Analysis of Exosomal Cargo Provides Accurate Clinical, Histologic and Mutational Information in Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Establishment of Primary Cell Cultures
2.3. Commercial NSCLC Cell Lines
2.4. Tumor Cell Culture Growth Conditions
2.5. In Silico Dataset Validation
2.6. Isolation of Exosomes from Cell Cultures
2.7. Negative-Staining and Transmission Electron Microscopy (TEM) of Exosomes
2.8. Nanoparticle Tracking Analysis (NTA)
2.9. Immunoblotting
2.10. Flow Cytometry Analysis
2.11. RNA Isolation and Integrity
2.12. Exosomal Mutational Status in Established NSCLC Primary Cultures
2.13. Whole mRNA Expression Profiling
2.14. DEGs Validation Using RT-qPCR
2.15. Immunofluorescence Analysis
2.16. Immunohistochemical Analysis
2.17. Statistical Analysis
3. Results
3.1. Characterization of Exosomes Derived from NSCLC Cell Cultures
3.2. Mutational Status of Exosomes Derived from Cell Cultures
3.3. Differential Expression Profiles of Tumor Cell Culture-Derived Exosomes
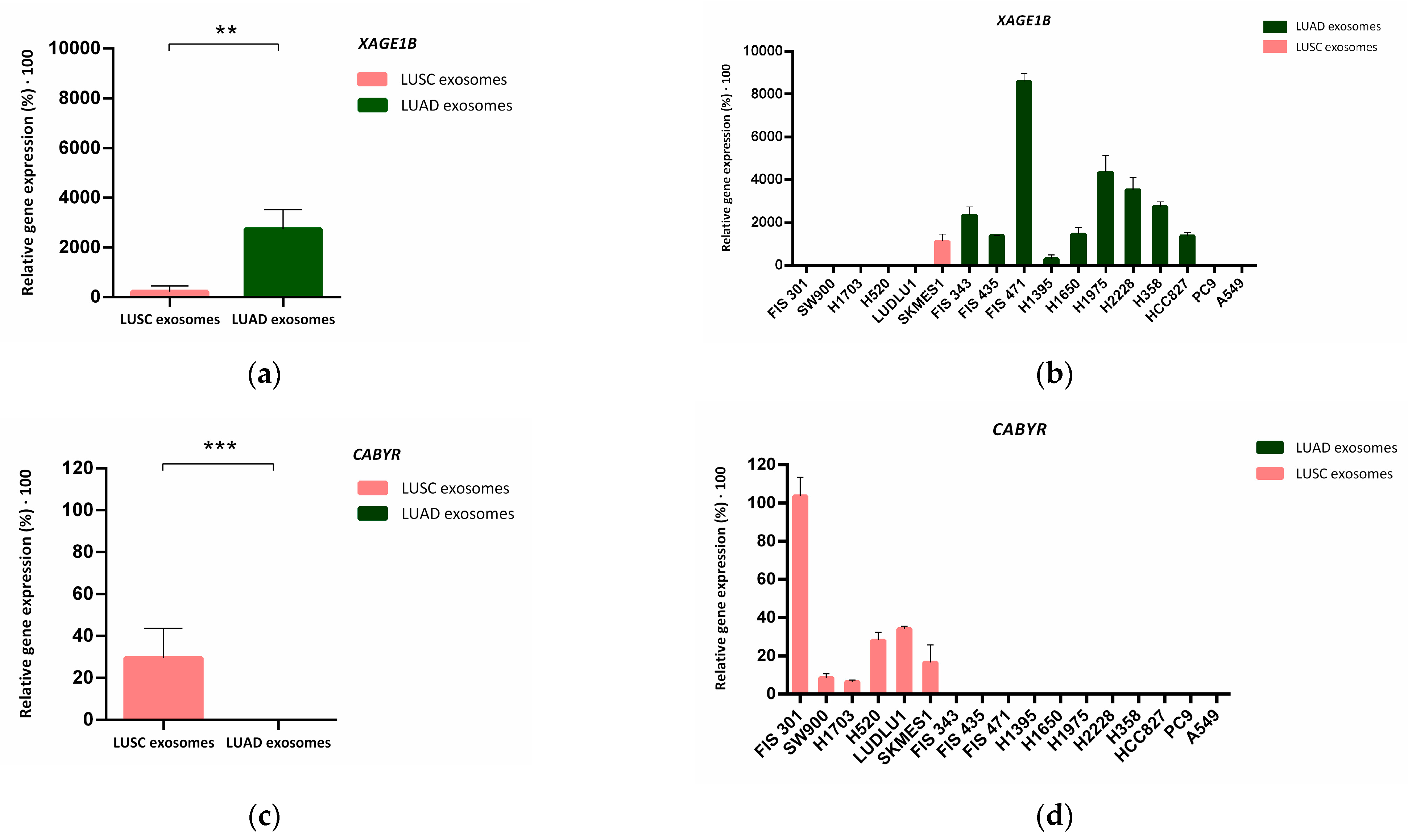
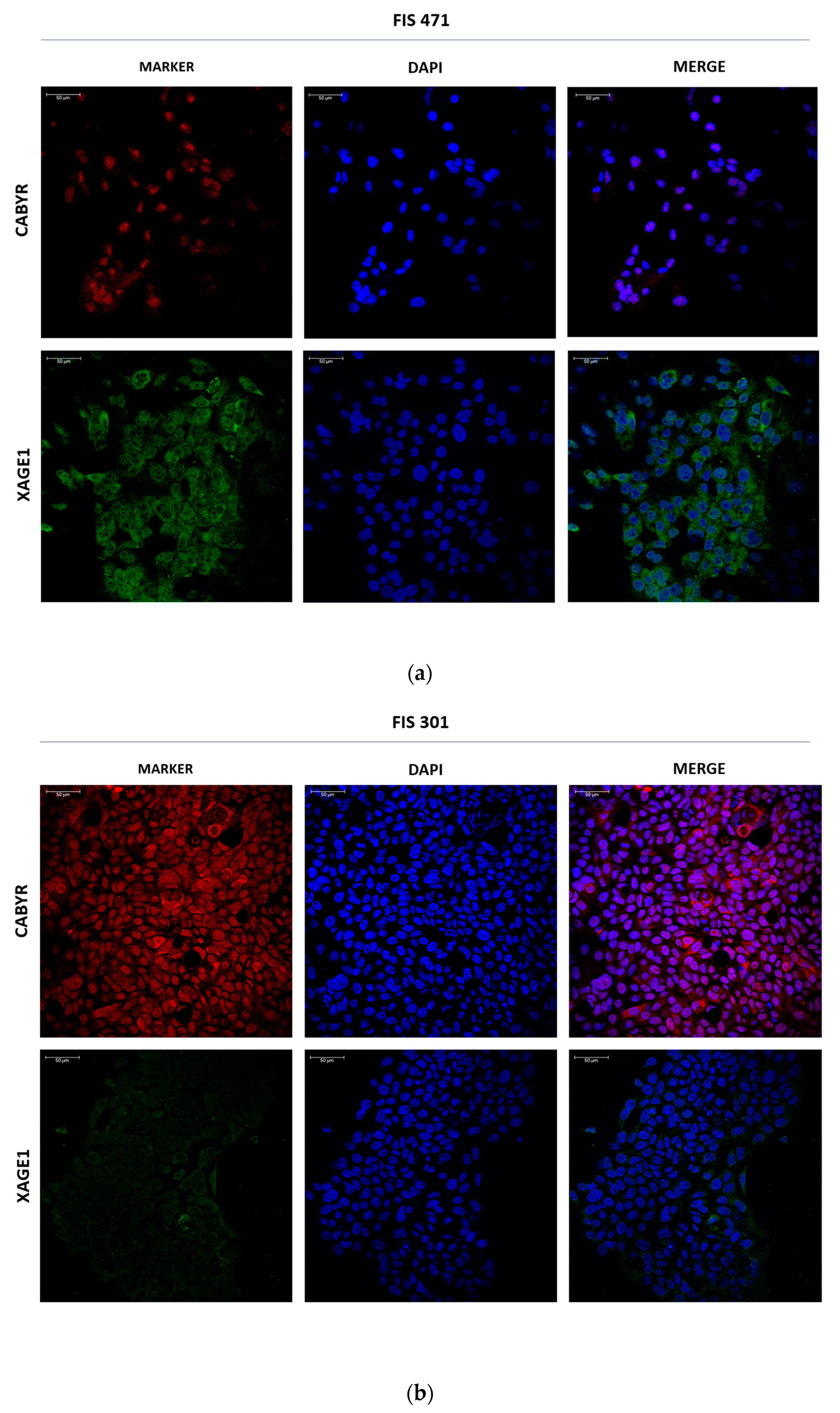
3.4. Biomarker Validation in Tumor Cell Culture-Derived Exosomes
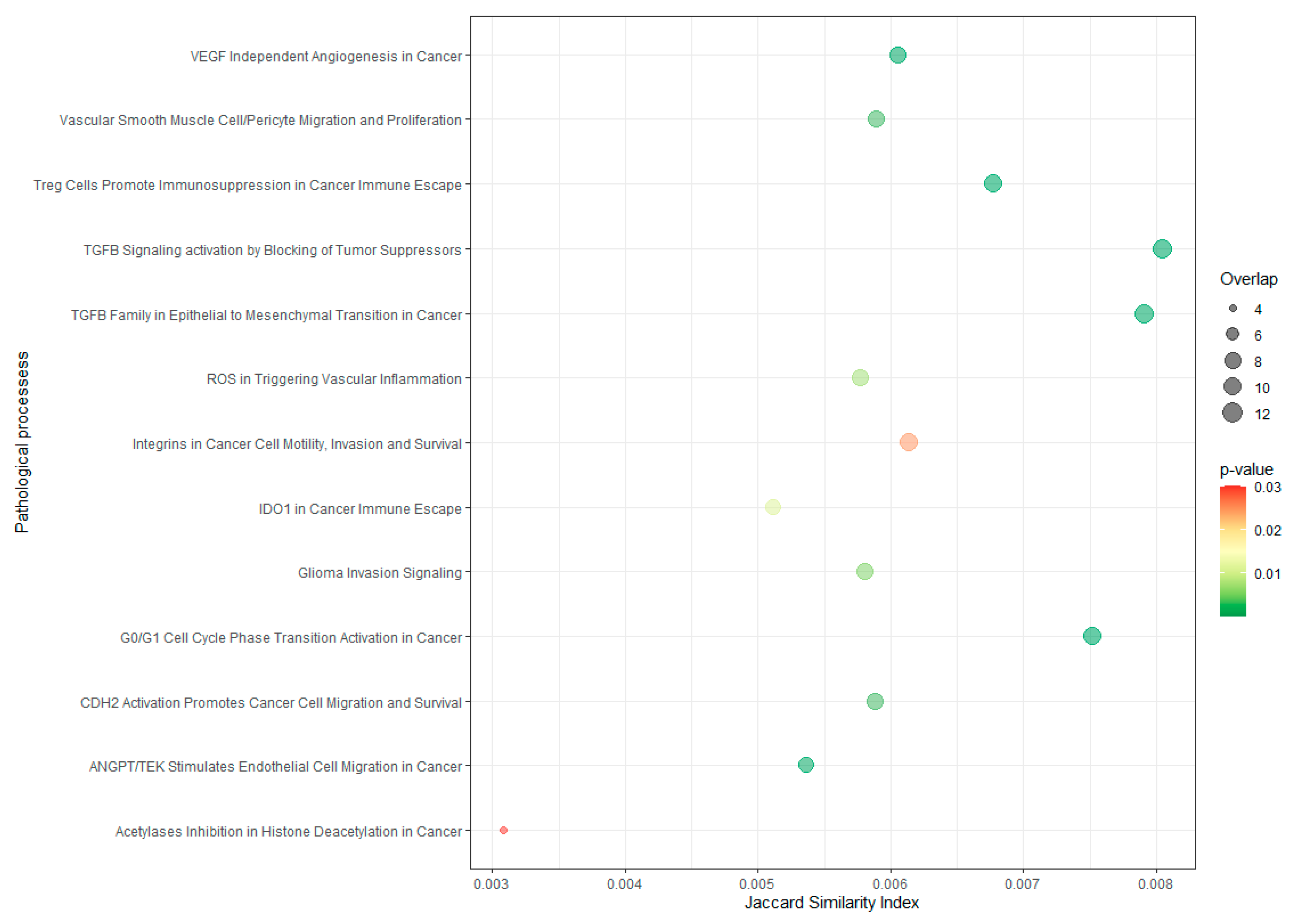
3.5. In Silico Validation of Exosomal Biomarkers in NSCLC
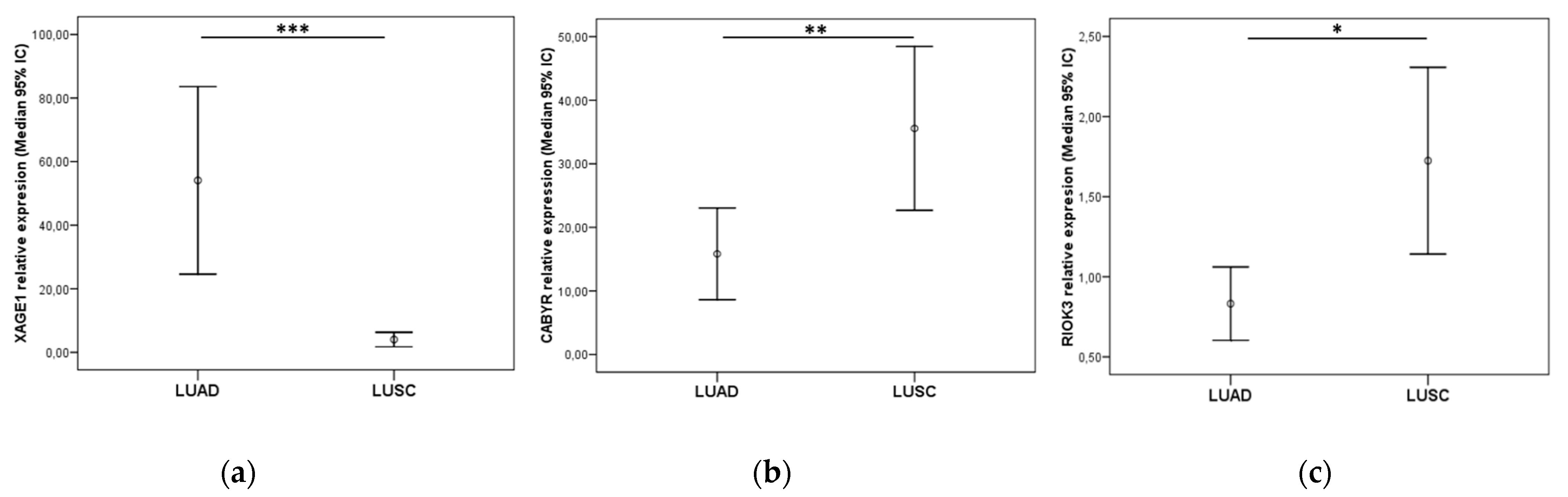
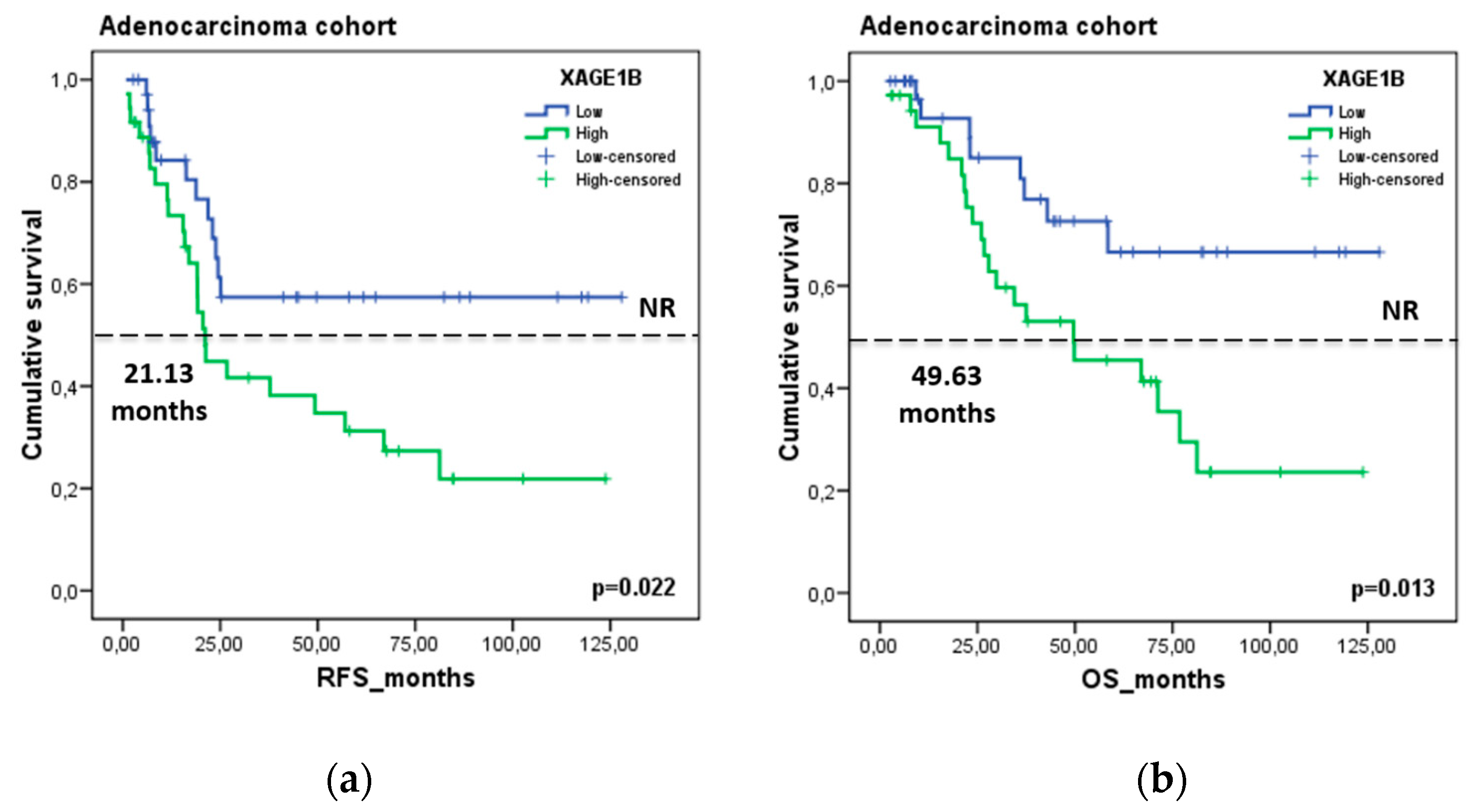
3.6. Validation of Exosomal Biomarkers in a Resected NSCLC Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 6478. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell. Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Huang, C.; Wu, Q.; Jiang, L.; Chen, S.; Chen, L. Circular RNAs expression profiles in plasma exosomes from early-stage lung adenocarcinoma and the potential biomarkers. J. Cell Biochem. 2020, 121, 2525–2533. [Google Scholar] [CrossRef]
- Kok, V.C.; Yu, C.C. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int. J. Nanomed. 2020, 15, 8019–8036. [Google Scholar] [CrossRef]
- Wan, Z.; Gao, X.; Dong, Y.; Zhao, Y.; Chen, X.; Yang, G.; Liu, L. Exosome-mediated cell-cell communication in tumor progression. Am. J. Cancer Res. 2018, 8, 1661–1673. [Google Scholar]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Pei, X.; Guo, G.; Qian, X.; Dou, D.; Zhang, Z.; Xu, X.; Duan, X. Exosome-mediated transfer of long noncoding RNA H19 induces doxorubicin resistance in breast cancer. J. Cell Physiol. 