Simple Summary
Immune checkpoint inhibitors have improved the tumor response and survival of patients with metastatic renal cell carcinoma. However, no predictive factor of response to immunotherapy is currently available. The aim of our study was to evaluate metabolism, assessed with resting energy expenditure as a biomarker of response, in patients with metastatic renal cell carcinoma treated with nivolumab. We found that the 15 patients (29% of the cohort) that had hypermetabolism also had decreased tumor control and tended to have a worse overall survival compared to patients with normal or low metabolism. We conclude that the measurement of resting energy expenditure could help identify patients who will benefit most from immunotherapy in metastatic renal cell carcinoma.
Abstract
Background: Nivolumab improved patients’ survival in metastatic renal cell carcinoma (mRCC). We aimed to evaluate resting energy expenditure (REE) (i.e., patients’ basal metabolism) to predict efficacy. Methods: We conducted a monocentric, observational study of mRCC patients receiving nivolumab between October 2015 and May 2020. REE was measured prior to initiating immunotherapy using indirect calorimetry to determine hypo, normo and hypermetabolism. Primary endpoint was 6-month, progression-free survival (PFS), and secondary endpoints were response rate, PFS and overall survival (OS). Results: Of the 51 consecutive patients, 15 (29%) were hypermetabolic, 24 (47%) normometabolic, and 12 (24%) hypometabolic. The 6-month PFS was 15% for hypermetabolic patients and 65% for non-hypermetabolic patients (p < 0.01). In the multivariate analysis, hypermetabolism was the only baseline factor predicting 6-month PFS (OR 9.91, 95%CI [1.62–60.55], p = 0.01). Disease progression was noted as the best response in 73% of hypermetabolic patients and 26% of non-hypermetabolic patients (p = 0.02). Median PFS was 2.8 and 8.7 months (p < 0.01), and median OS was 20.2 and 35.1 months (p = 0.13) in the hypermetabolic and non-hypermetabolic groups, respectively. Conclusions: Our study identifies an association between mRCC patients’ energy expenditure and nivolumab efficacy. The measurement of REE by indirect calorimetry in routine practice could help identify patients at risk of nivolumab failure.
1. Introduction
Renal cell carcinoma is the 6th and 10th most frequent cancer in men and women, respectively [1]. The prognosis of metastatic renal cell carcinoma (mRCC) has improved during recent decades with Vascular Epithelial Growth Factor Receptor (VEGFR), tyrosine kinase inhibitors (TKI), anti-PD-1 and anti-CTLA-4 immune checkpoint inhibitors (ICI). In the frontline setting, recent studies demonstrated the superiority of sunitinib over ipilimumab plus nivolumab [2], pembrolizumab–axitinib [3], cabozantinib–nivolumab [4] and lenvatinib–pembrolizumab [5], according to the International Metastatic Consortium Database (IMDC) risk subgroups. In the second line after previous TKI treatment, nivolumab as a monotherapy is the only approved ICI [6]. Despite these recent developments, survival remains poor in mRCC, with a survival rate of 5 years at 12%, and primary or secondary resistance to ICI occurring most of the time [7]. As of today, no robust predictive factor of response to ICI exists in mRCC. For example, the PD-L1 status, which is routinely used as a tumor-related biomarker of response in non-small lung cancer (NSCLC), has been inconstantly associated with response to ICI, and is not recommended in clinical practice [8,9,10]. There is an urgent need to identify the patients who will benefit from these treatments and those who are only at risk of toxicity or disease progression.
Cancer-associated weight loss results from negative energy balance, due to decreased food intake, increased resting energy expenditure (REE), or both. REE can be defined as the amount of energy that is spent in one day by a resting individual. Hypermetabolism is defined as an increase in measured REE compared to calculated REE from standard formulas [11] and can affect up to half of cancer patients [12]. It is considered as an early and major contributor to cancer cachexia and was determined as an independent prognostic factor for NSCLC patients’ survival [13]. Furthermore, hypermetabolism is associated with the production of inflammatory cytokines [14], and thus could possibly modulate the response to immunotherapy. We hypothesized that increased REE would result in a reduced ability of the patient to develop an active immune response following ICI infusion. This is because T-cell activation requires metabolic changes to leave the G0 stage and enter the G1 stage of the cell cycle, which includes enhanced glycolysis and is a highly ATP-dependent process [15]. We previously demonstrated that ICI have poor efficacy in hypermetabolic NSCLC patients with a worse prognosis compared to non-hypermetabolic patients [16].
