RCT | Before starting CT, both arms completed a baseline CGA and Fulmer SPICES assessment. In the intervention arm (GAIN), a geriatrics-trained multidisciplinary team (oncologist, nurse practitioner, social worker, physical/occupation therapist, nutritionist, and pharmacist) acted on CGA results and implemented interventions. In the control arm (SOC), CGA results were sent to treating oncologists for consideration without any input from the multidisciplinary team. | LoS ED visits Unplanned hospitalisations Hospital readmissions | No significant differences were observed in ED visits, unplanned hospitalisations, average LoS, unplanned hospital readmissions and OS between groups. | Neutral |
| ADEs CT discontinuation | Compared to SOC, GAIN arm experienced a significant 10.1% reduction in the incidence of grade 3+ CT-related toxic effects. Reductions were observed for hematologic-only toxic effects (8.0% reduction) as well as non-hematologic-only toxic effects (8.2% reduction). No significant differences in CT dose modifications or discontinuations. | Positive Neutral | ||||
| Nadaraja et al., 2020 [69] | 96 patients ≥70 years Median age: 75.4 years Female: 47.9% Type of cancer: GU cancer (ovarian 32.3%, endometrial 8.3%, prostate 32.3%), bladder 12.5%, kidney 6.3%, NSCLC 8.3% (starting CT or targeted therapy for primary or recurrent disease).
| RCT | Control group: treatment decision based on oncologist’s clinical judgement. Intervention group if G8 ≤ 14: treatment decision based on G8 screening followed by CGA, a multidisciplinary team conference and interventions. Intervention group if G8 > 14: treatment decision based on oncologist’s clinical judgement. | Treatment completion ADEs | No impact on completion rate of planned oncologic treatment, but the intervention resulted in a borderline significant lower incidence of grade 3–4 toxicity. | Neutral Positive |
| Nipp et al., 2020 [70] | 62 patients ≥65 years Median age: 72.3 years Female: 45% Type of cancer: incurable GI or lung cancer
| RCT | Random assignment to usual care or intervention. Transdisciplinary intervention integrating geriatric and palliative care with oncology care (two visits with a geriatrician trained about geriatric-specific and palliative care issues and CGA). | QoL | Intervention patients presented less decrease in QoL decrement (FACT-G). | Positive |
| Symptom burden Communication confidence | Intervention patients had reduced number of moderate/severe symptoms and improved confidence in communication compared to usual care. | Positive | ||||
| Ørum et al, 2021 [71] | 363 patients >70 years Median age: 75 years Female: 45% Type of cancer: head and neck, (4%), lung (47%), upper GI (23%), CRC (26%).
| RCT | All patients received CGA at baseline performed by a multidisciplinary team with evaluation of patient health status. Intervention group received a tailored follow-up by a multidisciplinary team. Follow-up lasted 90 days, performed in-hospital (either in the outpatient clinic or during hospitalisation), in the patient own home, or as phone calls. | Hospitalisations | No significant impact on hospitalisation (47% of intervention vs. 55% of controls). | Neutral |
| Completion of planned treatment ADLs Physical Performance | No differences in ability to complete the treatment, ADLs or physical performance were found. | Neutral | ||||
| Soo et al., 2020 [58,87] | 130 patients >70 years Type of cancer: solid organ cancer or diffuse large B-cell lymphoma [candidates for systemic anticancer therapy (CT, targeted therapy or immunotherapy)]. | RCT | Intervention group received integrated oncogeriatric care: CGA and management integrated with standard oncology care. The group was co-managed by a geriatrician during oncological treatment, undergoing a CGA, standardised personalised interventions and receiving referrals, supportive care information, encouragement of physical activity, management of comorbidities, medication reconciliation and advance care planning. Control group: managed by oncologist only, without input from geriatrician. | QoL Unplanned hospital admissions ED visits | Significant differences favouring the intervention group over the usual care group were seen in QoL—assessed using EORTC QLQ-C30 and EORTC QLQ-ELD14 at 0, 12, 18 and 24 weeks—and unplanned hospital admissions (−1.2 admissions person-years in intervention group; 41% less) and ED visits (39% less). Intervention group presented significantly better ELFI than usual care group at all follow-ups. | Positive |
| Treatment discontinuation | Significant differences favouring the intervention group over the usual care group were seen in early treatment discontinuation (32.9% vs. 53.2%, respectively). | Positive | ||||
| Ramsdale et al., 2018 [84] | 40 patients ≥70 years Mean and median age: 77 years Female: 45% Type of cancer: advanced solid cancer or lymphoma. Impairment in at least one geriatric domain other than PP.