2020, 235, 6896–6904. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer Stem Cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Pomares, A.; Diego De-Maya-Girones, J.; Calabuig-Fariñas, S.; Lucas, R.; Martínez, A.; Pardo-Sánchez, M.; Alonso, S.; Blasco, A.; Guijarro, R.; Martorell, M.; et al. Lung tumorspheres reveal cancer stem cell-like properties and a score with prognostic impact in resected non-small-cell lung cancer. Cell Death Dis. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Cheng, Z.; Qin, W.; Jiang, L. Exosomes as a liquid biopsy for lung cancer. Lung Cancer 2018, 116, 46–54. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumor heterogeneity and cancer cell plasticity. Nature 2013, 501, 328. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef]
- Remon, J.; García-Campelo, R.; de Álava, E.; Vera, R.; Rodríguez-Peralto, J.L.; Rodríguez-Lescure, A.; Bellosillo, B.; Garrido, P.; Rojo, F.; Álvarez-Alegret, R. Liquid biopsy in oncology: A consensus statement of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2020, 22, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.J.; Turner, R.; Chen, Y.W.; Rigas, J.R.; Fernandes, A.W.; Karve, S. Complications and economic burden associated with obtaining tissue for diagnosis and molecular analysis in patients with non-small-cell lung cancer in the United States. J. Oncol. Pract. 2019, 15, E717–E727. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.L.; Tan, S.Z.; Liu, G.; Tsao, M.S. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl. Lung Cancer Res. 2015, 4, 67–81. [Google Scholar] [PubMed]
- Lin, J.J.; Riely, G.J.; Shaw, A.T. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov. 2017, 7, 137–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Wang, M. MET Oncogene in Non-Small Cell Lung Cancer: Mechanism of MET Dysregulation and Agents Targeting the HGF/c-Met Axis. Onco Targets Ther. 2020, 13, 2491–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolfo, C.; Caglevic, C.; Santarpia, M.; Araujo, A.; Giovannetti, E.; Gallardo, C.D.; Pauwels, P.; Mahave, M. Immunotherapy in NSCLC: A Promising and Revolutionary Weapon. Adv. Exp. Med. Biol. 2017, 995, 97–125. [Google Scholar] [CrossRef]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’aquila, E.; Anesi, C.; Silvestris, N.; et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: The potentiality of blood samples. J. Exp. Clin. Cancer Res. 2020, 39, 95, Erratum in J. Exp. Clin. Cancer Res. 2020, 39, 120. [Google Scholar] [CrossRef]