In this context, we decided to evaluate the association between metabolism and efficacy outcomes in mRCC patients treated with nivolumab.
2. Patients and Methods
2.1. Patients
We conducted a prospective, monocentric, observational study, and thus neither randomized nor blinded, between 1 October 2015 and 31 May 2020 in Hôpital Cochin AP-HP, Paris. We enrolled consecutive patients who participated in the CERTIM (Immunomodulatory Therapies Multidisciplinary Study group) multidisciplinary risk assessment program for immunotherapy in the outpatient unit of the oncology department. This program is proposed to all cancer patients before ICI initiation and aims to provide personalized, supportive and optimized care. During this program, patients benefit from a multidisciplinary evaluation, including a nutritional assessment with a dietitian consultation and indirect calorimetry.
Male and female patients of at least 18 years of age with histologically proven metastatic RCC and indication of nivolumab were eligible. Patients were followed until death or last examination. Follow-up period for the current analysis ended on 2 September 2020.
Written informed consent was obtained for all patients. The study was approved by the Cochin institutional ethical review board (CLEP n°5120518) and procedures were performed according to the revised Declaration of Helsinki.
2.2. Data Collection
REE was determined prior to immunotherapy initiation under standard resting conditions, i.e., after 12 h of fasting (overnight), between 8 and 9 a.m. in a thermo-neutral environment by a trained nurse. Before the start of the measurements, patients rested quietly for 15 min while the indirect calorimeter was calibrated and a steady state was obtained [17]. Patients were asked to remain awake for the duration of the measurement. For each patient, oxygen consumption (VO2) was measured for 15 min by indirect calorimetry using a face mask connected to an oxygen analyzer (Fitmate, COSMED, Italy). The calorimeter was calibrated before each measurement. Measured REE (mREE, kcal/d) was determined from VO2 using Weir’s equation [18], and the results were immediately displayed in software attached to the system.
To evaluate the extent of REE alteration compared to healthy individuals, mREE was compared to predicted REE (pREE), calculated with revised Harris and Benedict equations [19]:
Males: pREE (kcal/d) = 66.5 + 13.75 × weight (kg) + 500 × height (m) − 6.78 × age
Females: pREE (kcal/d) = 655 + 9.56 × weight + 185 × height − 4.68 × age
Hypermetabolism is defined as mREE over pREE ≥ 110%, normometabolism is defined as mREE/pREE between 90 and 110%, and hypometabolism is defined as mREE/pREE < 90% [11]. In our study, hypermetabolic patients were compared to the other patients (normometabolic and hypometabolic patients).
During the multidisciplinary risk assessment program, multiple clinical and biological measurements were performed. Concomitant medication that could impact the immune response was recorded [20,21]. Risk groups for survival were defined as favorable, intermediate or poor using the IMDC classification [22].
Response to treatment was evaluated by computed tomography at two to four months after treatment initiation, at the discretion of the treating oncologist.
Treatment-related adverse events (TRAE) were defined as all-grade adverse events that could be related to nivolumab, according to the oncologist. A serious adverse event was defined as an adverse event requiring treatment interruption, corticosteroid use and/or hospitalization.
2.3. Outcomes
Six-month progression-free survival (6 m PFS), i.e., the proportion of patients that did not have disease progression at 6 months, was the main evaluation criterion since it was determined to be a fair surrogate for the evaluation of efficacy of ICI [23]. Secondary criteria were response rate, PFS and overall survival (OS).
2.4. Statistical Analysis
Calculations were performed using R statistical software (version 4.1.0, R Stats, survival and survcomp packages).
