18FDG PET Assessment of Therapeutic Response in Patients with Advanced or Metastatic Melanoma Treated with First-Line Immune Checkpoint Inhibitors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
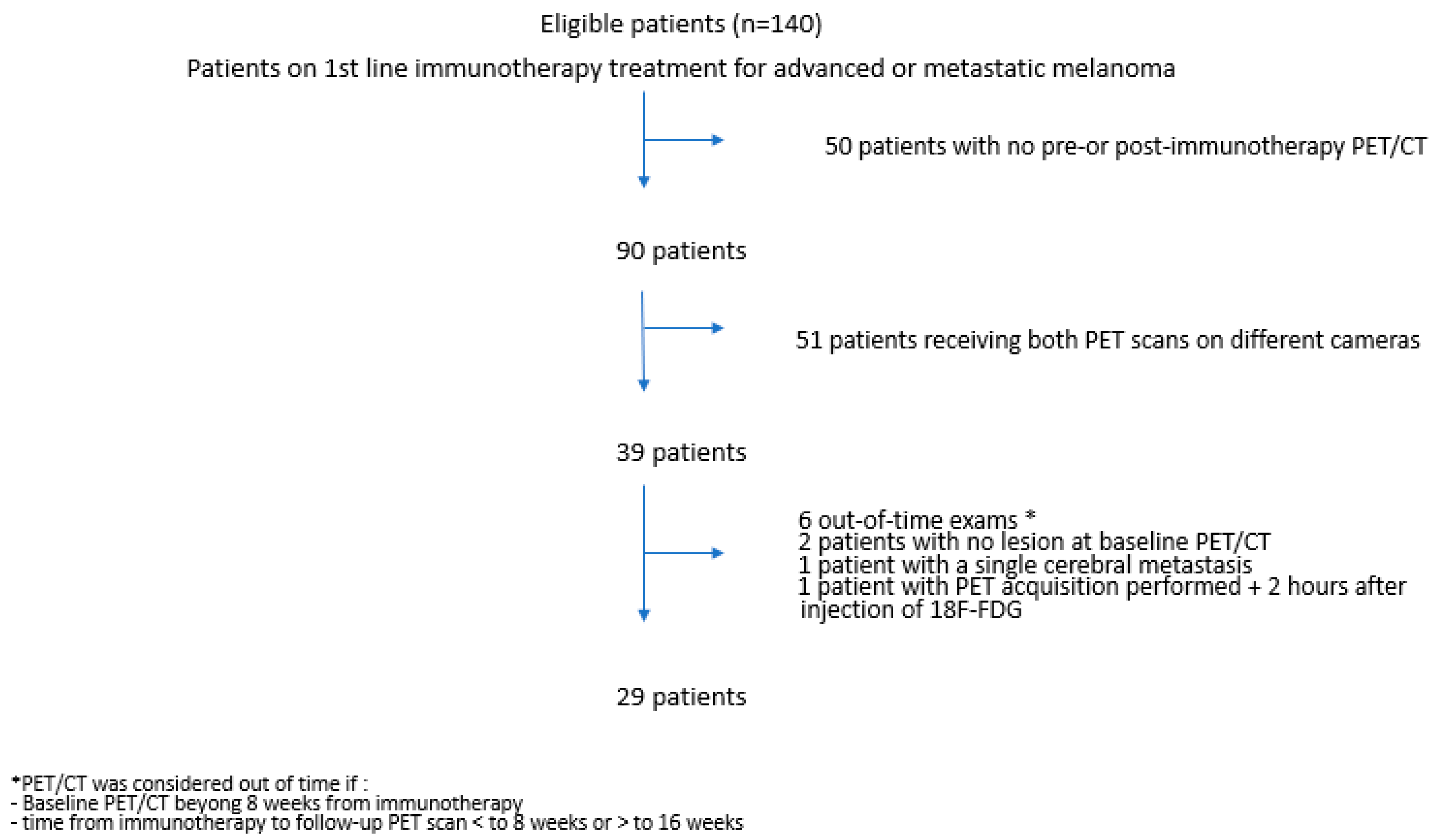
2.1. Patients
2.2. 18F-FDG PET/CT Protocol
2.3. Image Analysis
- -
- PERCIST5 criteria (PET Response Criteria in Solid Tumors): nuclear physicians measured SULpeak on up to 5 metastatic lesions and no more than 2 per organ. The lesions could be different on baseline and follow-up PET/CT. The target lesions had to have a SULpeak greater than the reference threshold defined as follows: on the healthy liver: reference threshold = 1.5* SULmean liver +2* σSULliver; on the metastatic liver: reference threshold = 2* SULmean mediastinum +2* σSULmediastinum. CR was defined as complete resolution of 18F-FDG within measurable target lesion so that it is less than mean liver activity and indistinguishable from surrounding background blood-pool levels. PR was defined as a decrease of more than 30% in the sum of SULpeak. PD was defined as an increase of more than 30% in the sum of SULpeak or the appearance of new lesions. Finally, SD applies to patients who do not fit into the definitions of CR, PR or PD.