- De Rubis, G.; Rajeev Krishnan, S.; Bebawy, M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol. Sci. 2019, 40, 172–186. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef] [Green Version]
- Pfohl, U.; Pflaume, A.; Regenbrecht, M.; Finkler, S.; Adelmann, Q.G.; Reinhard, C.; Regenbrecht, C.R.A.; Wedeken, L. Precision Oncology Beyond Genomics: The Future Is Here—It Is Just Not Evenly Distributed. Cells 2021, 10, 928. [Google Scholar] [CrossRef]
- Collisson, E.A.; Campbell, J.D.; Brooks, A.N.; Berger, A.H.; Lee, W.; Chmielecki, J.; Beer, D.G.; Cope, L.; Creighton, C.J.; Danilova, L.; et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Hammerman, P.S.; Voet, D.; Lawrence, M.S.; Voet, D.; Jing, R.; Cibulskis, K.; Sivachenko, A.; Stojanov, P.; McKenna, A.; Lander, E.S.; et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef]
- Applications of Nanoparticle Tracking Analysis (NTA) in Nanoparticle Research. Available online: http://www.schaefer-tec.com/fileadmin/user_up-load/sortiment/nanopartikel/NanoSight/NANOSIGHT_Applicati-on_Review_NTA_April_2009_M201B.pdf (accessed on 26 September 2009).
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic. Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Vanni, I.; Alama, A.; Grossi, F.; Dal Bello, M.G.; Coco, S. Exosomes: A new horizon in lung cancer. Drug Discov. Today 2017, 22, 927–936. [Google Scholar] [CrossRef]
- Srivastava, A.; Amreddy, N.; Razaq, M.; Towner, R.; Zhao, Y.D.; Ahmed, R.A.; Munshi, A.; Ramesh, R. Exosomes as Theranostics for Lung Cancer. Adv. Cancer Res. 2018, 139, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Reclusa, P.; Taverna, S.; Pucci, M.; Durendez, E.; Calabuig, S.; Manca, P.; Serrano, M.J.; Sober, L.; Pauwels, P.; Russo, A.; et al. Exosomes as diagnostic and predictive biomarkers in lung cancer. J. Thorac. Dis. 2017, 9, S1373–S1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct. Target Ther. 2020, 5, 144. [Google Scholar] [CrossRef]
- Song, P.; Wu, L.R.; Yan, Y.H.; Zhang, J.X.; Chu, T.; Kwong, L.N.; Patel, A.A.; Zhang, D.Y. Limitations and opportunities of technologies for the analysis of cell-free DNA in cancer diagnostics. Nat. Biomed. Eng. 2022, 6, 232–245. [Google Scholar] [CrossRef]