| Descriptive comparison study (subset of patients enrolled in RCT) | All patients received CGA at baseline, prior to starting antineoplastic therapy. In the intervention group, oncologists were given results of CGA. In the control group, they received no information. | Addressing PIM and PP | Physician-initiated discussions were higher in intervention (73% vs. 49%, p = 0.006). More PP concerns brought up per patient (4.1 vs. 2.6, p = 0.07) and “addressed” in intervention compared with control (59% vs. 45%, p = 0.1). Medication management concerns were addressed more commonly in intervention (79% vs. 38%, p = 0.003). Supportive care medication concerns were more often addressed in control group (58% vs. 18%, p = 0.008). | Positive |
| Magnuson et al., 2018 [83] | 71 patients ≥70 years Mean age: 76 years Female: 44 % Type of cancer: advanced (stages III or IV) solid tumour malignancy.
| Prospective randomised pilot study | In intervention arm an algorithm was used to guide GA management recommendations. The coordinator scored the GA and identified impairments, then summarised GA impairments with management recommendations and delivered recommendations to the patient’s primary oncologist within 1 week of assessment. At the 3-month follow-up timepoint, the primary oncologist reported whether these recommendations had been implemented. | Hospitalisations | Prevalence of hospitalisation did not differ between the two groups. | Neutral |
| ADEs Treatment continuity | Incidence of grade 3–5 CT toxicity did not differ between the two groups. Dose reductions, dose delays, and early treatment discontinuation also did not differ between the two groups. | Neutral | ||||
| Puts et al., 2018 [73] and Sattar et al., 2019 [88] | 61 patients ≥70 years Mean age: 75 years Female: 36% Type of cancer: (stage I–IV) GI, GU, or breast cancer.
| RCT | Intervention: CGA, interventions, first follow up. 3 months after CGA, follow-up appointment if needed. Treating physician received the summary of findings and interventions that would be implemented by the clinical intervention team. Control group: usual care from the oncology team. | QoL ED visits Family physician visit | Slight benefit in QoL for intervention patients, but results at all timepoints presented no statistically significant difference. No significant differences in number of ED and GP visits, even if slightly lower rates in intervention group. | Neutral |
| IADL impairment | Fewer patients with IADL impairment ≥ 1 in intervention than in control group at baseline. At six months, the proportion of those with ≥ 1 IADL impairment was similar. | Neutral | ||||
| Corre et al, 2016 [66] | 494 patients ≥70 years Median age: 77 years Female: 26% Type of cancer: advanced (stage IV) NSCLC (starting CT). Patients with PS of 0 to 2.
| RCT | All patients had a CGA performed by their regular cancer physician. Intervention group: experimental CGA-based allocation of the same CT or BSC. Control group: standard strategy of treatment allocation (based on PS and age). | QoL QoL-adjusted survival | Although QoL utility scores at baseline were not different between the arms, they always were higher (although not significantly) in the CGA arm than in the standard arm at each subsequent evaluation, with no evident negative impact of the 23% of patients who received exclusive BSC. The difference in QoL utility scores was significant only at week 36 (p = 0.02). | Neutral |
| ADEs CT failures for toxicity | Treatment objective response rate and disease control rate in CGA arm and control showed no difference. Intervention had significantly less all grade toxicity than control (85.6% vs. 93.4%, p = 0.015) and fewer treatment failures as result of toxicity (4.8% vs. 11.8%, p = 0.007). Percentage of patients with all grade ADEs was significantly higher in control than intervention (93.4% vs. 85.6%, p = 0.015), but not significantly for grade III-IV (71.3% vs. 67.9%, p = 0.41). | Positive | ||||
| Kalsi et al., 2015 [74] | 135 patients ≥70 years Mean age: 75 years Female: 45% Type of cancer: GI (55%), other (45%) (starting CT with or without RT).