- -
- imPERCIST5 criteria (modified PERCIST criteria for immunotherapy): the analysis of the 5 lesions was performed similarly to PERCIST5, with the difference that the appearance of a new lesion did not lead automatically to PD. Thus, the SULpeak of the new lesion(s) had to be included in the sum of the SULpeak of the 5 hottest tumor lesions, and PD was only defined by an increase of more than 30% in the sum of the SULpeak. New lesion(s) were included in the sum of total SULpeak if they had a SULpeak greater than pre-existing target lesions or if less than 5 target lesions were detected at baseline.
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. PET/CT Acquisitions
3.3. Baseline PET/CT
3.4. Metabolic Response at 3 Months
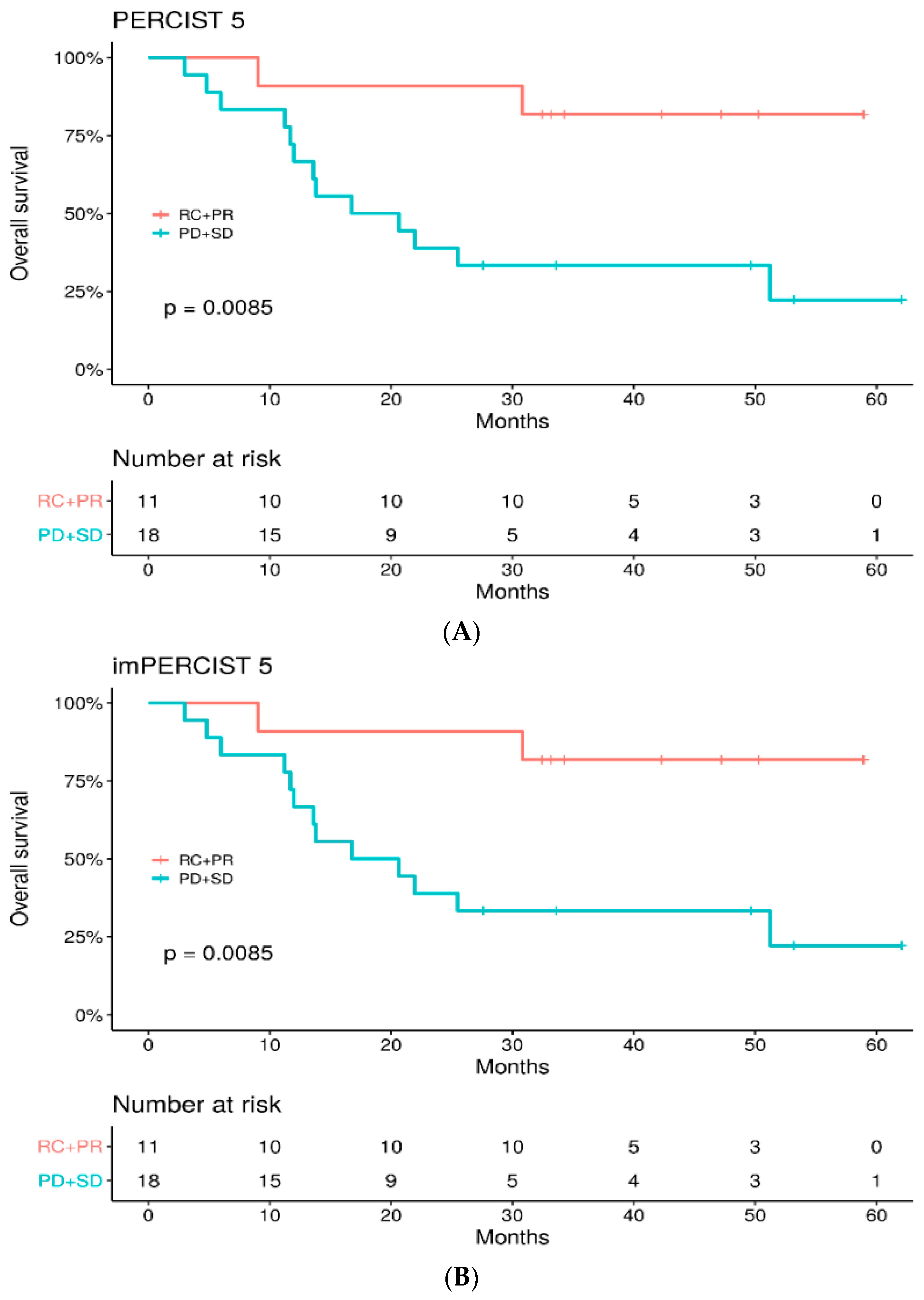
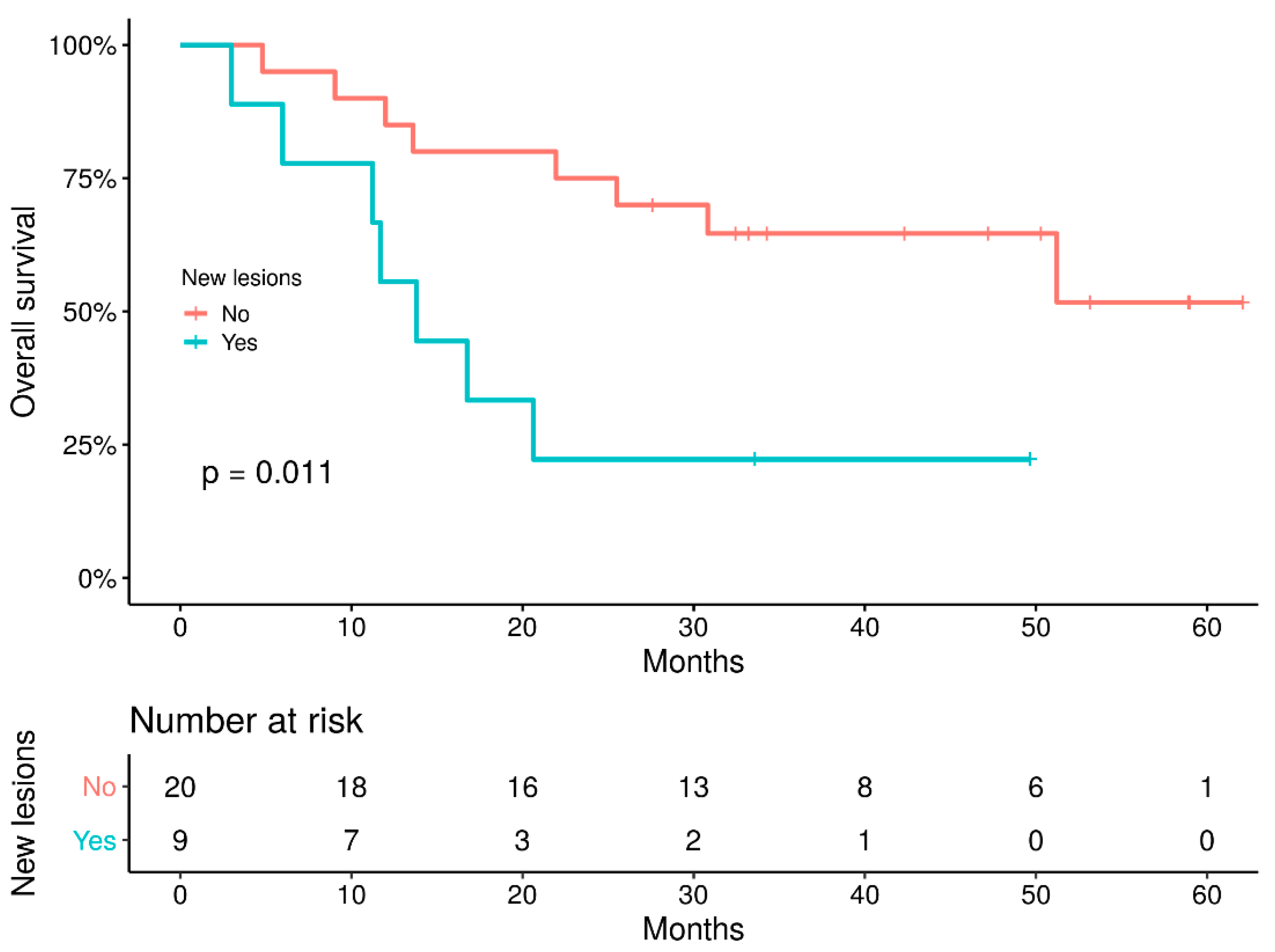
3.5. Survival Study
3.6. Patients’ Outcome
3.7. Assessment of Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; Fargnoli, M.C.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 2: Treatment—Update 2019. Eur. J. Cancer 2020, 126, 159–177. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma (KEYNOTE-006): Post-Hoc 5-Year Results from an Open-Label, Multicentre, Randomised, Controlled, Phase 3 Study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-Year Survival Outcomes for Patients with Advanced Melanoma Treated with Pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbe, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [Green Version]
- Hoos, A.; Wolchok, J.D.; Humphrey, R.W.; Hodi, F.S. CCR 20th Anniversary Commentary: Immune-Related Response Criteria--Capturing Clinical Activity in Immuno-Oncology. Clin. Cancer Res. 2015, 21, 4989–4991. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Bronstein, Y.; Ross, M.I.; Askew, R.L.; Lee, J.E.; Gershenwald, J.E.; Royal, R.; Cormier, J.N. Contemporary Diagnostic Imaging Modalities for the Staging and Surveillance of Melanoma Patients: A Meta-Analysis. J. Natl. Cancer Inst. 2011, 103, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Schroer-Gunther, M.A.; Wolff, R.F.; Westwood, M.E.; Scheibler, F.J.; Schurmann, C.; Baumert, B.G.; Sauerland, S.; Kleijnen, J. F-18-Fluoro-2-Deoxyglucose Positron Emission Tomography (PET) and PET/Computed Tomography Imaging in Primary Staging of Patients with Malignant Melanoma: A Systematic Review. Syst. Rev. 2012, 1, 62. [Google Scholar] [CrossRef] [Green Version]
- Sachpekidis, C.; Larribere, L.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. Predictive Value of Early 18F-FDG PET/CT Studies for Treatment Response Evaluation to Ipilimumab in Metastatic Melanoma: Preliminary Results of an Ongoing Study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 386–396. [Google Scholar] [CrossRef]
- Amrane, K.; Le Goupil, D.; Quere, G.; Delcroix, O.; Gouva, S.; Schick, U.; Salaun, P.-Y.; Abgral, R.; Alavi, Z.; Keromnes, N.; et al. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using 18F-FDG PET/CT in Patients with Melanoma. Medicine 2019, 98, e16417. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Anwar, H.; Winkler, J.; Kopp-Schneider, A.; Larribere, L.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. The Role of Interim 18F-FDG PET/CT in Prediction of Response to Ipilimumab Treatment in Metastatic Melanoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1289–1296. [Google Scholar] [CrossRef]
- Anwar, H.; Sachpekidis, C.; Winkler, J.; Kopp-Schneider, A.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Absolute Number of New Lesions on 18F-FDG PET/CT Is More Predictive of Clinical Response than SUV Changes in Metastatic Melanoma Patients Receiving Ipilimumab. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 376–383. [Google Scholar] [CrossRef]