- Li, M.Y.; Liu, L.Z.; Dong, M. Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Mol. Cancer 2021, 20, 22. [Google Scholar] [CrossRef]
- Hasan, H.; Sohal, I.S.; Soto-Vargas, Z.; Byappanahalli, A.M.; Humphrey, S.E.; Kubo, H.; Kitdumrongthum, S.; Copeland, S.; Tian, F.; Chairoungdua, A.; et al. Extracellular vesicles released by non-small cell lung cancer cells drive invasion and permeability in non-tumorigenic lung epithelial cells. Sci. Rep. 2022, 12, 972. [Google Scholar] [CrossRef]
- Kodack, D.P.; Farago, A.F.; Dastur, A.; Held, M.A.; Dardaei, L.; Friboulet, L.; von Flotow, F.; Damon, L.J.; Lee, D.; Parks, M.; et al. Primary Patient-Derived Cancer Cells and Their Potential for Personalized Cancer Patient Care. Cell Rep. 2017, 21, 3298–3309. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, H.; Ding, Q.; Xing, Y.; Xu, Z.; Lu, C.; Luo, D.; Xu, L.; Xia, W.; Zhou, C.; et al. Establishment of patient-derived tumor spheroids for non-small cell lung cancer. PLoS ONE 2018, 13, e0194016. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Yeon Lee, J.; Hwi Kim, D.; Seok Joo, H.; Ran Yun, M.; Jung, D.; Yun, J.; Heo, S.G.; Ahn, B.C.; Park, C.W.; et al. Patient-derived cells to Guide targeted therapy for Advanced Lung Adenocarcinoma. Sci. Rep. 2019, 9, 19909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitcholtan, K.; Asselin, E.; Parent, S.; Sykes, P.H.; Evans, J.J. Differences in growth properties of endometrial cancer in three-dimensional (3D) culture and 2D cell monolayer. Exp. Cell Res. 2013, 319, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Pomares, A.; Zhou, X.; Calabuig-Fariñas, S.; Lee, S.J.; Torres, S.; Esworthy, T.; Hann, S.Y.; Jantus-Lewintre, E.; Camps, C.; Zhang, L.G. 3D printing novel in vitro cancer cell culture model systems for lung cancer stem cell study. Mater. Sci. Eng. C 2021, 122, 111914. [Google Scholar] [CrossRef]
- Soto-Cerrato, V.; Manuel-Manresa, P.; Hernando, E.; Calabuig-Fariñas, S.; Martínez-Romero, A.; Fernández-Dueñas, V.; Sahlholm, K.; Knöpfel, T.; García-Valverde, M.; Rodilla, A.M.; et al. Facilitated Anion Transport Induces Hyperpolarization of the Cell Membrane That Triggers Differentiation and Cell Death in Cancer Stem Cells. J. Am. Chem. Soc. 2015, 137, 15892–15898. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.G.; D’Arcangelo, E.; Tsao, M.S. Patient-derived cell line, xenograft and organoid models in lung cancer therapy. Transl. Lung Cancer Res. 2020, 9, 2214–2232. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Castellanos-Rizaldos, E.; Grimm, D.G.; Tadigotla, V.; Hurley, J.; Healy, J.; Neal, P.L.; Sher, M.; Venkatesan, R.; Karlovich, C.; Raponi, M.; et al. Exosome-based detection of EGFR T790M in plasma from non–small cell lung cancer patients. Clin. Cancer Res. 2018, 24, 2944–2950. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Shin, S.; Kim, B.; Lee, K.A. Selecting short length nucleic acids localized in exosomes improves plasma EGFR mutation detection in NSCLC patients. Cancer Cell Int. 2019, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahrmann, J.F.; Mao, X.; Irajizad, E.; Katayama, H.; Capello, M.; Tanaka, I.; Kato, T.; Wistuba, I.I.; Maitra, A.; Ostrin, E.J.; et al. Plasma-derived extracellular vesicles convey protein signatures that reflect pathophysiology in lung and pancreatic adenocarcinomas. Cancers 2020, 12, 1147. [Google Scholar] [CrossRef] [PubMed]
- Duréndez-Sáez, E.; Torres-Martinez, S.; Calabuig-Fariñas, S.; Meri-Abad, M.; Ferrero-Gimeno, M.; Camps, C. Exosomal microRNAs in non-small cell lung cancer. Transl. Cancer Res. 2021, 10, 3128–3139. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, P.; Gu, L.; Liu, J. Use of four genes in exosomes as biomarkers for the identification of lung adenocarcinoma and lung squamous cell carcinoma. Oncol. Lett. 2021, 21, 1. [Google Scholar] [CrossRef]
- Chen, J.W.; Dhahbi, J. Lung adenocarcinoma and lung squamous cell carcinoma cancer classification, biomarker identification, and gene expression analysis using overlapping feature selection methods. Sci. Rep. 2021, 11, 13323. [Google Scholar] [CrossRef]
- Lucchetta, M.; Da Piedade, I.; Mounir, M.; Vabistsevits, M.; Terkelsen, T.; Papaleo, E. Distinct signatures of lung cancer types: Aberrant mucin O-glycosylation and compromised immune response. BMC Cancer 2019, 19, 824. [Google Scholar] [CrossRef]
- Moreno-Manuel, A.; Jantus-Lewintre, E.; Simões, I.; Aranda, F.; Calabuig-Fariñas, S.; Carreras, E.; Zúñiga, S.; Saenger, Y.; Rosell, R.; Camps, C.; et al. CD5 and CD6 as immunoregulatory biomarkers in non-small cell lung cancer. Transl. Lung Cancer Res. 2020, 9, 1074–1083. [Google Scholar] [CrossRef]