Comparisons between groups were performed with the Mann–Whitney–Wilcoxon test for quantitative variables and Fisher’s exact test for qualitative variables.
Based on previous results of the CheckMate-025 study [6], we calculated that we would need to enroll 17 patients in the hypermetabolic group and 33 patients in the normo- and hypometabolic group to show a 50% difference of 6m PFS (80% vs. 40%, respectively) with a two-sided 5% significance level and an 80% statistical power.
Logistic regression was used to test the association of clinical and biological variables with 6-month PFS. Variables not normally distributed were transformed into categorical variables for logistic regression analyses. Significant variables in univariate analyses and clinically relevant analyses such as the IMDC prognostic risk groups were included into multivariable models. Interaction tests revealed no significant subgroup differences. The calibration of multivariate models was checked using the Hosmer–Lemeshow test (adequate calibration with p > 0.05 for all models). Finally, likelihood ratio test was used to check the discrimination performance of nested models (p < 0.05 for models adding mREE/pREE).
Survival curves were obtained with Kaplan–Meier estimates and compared with log-rank test.
All p-values were two-sided, and the level of significance was set at p < 0.05.
3. Results
3.1. Patient Characteristics
Between October 2015 and September 2020, 58 mRCC patients were included in the CERTIM multidisciplinary assessment before nivolumab treatment, and 51 patients were included in the study cohort (Figure 1).
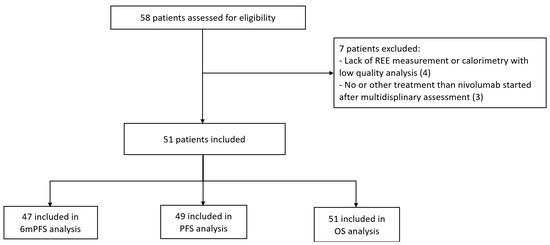
Figure 1.
Flow chart. REE: resting energy expenditure; PFS: progression-free survival; 6 m PFS: 6-month progression-free survival; OS: overall survival.
Among them, two patients were lost to follow-up and two had discontinuation of nivolumab before 6 months because of a serious TRAE.
Patient characteristics are presented in Table 1.

Table 1.
Patient characteristics.
Using the standard mREE/pREE ≥ 110% and <90% cut-off, a total of 15 (29%), 24 (47%) and 12 (24%) patients were classified as hypermetabolic, normometabolic and hypometabolic, respectively. Patients were predominantly men, median age was 63 years, and performance status (PS) was good. Most patients (60%) had received one previous TKI regimen. Eight patients received frontline nivolumab due to TKI ineligibility or sarcomatoid features on the tumor [24]. Characteristics were well-balanced between the hypermetabolic and non-hypermetabolic groups. Neutrophil-to-lymphocyte ratio (NLR) at four to six weeks of treatment did not differ between the two groups (p = 0.74 and 0.67, respectively; data not shown).
Two patients had a papillary renal cell carcinoma. They were in the non-hypermetabolic group, and nivolumab was used as second-line treatment in both patients.
3.2. Efficacy
The median follow-up was 19.2 months. The 6m PFS was 15% in hypermetabolic patients and 65% in non-hypermetabolic patients (p < 0.01). In the univariate analysis, increased REE and the absence of TRAE were unfavorably associated with 6m PFS (Table 2).

Table 2.
Univariate analysis of 6-month progression-free survival.
In multivariate analysis, these two parameters were associated with 6m PFS, with an OR of 9.91 for hypermetabolism (95%CI [1.62–60.55], p = 0.01) (Table 3).

Table 3.
Multivariate analysis for 6-month progression-free survival.
Disease control rate, defined as the proportion of patients with complete response, partial response, or stable disease, was statistically higher in the non-hypermetabolic group, with 74% and 27% in the non-hypermetabolic and hypermetabolic groups, respectively (p < 0.01, Table 4). The two patients with a papillary histology subtype had stable disease as the best response, and the duration of response was 4 and 5 months.

Table 4.
Efficacy of nivolumab.