| Prospective cohort comparison study | The intervention group underwent risk stratification using a patient-completed screening questionnaire and high-risk patients received geriatrician-delivered CGA. The observational control group received standard oncology care. | CT outcome ADEs CT completion | Geriatrician-delivered CGA was associated with better outcomes. No significant trend for a lower grade 3+ toxicity rate in intervention (43.8% 3+ toxicity rate in the intervention group and 52.9% in the control). More participants in intervention completed treatment as planned (p = 0.006) and fewer required treatment modifications (p = 0.006). | Positive Neutral Positive |
| Bourdel-Marchasson et al., 2014 [68], Regueme et al., 2021 [89] | 336 patients ≥70 years Mean age: 78 years Female: 49% Type of cancer: carcinomas (colon, stomach, pancreas and biliary tract, ovary, prostate, bladder, and lung) and lymphomas (treated with CT). Patients with at least KPS ≥ 50% and at risk of malnutrition (17 ≤ MNA ≤ 23.5). | RCT | The usual care received usual dietary recommendations. The intervention group received usual care and nutritional intervention. Counselling was based on face-to face interviewing and dietary advice cards and involved caregivers or relatives if possible. | Hospitalisation QoL | Analyses were performed on an ITT basis. The intervention was no beneficial for hospitalisation (intervention presented not significantly lower hospitalisations) and 1 and 2-year mortality (similar in both groups). Cancer cachexia anti-anabolism may explain this lack of effect. The intervention did not modify the HRQoL changes in comparison with routine care. | Neutral |
| ADEs CT management Dietary intake | Diet counselling was efficient in increasing dietary intake but had no beneficial effect on CT management (dosage, changes, arrest). There were more usual care patients with grade 3 to 4 infections than in intervention group, but the robustness analysis did not confirm the difference in the incidence of severe infections. | Neutral | ||||
| Rao et al., 2005 [86] | 99 frail patients ≥65 years Mean age: 74 years Female: 2% Type of cancer: prostate, lung, hematologic, GI, head/neck, bladder, renal cancer, ill-defined malignancies. Frailty is at least 2 among: dependence in ADL, stroke/unplanned admission (last 3 months), previous falls, critical ambulation, malnutrition, dementia, depression, prolonged bed rest, incontinence. | Secondary analysis (of RCT) | Hospitalised on a medical or surgical ward, after stabilisation of acute illness, randomised to receive care in a geriatric inpatient unit/geriatric outpatient clinic/both/neither. In geriatric evaluation and management units (inpatient and outpatient geriatric units) core teams provided CGA and patient management. | LoS Direct costs for care QoL | Geriatric evaluation and management inpatient units impacts the QoL (SF-36) in the management of bodily pain and mental and emotional health (no difference in SF-36 general scores between groups). These effects were achieved with no overall increase in hospitalisation or cost of care over the year of the study. No significant differences in LoS. No effect on mortality. | Neutral Positive Positive |
| Measures | Evidence in Identified Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LoS | Nipp et al., 2022 [72] RCT (98 patients) | Koh et al., 2021 [81] Before-and-after (81 patients) | DuMontier et al., 2020 [65] RCT (160 patients) | Li et al., 2020 [61] RCT (605 patients) | Shahrokni et al., 2020 [76] Retrospective (1892 patients) | van der Vlies et al., 2020 [78] Retrospective (433 patients) | Janssen et al., 2019 [80] Before-and-after (627 patients) | Tarazona-Santabalbina et al., 2019 [75] Retrospective (310 patients) | |
| positive | positive | neutral | neutral | negative | neutral * | neutral | neutral * | ||