- Usó, M.; Jantus-Lewintre, E.; Calabuig-Fariñas, S.; Blasco, A.; Del Olmo, E.; Guijarro, R.; Martorell, M.; Camps, C.; Sirera, R. Analysis of the prognostic role of an immune checkpoint score in resected non-small cell lung cancer patients. Oncoimmunology 2017, 6, e1260214. [Google Scholar] [CrossRef] [Green Version]
- Usó, M.; Jantus-Lewintre, E.; Bremnes, R.M.; Calabuig, S.; Blasco, A.; Pastor, E.; Borreda, I.; Molina-Pinelo, S.; Paz-Ares, L.; Guijarro, R.; et al. Analysis of the immune microenvironment in resected non-small cell lung cancer: The prognostic value of different T lymphocyte markers. Oncotarget 2016, 7, 52849–52861. [Google Scholar] [CrossRef] [Green Version]
- Gallach, S.; Jantus-Lewintre, E.; Calabuig-Fariñas, S.; Montaner, D.; Alonso, S.; Sirera, R.; Blasco, A.; Usó, M.; Guijarro, R.; Martorell, M.; et al. MicroRNA profiling associated with non-small cell lung cancer: Next generation sequencing detection, experimental validation, and prognostic value. Oncotarget 2017, 8, 56143–56157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, A.J.G.; Caballero, O.L.; Jungbluth, A.; Chen, Y.T.; Old, L.J. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 2005, 5, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Yokoe, T.; Tanaka, F.; Mimori, K.; Inoue, H.; Ohmachi, T.; Kusunoki, M.; Mori, M. Efficient identification of a novel cancer/testis antigen for immunotherapy using three-step microarray analysis. Cancer Res. 2008, 68, 1074–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero, O.L.; Chen, Y.T. Cancer/testis (CT) antigens: Potential targets for immunotherapy. Cancer Sci. 2009, 100, 2014–2021. [Google Scholar] [CrossRef]
- Gure, A.O.; Chua, R.; Williamson, B.; Gonen, M.; Ferrera, C.A.; Gnjatic, S.; Ritter, G.; Simpson, A.J.G.; Chen, Y.T.; Old, L.J.; et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin. Cancer Res. 2005, 11, 8055–8062. [Google Scholar] [CrossRef] [Green Version]
- Ohue, Y.; Kurose, K.; Karasaki, T.; Isobe, M.; Yamaoka, T.; Futami, J.; Irei, I.; Masuda, T.; Fukuda, M.; Kinoshita, A.; et al. Serum Antibody Against NY-ESO-1 and XAGE1 Antigens Potentially Predicts Clinical Responses to Anti–Programmed Cell Death-1 Therapy in NSCLC. J. Thorac. Oncol. 2019, 14, 2071–2083. [Google Scholar] [CrossRef] [Green Version]