At data cut-off, 13 of 14 patients (93%) in the hypermetabolic group had disease progression compared to 25 of 35 (71%) in the non-hypermetabolic group. Median PFS was 2.8 months in the hypermetabolic group and 8.7 months in the non-hypermetabolic group (p < 0.01, Figure 2).
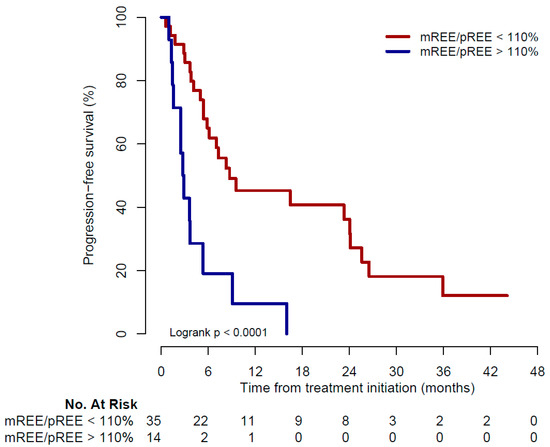
Figure 2.
Kaplan–Meier curve for progression-free survival. mREE: measured resting energy expenditure; pREE: predicted resting energy expenditure.
Twenty-seven deaths were observed: 11 in the hypermetabolic group (73%) and 16 in the non-hypermetabolic group (44%). Median OS was 20.2 months (range 1.3–45) and 35.1 months (range 1–48.4) in the hypermetabolic and non-hypermetabolic groups, respectively (p = 0.13, Figure 3).
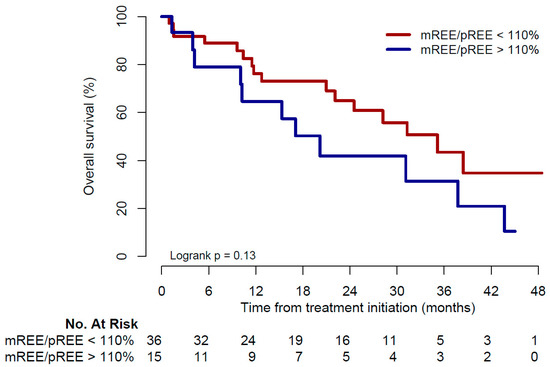
Figure 3.
Kaplan–Meier curve for overall survival. mREE: measured resting energy expenditure; pREE: predicted resting energy expenditure.
3.3. Toxicity
All-grade TRAE occurred in 4 (27%) and 19 (53%) of the hypermetabolic and non-hypermetabolic patients, respectively (p = 0.13). The main TRAE were cutaneous lesions (33%), dysthyroidism (8%) and pneumonitis (8%). Among patients presenting a TRAE, seven presented a serious adverse event: five patients in the non-hypermetablic group (two interstitial pneumonitis, three serious cutaneous reaction); and two patients in the hypermetabolic group (one pneumonitis and one myositis with cardiac insufficiency) (p = 0.57); with no death associated with the reported treatment toxicity.
4. Discussion
To our best knowledge, this is the first study evaluating the association of resting energy expenditure and efficacy of ICI in mRCC. We found that non-hypermetabolic patients receiving nivolumab had better outcomes than hypermetabolic patients, with a 6m PFS of 65% and 15%, respectively. The objective response rate was also two-fold higher in the non-hypermetabolic group than in the hypermetabolic group, and hypermetabolism resulted in a significantly decreased disease control rate as compared to non-hypermetabolism.