| Ommundsen et al., 2018 [64] RCT (122 patients) | Shipway et al., 2018 [82] Before-and-after (682 patients) | Souwer et al., 2018 [77] Retrospective (86 patients) | Ho et al., 2017 [59] RCT (74 patients) | Indrakusuma et al., 2015 [79] Retrospective cohort study (100 patients) | Mak et al., 2014 [60] Prospective (78 patients) | Hempenius et al., 2013 [63] RCT (260 patients) | Rao et al.2005 [86] Secondary analysis (of RCT) (99 patients) | ||
| neutral | positive | positive | positive | neutral | positive | neutral * | neutral | ||
| ADEs | Lund et al., 2021 [67] RCT (412 patients) | Mohile et al., 2021 [62] RCT (718 patients) | Choukroun et al., 2021 [85] Prospective (51 patients) | Li et al., 2020 [61] RCT (605 patients) | Nadaraja et al., 2020 [69] RCT (96 patients) | Magnuson et al., 2018 [83] Prospective (71 patients) | Corre et al., 2016 [66] RCT (492 patients) | Kalsi et al., 2015 [74] Prospective (135 patients) | Bourdel-Marchasson et al., 2014 [68] RCT (336 patients) |
| positive | positive | positive | positive | positive | neutral | positive | neutral | neutral | |
| Country | Cost Estimates |
|---|---|
| United States [98] | Median cost per day of inpatient visits for older patients with cancer: USD 2108—USD 3468 |
| United States [97] | Direct cost of neutropenia per episode: USD 1893 (outpatient)/USD 2893 (inpatient)—USD 38,583 (febrile neutropenia hospitalisation) |
| UK and Europe [97] | Direct cost of neutropenia per episode: USD 300 (non-febrile cases)—USD 32,395 (older breast cancer patients) |
| United States [97] | Direct cost of thrombocytopenia per cycle/episode: USD 1035—USD 5328 |
| Europe [97] | Direct cost of thrombocytopenia per cycle/episode: USD 790—USD 2523 |
| United States [97] | Direct cost attributable of anaemia per year: USD 18,418—USD 69,478 |
| Canada and Europe [97] | Total cost of anaemia per episode: USD 124—USD 2704 |
| United States and Europe [99] | Average total cost per ICU/day: estimated at EUR 1200 |
| Europe [99] | Direct costs per sepsis patient (ICU): estimates of EUR 23,000—EUR 29,000. |
| United States [99] | Direct costs per sepsis patient (ICU): estimates of EUR 34,000 |
| United States [100] | Costs for severe sepsis cancer hospitalisation: USD 27,400 Costs for surgical cancer severe sepsis hospitalisation: USD 48,000 Costs for medical cancer severe sepsis hospitalisation: USD 18,200 Costs for non-severe sepsis cancer hospitalisation: USD 8700 |
| United States [101] | Healthcare costs per delirious patient per year: USD 60,516—USD 64,421 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccarino, S.; Monacelli, F.; Antognoli, R.; Nencioni, A.; Monzani, F.; Ferrè, F.; Seghieri, C.; Antonelli Incalzi, R. Exploring Cost-Effectiveness of the Comprehensive Geriatric Assessment in Geriatric Oncology: A Narrative Review. Cancers 2022, 14, 3235. https://doi.org/10.3390/cancers14133235
Zuccarino S, Monacelli F, Antognoli R, Nencioni A, Monzani F, Ferrè F, Seghieri C, Antonelli Incalzi R. Exploring Cost-Effectiveness of the Comprehensive Geriatric Assessment in Geriatric Oncology: A Narrative Review. Cancers. 2022; 14(13):3235. https://doi.org/10.3390/cancers14133235
Chicago/Turabian StyleZuccarino, Sara, Fiammetta Monacelli, Rachele Antognoli, Alessio Nencioni, Fabio Monzani, Francesca Ferrè, Chiara Seghieri, and Raffaele Antonelli Incalzi. 2022. "Exploring Cost-Effectiveness of the Comprehensive Geriatric Assessment in Geriatric Oncology: A Narrative Review" Cancers 14, no. 13: 3235. https://doi.org/10.3390/cancers14133235
APA StyleZuccarino, S., Monacelli, F., Antognoli, R., Nencioni, A., Monzani, F., Ferrè, F., Seghieri, C., & Antonelli Incalzi, R. (2022). Exploring Cost-Effectiveness of the Comprehensive Geriatric Assessment in Geriatric Oncology: A Narrative Review. Cancers, 14(13), 3235. https://doi.org/10.3390/cancers14133235