- Shan, Q.; Lou, X.; Xiao, T.; Zhang, J.; Sun, H.; Gao, Y.; Cheng, S.; Wu, L.; Xu, N.; Liu, S. A cancer/testis antigen microarray to screen autoantibody biomarkers of non-small cell lung cancer. Cancer Lett. 2013, 328, 160–167. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Fu, Q. Exosomes from CD133+ cells carrying circ-ABCC1 mediate cell stemness and metastasis in colorectal cancer. J. Cell Biochem. 2020, 121, 3286–3297. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Guo, B.; Deng, J.; Wu, S.; Li, F.; Wang, Y.; Lu, J.; Zhou, Y. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc signaling in female esophageal carcinoma. Mol. Cancer 2019, 18, 22. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Zhou, Y.; Dong, L.; Ma, W.; Lv, L.; Zhang, J.; Wang, X. Glioblastoma Stem Cell-Derived Exosomes Enhance Stemness and Tumorigenicity of Glioma Cells by Transferring Notch1 Protein. Cell Mol. Neurobiol. 2020, 40, 767–784. [Google Scholar] [CrossRef]
- Xiang, H.; Li, F.; Luo, J.; Long, W.; Hong, L.; Hu, Y.; Du, H.; Yuan, Y.; Luo, M. A meta-analysis on the relationship of exosomes and the prognosis of lung cancer. Medicine 2021, 100, e25332. [Google Scholar] [CrossRef] [PubMed]
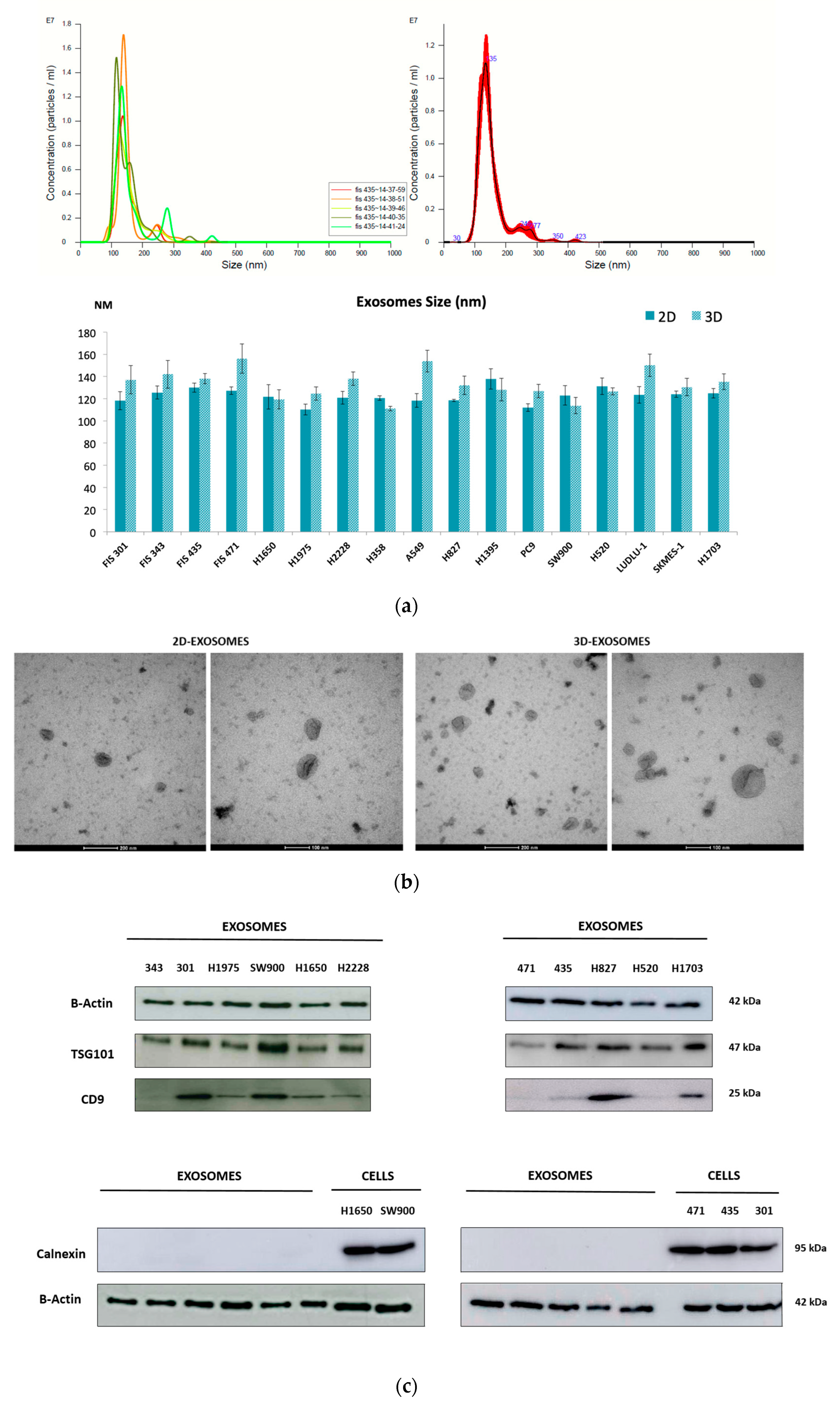
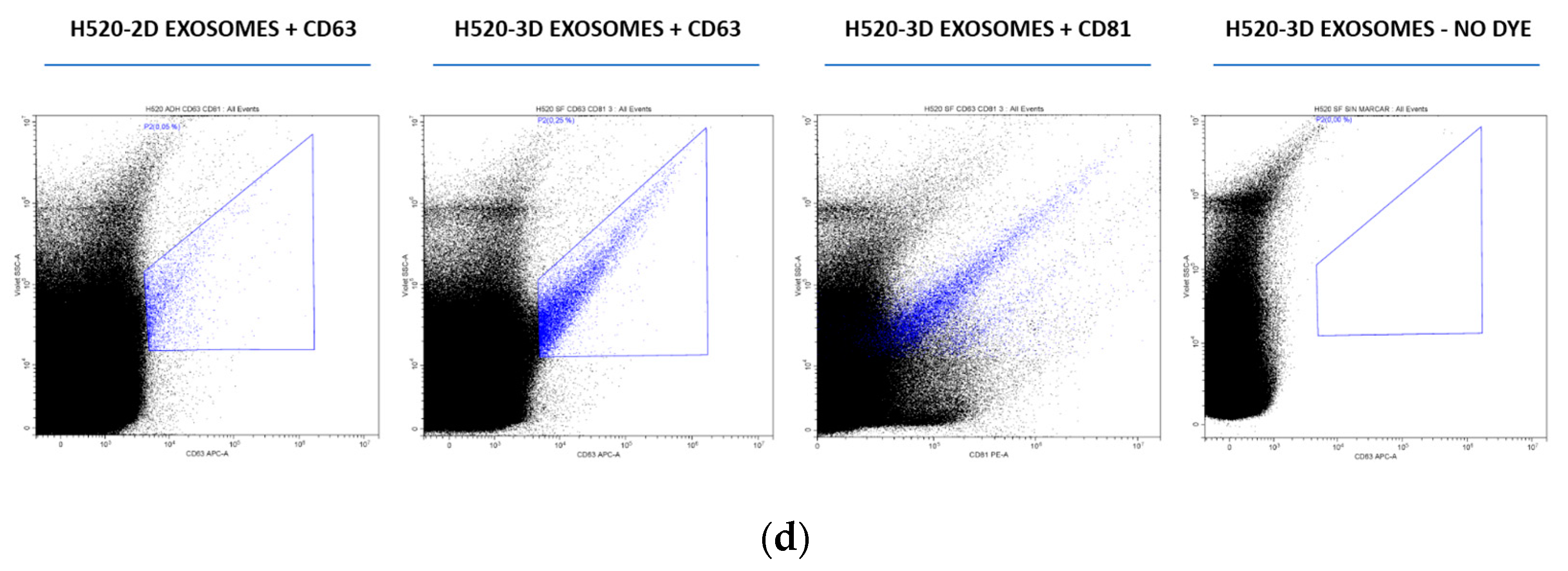
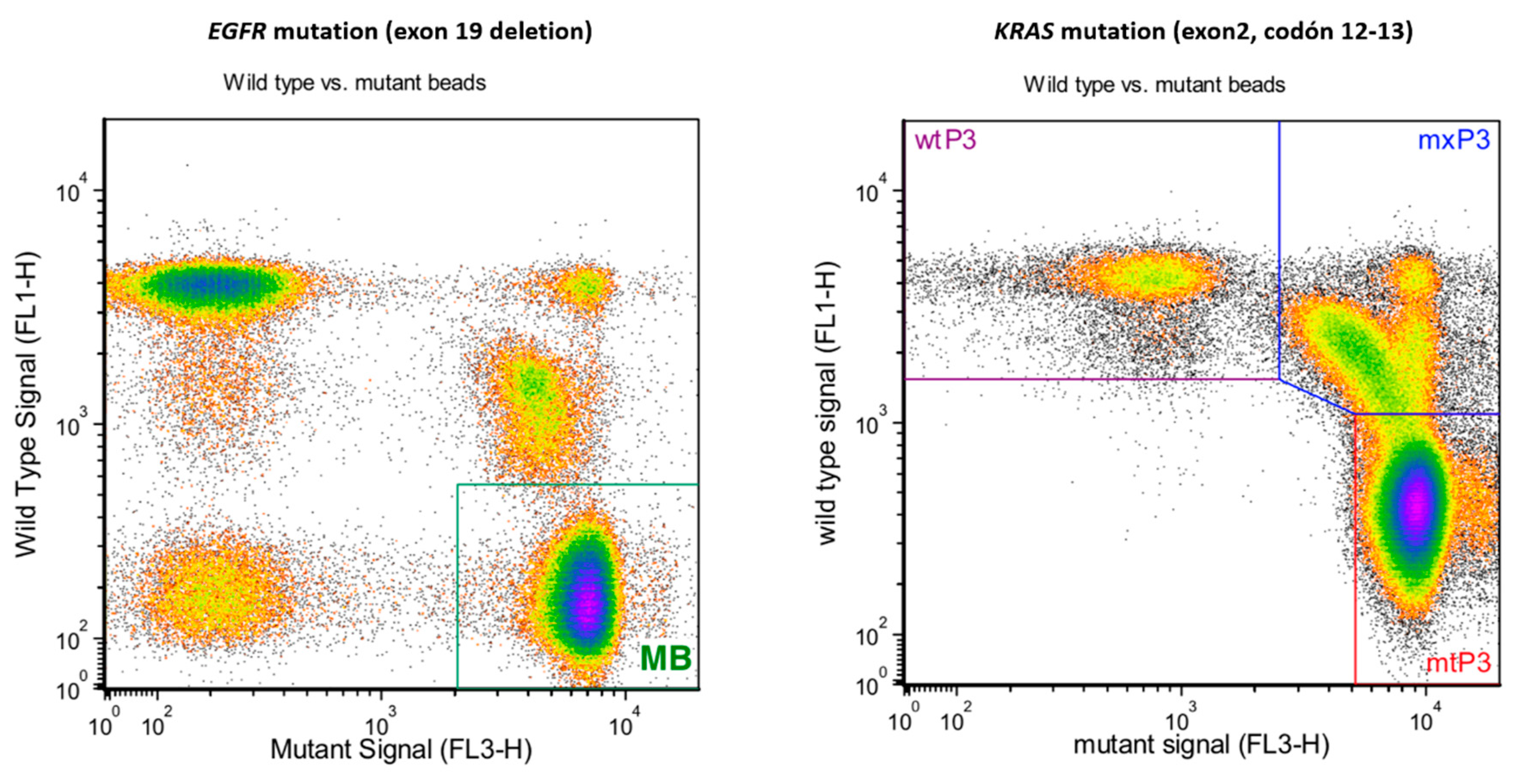
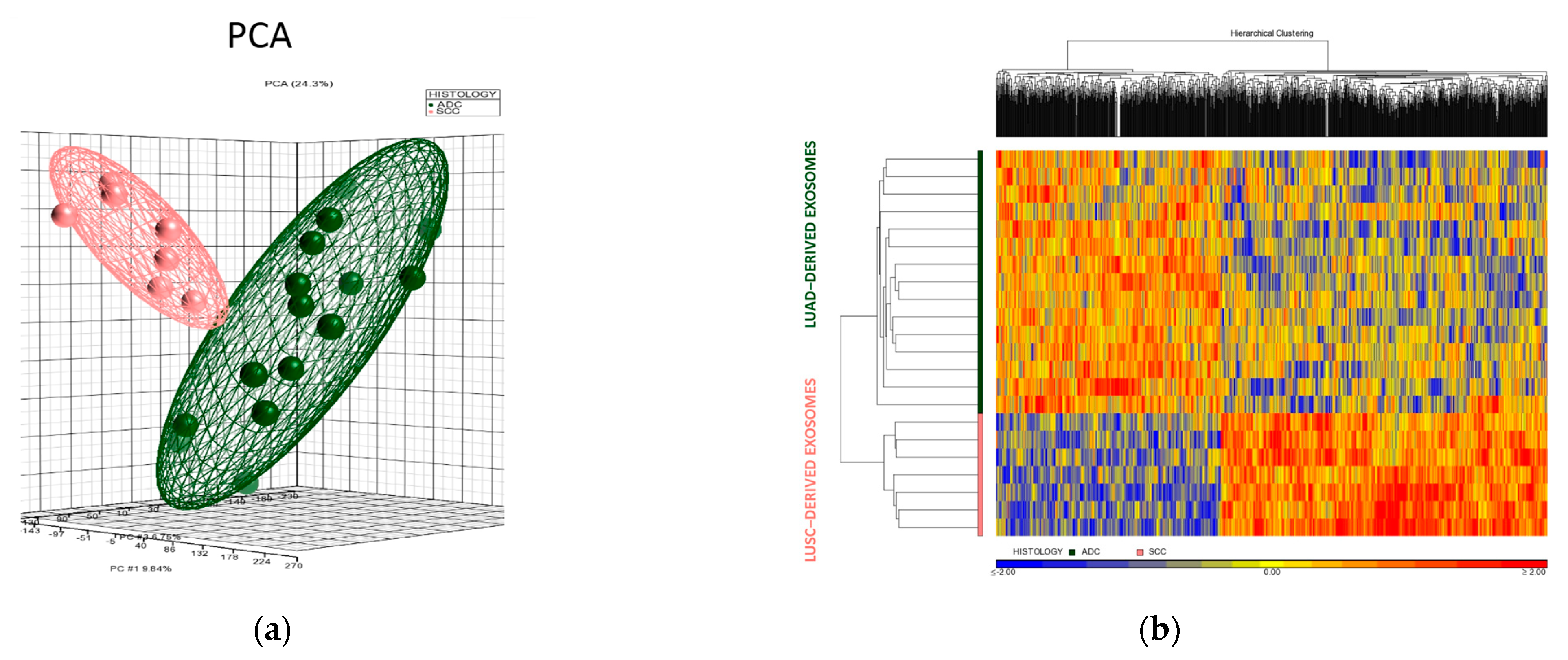

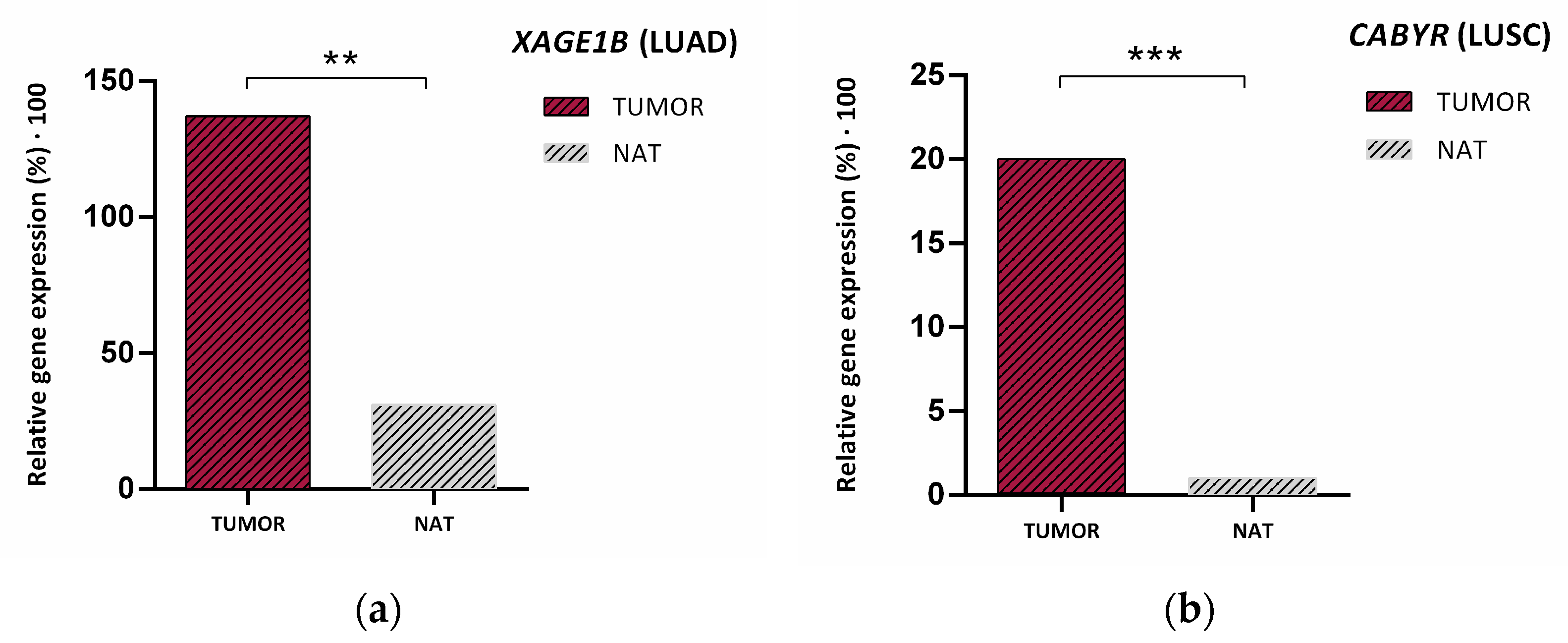
| Gene Symbol | RefSeq | p-Value | Fold Change | Description |
|---|---|---|---|---|
| XAGE1B | NM_001097604 | 0.0002 | 4.38139 | LUAD up vs. LUSC |
| SEPP1 | NM_001085486 | 0.0042 | 1.75403 | LUAD up vs. LUSC |
| NKX2-1 (TTF-1) | NM_001079668 | 0.0022 | 1.27234 | LUAD up vs. LUSC |
| CAPRIN1 RIOK3 | NM_005898 NM_003831 | 8.94 × 105 0.0001 | 2.47485 2.56426 | LUSC up vs. LUAD LUSC up vs. LUAD |
| CABYR | NM_001308231 | 2.04 × 105 | 4.72413 | LUSC up vs. LUAD |
| Characteristics | Total | % |
|---|---|---|
| (N = 661) | ||
| Age at surgery | (median, range) | |
| 66 (38–88) | ||
| Gender | ||
| Male | 395 | 59.76 |
| Female | 266 | 40.24 |
| Smoking status | ||
| Current | 165 | 24.96 |
| Former | 382 | 57.79 |
| Never | 114 | 17.25 |
| Stage | ||
| IA | 152 | 22.99 |
| IB | 223 | 33.74 |
| IIA | 63 | 9.53 |
| IIB | 116 | 17.55 |
| IIIA | 107 | 16.19 |
| Histology | ||
| Adenocarcinoma | 345 | 52.19 |