This report is the first study including a measurement of REE in mRCC patients treated with ICI. The diagnosis of hypermetabolism can be obtained in routine practice in the outpatient setting using indirect calorimetry, with a result immediately available for the physician. REE reflects the total amount of energy used by an individual per day. It depends, for example, on weight, sex and age, and its value varies from one person to another. A vast array of phenomena can increase REE in cancer patients. The energetic demand of the tumor itself, changes in inflammation and body composition, will result in increased REE. It is estimated that up to half of cancer patients may be hypermetabolic [12,16,25]. We confirmed in this study the high prevalence of hypermetabolism with 29% of patients being hypermetabolic. Cancer cachexia is a multifactorial syndrome with an ongoing loss of skeletal muscle mass that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment [26]. Hypermetabolism has been correlated with clinical and biological markers of cancer cachexia such as weight loss, PS ≥ 2, CRP ≥ 10 mg/L and associated with reduced survival [12,13]. Hypermetabolism is an early feature of cachexia. Therefore, indirect calorimetry should be encouraged and performed early and systematically for need and risk assessments of cancer patients, prior to initiation of cancer therapies.
Nivolumab was approved for the treatment of mRCC following the pivotal CheckMate-025 study [6]. Patients’ characteristics in both studies are comparable, with a high proportion of males, 16% in the unfavorable IMDC risk group, and patients that received mainly one or two prior lines of treatment before nivolumab. Efficacy outcomes for the overall population of our study were similar to the nivolumab group in the CheckMate-025 study, with 45% and 40% of 6m PFS, and 33% and 25% of objective response rate, respectively. Furthermore, primary resistance to nivolumab was reported in 39% of our overall study population (73% of hypermetabolic patients and 26% of non-hypermetabolic patients), and in 35% of the nivolumab cohort of the CheckMate-025 study. Interestingly, survival surrogates were better in the non-hypermetabolic group of our study than in the nivolumab group in the CheckMate-025 study: median PFS was 8.7 months in our study group versus 4.6 months in the CheckMate-025 study; and median OS was 35 months versus 25 months.
In our study, we did not find any predictive marker of nivolumab efficacy other than hypermetabolism and occurrence of TRAE. In mRCC, no easily available biomarker of response to immunotherapy has been identified, but multiple studies are currently trying to evaluate the tumor, tumor microenvironment and host-related biomarkers. Promising biomarkers related to the tumor include molecular signatures, such as in the BIONIKK trial [27]. Host-related factors offer new potential biomarkers in mRCC such as the gut microbiome [28] and systemic inflammation reflected by NLR [29,30]. Finally, the IMDC risk group has been identified as a predictive factor of response to nivolumab plus ipilimumab [2]. The predictive value of TRAE has also been studied [31,32] but it cannot help as a predictive marker before initiation of treatment. Interestingly, we previously reported an association between patients’ hypermetabolism and acute toxicity under nivolumab [33].
The main limitations of our study are its monocentric nature and its limited sample size. In our cohort, eight patients received nivolumab as frontline treatment, which may not have a comparable efficacy as in the post TKI setting [34,35,36]. The value of hypermetabolism as a biomarker of response under TKI treatment or ICI combination with ICI or TKI is currently unknown. We decided to include all mRCC patients in our cohort, including two patients with papillary histology subtype since we believe that metabolism is a host-related, histology-independent marker. Although the efficacy of ICI in papillary renal carcinoma may differ from that of ICI in clear cell carcinoma, these two non-hypermetabolic patients had stable disease as a best response.
This study also has strengths. Despite a limited number of patients, there is a strong indication that hypermetabolism should be investigated as a marker of efficacy in larger cohorts. Our population is representative of the real-life setting in mRCC, with patient characteristics and outcomes consistent with the literature and similar to that of the pivotal CheckMate-025 study [22,37].
It can be highlighted that, in our study, hypermetabolism is not only associated with a poor PFS but also with a reduced tumor response rate, suggesting a direct link between whole-body energy and the possibility of a pharmacodynamic effect of ICI. Generating an immune response is a considerable bioenergetic challenge [15]. In order to respond appropriately to the tumors, T-cells have to metabolically switch between resting and proliferative states and actively acquire metabolic substrates from their environment to meet these energy demands [38]. Since hypermetabolism indicates that increased energy is spent by the patient in resting conditions, to maintain vital homeostasis, we hypothesized that hypermetabolic patients might have less remaining energy to fuel lymphocytes, and therefore may experience less lymphocytes-mediated tumor responses [39,40].
In conclusion, indirect calorimetry allows for an easy and reproducible measure of REE, and our findings point toward its interest as a marker of efficacy for immunotherapy in mRCC patients. Future studies will need to explore this biomarker in the frontline setting in different ICI combinations and determine the efficacy of strategies to reverse patients’ hypermetabolism.
Author Contributions
J.N.: data curation; methodology; formal analysis; writing—original draft; writing—review and editing; A.J.: methodology; formal analysis; writing—review and editing; J.A. (Jérôme Alexandre): investigation; supervision; writing—review and editing; G.U.: investigation; writing—review and editing; M.B.: data curation; writing—review and editing; Z.C.-A.: data curation; writing—review and editing; S.D.P.: investigation; writing—review and editing; C.V.-V.: investigation; writing—review and editing; M.-P.R.: writing—review and editing; M.P.: writing—review and editing; P.B.-R.: investigation; writing—review and editing; J.A. (Jennifer Arrondeau): investigation; writing—review and editing; I.G.: writing—review and editing; J.-P.D.: writing—review and editing; F.G.: investigation; methodology; project administration; resources; supervision; validation; writing—review and editing; O.H.: methodology; supervision; validation; writing—original draft; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Cochin Hospital CLEP n°5120518.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
Pr. Alexandre reports personal fees from MSD, ASTRA ZENECA, EISAI, CLOVIS, grants and non-financial support from JANSSEN, personal fees and non-financial support from GSK, outside the submitted work. Dr. Boudou-Rouquette reports personal fees from TAKEDA, PHARMAMAR, outside the submitted work. Pr. Goldwasser reports grants and personal fees from BAXTER, personal fees from FRESENIUS KABI, NUTRICIA, outside the submitted work. Dr. Huillard reports personal fees from BAYER, SANOFI, MSD, BMS, IPSEN, PFIZER, EISAI, JANSSEN, ASTRA ZENECA, outside the submitted work. Dr. Noel, Dr. Jouinot, Dr. Ulmann, Dr. Bretagne, Dr. Castel-Ajgal, Dr. De Percin, Dr. Vaqui-Villeminey, Pr. Revel, Pr. Peyromaure, Dr. Arrondeau, Dr. Gataa, Dr. Durand have nothing to disclose.
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [Green Version]
- Survival Rates for Kidney Cancer. Available online: https://www.cancer.org/cancer/kidney-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 9 July 2020).
- Albiges, L.; Flippot, R.; Escudier, B. Immune Checkpoint Inhibitors in Metastatic Clear-Cell Renal Cell Carcinoma: Is PD-L1 Expression Useful? Eur. Urol. 2021, 79, 793–795. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Noel, J.; Huillard, O.; Goldwasser, F. Re: Keiichiro Mori, Mohammad Abufaraj, Hadi Mostafaei; et al. The Predictive Value of Programmed Death Ligand 1 in Patients with Metastatic Renal Cell Carcinoma Treated with Immune-Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Eur Urol. In Press. https://doi.org/10.1016/j.Eururo.2020.10.006. Clinical Activity of Immune Checkpoint Inhibitors: Is the Host the Answer? Eur. Urol. 2021, 79, e112. [Google Scholar] [CrossRef]
- Boothby, W.M.; Sandiford, I. Summary of the Basal Metabolism Data on 8614 Subjects with Especial Reference to the Normal Standards for the Estimation of the Basal Metabolic Rate. J. Biol. Chem. 1922, 54, 783–803. [Google Scholar] [CrossRef]