| Squamous cell carcinoma | 316 | 47.81 |
| Exitus | ||
| Yes | 261 | 39.49 |
| No | 400 | 60.51 |
| Characteristics | Total | % |
|---|---|---|
| (N = 186) | ||
| Age at surgery | (Median, range) | |
| 65 (26–85) | ||
| Gender | ||
| Male | 158 | 84.95 |
| Female | 28 | 15.05 |
| Smoking status | ||
| Current | 91 | 48.65 |
| Former | 74 | 39.78 |
| Never | 21 | 11.29 |
| Stage | ||
| I | 96 | 51.61 |
| II | 55 | 29.57 |
| IIIA | 35 | 18.82 |
| Histology | ||
| Adenocarcinoma | 79 | 42.48 |
| Squamous cell carcinoma | 90 | 48.38 |
| Others | 17 | 9.14 |
| Relapse | ||
| Yes | 85 | 45.7 |
| No | 101 | 54.3 |
| Exitus | ||
| Yes | 91 | 48.92 |
| No | 95 | 51.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duréndez-Sáez, E.; Calabuig-Fariñas, S.; Torres-Martínez, S.; Moreno-Manuel, A.; Herreros-Pomares, A.; Escorihuela, E.; Mosqueda, M.; Gallach, S.; Guijarro, R.; Serna, E.; et al. Analysis of Exosomal Cargo Provides Accurate Clinical, Histologic and Mutational Information in Non-Small Cell Lung Cancer. Cancers 2022, 14, 3216. https://doi.org/10.3390/cancers14133216
Duréndez-Sáez E, Calabuig-Fariñas S, Torres-Martínez S, Moreno-Manuel A, Herreros-Pomares A, Escorihuela E, Mosqueda M, Gallach S, Guijarro R, Serna E, et al. Analysis of Exosomal Cargo Provides Accurate Clinical, Histologic and Mutational Information in Non-Small Cell Lung Cancer. Cancers. 2022; 14(13):3216. https://doi.org/10.3390/cancers14133216
Chicago/Turabian StyleDuréndez-Sáez, Elena, Silvia Calabuig-Fariñas, Susana Torres-Martínez, Andrea Moreno-Manuel, Alejandro Herreros-Pomares, Eva Escorihuela, Marais Mosqueda, Sandra Gallach, Ricardo Guijarro, Eva Serna, and et al. 2022. "Analysis of Exosomal Cargo Provides Accurate Clinical, Histologic and Mutational Information in Non-Small Cell Lung Cancer" Cancers 14, no. 13: 3216. https://doi.org/10.3390/cancers14133216
APA StyleDuréndez-Sáez, E., Calabuig-Fariñas, S., Torres-Martínez, S., Moreno-Manuel, A., Herreros-Pomares, A., Escorihuela, E., Mosqueda, M., Gallach, S., Guijarro, R., Serna, E., Suárez-Cabrera, C., Paramio, J. M., Blasco, A., Camps, C., & Jantus-Lewintre, E. (2022). Analysis of Exosomal Cargo Provides Accurate Clinical, Histologic and Mutational Information in Non-Small Cell Lung Cancer. Cancers, 14(13), 3216. https://doi.org/10.3390/cancers14133216