- Vazeille, C.; Jouinot, A.; Durand, J.-P.; Neveux, N.; Boudou-Rouquette, P.; Huillard, O.; Alexandre, J.; Cynober, L.; Goldwasser, F. Relation between Hypermetabolism, Cachexia, and Survival in Cancer Patients: A Prospective Study in 390 Cancer Patients before Initiation of Anticancer Therapy. Am. J. Clin. Nutr. 2017, 105, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Jouinot, A.; Ulmann, G.; Vazeille, C.; Durand, J.-P.; Boudou-Rouquette, P.; Arrondeau, J.; Tlemsani, C.; Fournel, L.; Alifano, M.; Wislez, M.; et al. Hypermetabolism Is an Independent Prognostic Factor of Survival in Metastatic Non-Small Cell Lung Cancer Patients. Clin. Nutr. Edinb. Scotl. 2020, 39, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Roubenoff, R.A.; Cannon, J.G.; Kehayias, J.J.; Zhuang, H.; Dawson-Hughes, B.; Dinarello, C.A.; Rosenberg, I.H. Rheumatoid Cachexia: Cytokine-Driven Hypermetabolism Accompanying Reduced Body Cell Mass in Chronic Inflammation. J. Clin. Investig. 1994, 93, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.; Priyadharshini, B.; Turka, L.A. Immunometabolism of Regulatory T Cells. Nat. Immunol. 2016, 17, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Boudou-Rouquette, P.; Arrondeau, J.; Gervais, C.; Durand, J.-P.; Fabre, E.; De Percin, S.; Villeminey, C.V.; Piketty, A.-C.; Rassy, N.; Ulmann, G.; et al. Development and Validation of a Host-Dependent, PDL1-Independent, Biomarker to Predict 6-Month Progression-Free Survival in Metastatic Non-Small Cell Lung Cancer (MNSCLC) Patients Treated with Anti-PD1 Immune Checkpoint Inhibitors (ICI) in the CERTIM Cohort: The ELY Study. EBioMedicine 2021, 73, 103630. [Google Scholar] [CrossRef]
- Achamrah, N.; Delsoglio, M.; De Waele, E.; Berger, M.M.; Pichard, C. Indirect Calorimetry: The 6 Main Issues. Clin. Nutr. 2021, 40, 4–14. [Google Scholar] [CrossRef]
- de Weir, J.B.V. New Methods for Calculating Metabolic Rate with Special Reference to Protein Metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Roza, A.M.; Shizgal, H.M. The Harris Benedict Equation Reevaluated: Resting Energy Requirements and the Body Cell Mass. Am. J. Clin. Nutr. 1984, 40, 168–182. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Zhang, Y.; Sivik, J.M.; Schmeck, C.; Zhu, J.; Repasky, E.A.; Drabick, J.J.; Schell, T.D. Beta Blocker Use Correlates with Better Overall Survival in Metastatic Melanoma Patients and Improves the Efficacy of Immunotherapies in Mice. Oncoimmunology 2017, 7, e1405205. [Google Scholar] [CrossRef] [Green Version]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative Association of Antibiotics on Clinical Activity of Immune Checkpoint Inhibitors in Patients with Advanced Renal Cell and Non-Small-Cell Lung Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.-H.; et al. External Validation and Comparison with Other Models of the International Metastatic Renal-Cell Carcinoma Database Consortium Prognostic Model: A Population-Based Study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, G.; Gasper, H.; Man, J.; Lord, S.; Marschner, I.; Friedlander, M.; Lee, C.K. Defining the Most Appropriate Primary End Point in Phase 2 Trials of Immune Checkpoint Inhibitors for Advanced Solid Cancers: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 522–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannir, N.M.; Signoretti, S.; Choueiri, T.K.; McDermott, D.F.; Motzer, R.J.; Flaifel, A.; Pignon, J.-C.; Ficial, M.; Frontera, O.A.; George, S.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab versus Sunitinib in First-Line Treatment of Patients with Advanced Sarcomatoid Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 78–86. [Google Scholar] [CrossRef]
- Xu, W.P.; Cao, D.X.; Lin, Z.M.; Wu, G.H.; Chen, L.; Zhang, J.P.; Zhang, B.; Yang, Z.A.; Jiang, Y.; Han, Y.S.; et al. Analysis of Energy Utilization and Body Composition in Kidney, Bladder, and Adrenal Cancer Patients. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Epaillard, N.; Simonaggio, A.; Elaidi, R.; Azzouz, F.; Braychenko, E.; Thibault, C.; Sun, C.-M.; Moreira, M.; Oudard, S.; Vano, Y.-A. BIONIKK: A Phase 2 Biomarker Driven Trial with Nivolumab and Ipilimumab or VEGFR Tyrosine Kinase Inhibitor (TKI) in Naïve Metastatic Kidney Cancer. Bull. Cancer 2020, 107, eS22–eS27. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Lalani, A.-K.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; Van Allen, E.M.; et al. Change in Neutrophil-to-Lymphocyte Ratio (NLR) in Response to Immune Checkpoint Blockade for Metastatic Renal Cell Carcinoma. J. Immunother. Cancer 2018, 6, 5. [Google Scholar] [CrossRef]
- Simonaggio, A.; Elaidi, R.; Fournier, L.; Fabre, E.; Ferrari, V.; Borchiellini, D.; Thouvenin, J.; Barthelemy, P.; Thibault, C.; Tartour, E.; et al. Variation in Neutrophil to Lymphocyte Ratio (NLR) as Predictor of Outcomes in Metastatic Renal Cell Carcinoma (MRCC) and Non-Small Cell Lung Cancer (MNSCLC) Patients Treated with Nivolumab. Cancer Immunol. Immunother. 2020, 69, 2513–2522. [Google Scholar] [CrossRef]
- Verzoni, E.; Cartenì, G.; Cortesi, E.; Giannarelli, D.; De Giglio, A.; Sabbatini, R.; Buti, S.; Rossetti, S.; Cognetti, F.; Rastelli, F.; et al. Real-World Efficacy and Safety of Nivolumab in Previously-Treated Metastatic Renal Cell Carcinoma, and Association between Immune-Related Adverse Events and Survival: The Italian Expanded Access Program. J. Immunother. Cancer 2019, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, H.; Takagi, T.; Kondo, T.; Homma, C.; Tachibana, H.; Fukuda, H.; Yoshida, K.; Iizuka, J.; Kobayashi, H.; Okumi, M.; et al. Association between Immune-Related Adverse Events and Prognosis in Patients with Metastatic Renal Cell Carcinoma Treated with Nivolumab. Urol. Oncol. 2019, 37, 355.e21–355.e29. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.; Bellesoeur, A.; Boudou-Rouquette, P.; Arrondeau, J.; Thomas-Schoemann, A.; Kirchgesner, J.; Gervais, C.; Jouinot, A.; Chapron, J.; Giraud, F.; et al. The Impact of Body Composition Parameters on Severe Toxicity of Nivolumab. Eur. J. Cancer 2020, 124, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Davis, I.D.; Mardiak, J.; Szczylik, C.; Lee, E.; Wagstaff, J.; Barrios, C.H.; Salman, P.; Gladkov, O.A.; Kavina, A.; et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Halabi, S.; Sanford, B.L.; Hahn, O.; Michaelson, M.D.; Walsh, M.K.; Feldman, D.R.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib Versus Sunitinib as Initial Targeted Therapy for Patients with Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 591–597. [Google Scholar] [CrossRef]
- Ulmann, G.; Bergoin, E.; Neveux, N.; Jouinot, A.; Durand, J.-P.; Boudou-Rouquette, P.; Goldwasser, F.; Cynober, L. Creatinine to Cystatin c Ratio (Sarcopenia Index) Is a Predictor of Mortality in Cancer Patients. Clin. Nutr. ESPEN 2020, 40, 462. [Google Scholar] [CrossRef]
- Fox, C.J.; Hammerman, P.S.; Thompson, C.B. Fuel Feeds Function: Energy Metabolism and the T-Cell Response. Nat. Rev. Immunol. 2005, 5, 844–852. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro-Grillo, I.; Camilo, M. Colorectal Cancer: Intrinsic Characteristics Modulate Cancer Energy Expenditure and the Risk of Cachexia. Cancer Investig. 2007, 25, 308–314. [Google Scholar] [CrossRef]
- Liu, A.; Curran, M.A. Tumor Hypermetabolism Confers Resistance to Immunotherapy. Semin. Cancer Biol. 2020, 65, 155–163. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).