- Ito, K.; Teng, R.; Schöder, H.; Humm, J.L.; Ni, A.; Michaud, L.; Nakajima, R.; Yamashita, R.; Wolchok, J.D.; Weber, W.A. 18 F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma. J. Nucl. Med. 2019, 60, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Goldfarb, L.; Duchemann, B.; Chouahnia, K.; Zelek, L.; Soussan, M. Monitoring Anti-PD-1-Based Immunotherapy in Non-Small Cell Lung Cancer with FDG PET: Introduction of IPERCIST. EJNMMI Res. 2019, 9, 8. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Schöder, H.; Teng, R.; Humm, J.L.; Ni, A.; Wolchok, J.D.; Weber, W.A. Prognostic Value of Baseline Metabolic Tumor Volume Measured on 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Melanoma Patients Treated with Ipilimumab Therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 930–939. [Google Scholar] [CrossRef]
- Nakamoto, R.; Zaba, L.C.; Rosenberg, J.; Reddy, S.A.; Nobashi, T.W.; Davidzon, G.; Aparici, C.M.; Nguyen, J.; Moradi, F.; Iagaru, A.; et al. Prognostic Value of Volumetric PET Parameters at Early Response Evaluation in Melanoma Patients Treated with Immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2787–2795. [Google Scholar] [CrossRef]
- Awada, G.; Jansen, Y.; Schwarze, J.K.; Tijtgat, J.; Hellinckx, L.; Gondry, O.; Vermeulen, S.; Warren, S.; Schats, K.; van Dam, P.-J.; et al. A Comprehensive Analysis of Baseline Clinical Characteristics and Biomarkers Associated with Outcome in Advanced Melanoma Patients Treated with Pembrolizumab. Cancers 2021, 13, 168. [Google Scholar] [CrossRef]
- Annovazzi, A.; Vari, S.; Giannarelli, D.; Pasqualoni, R.; Sciuto, R.; Carpano, S.; Cognetti, F.; Ferraresi, V. Comparison of 18F-FDG PET/CT Criteria for the Prediction of Therapy Response and Clinical Outcome in Patients with Metastatic Melanoma Treated With Ipilimumab and PD-1 Inhibitors. Clin. Nucl. Med. 2020, 45, 187–194. [Google Scholar] [CrossRef]
- Vermeulen, S.; Awada, G.; Keyaerts, M.; Neyns, B.; Everaert, H. Early Reassessment of Total Metabolic Tumor Volume on FDG-PET/CT in Advanced Melanoma Patients Treated with Pembrolizumab Predicts Long-Term Outcome. Curr. Oncol. 2021, 28, 1630–1640. [Google Scholar] [CrossRef]
- Schweighofer-Zwink, G.; Manafi-Farid, R.; Kölblinger, P.; Hehenwarter, L.; Harsini, S.; Pirich, C.; Beheshti, M. Prognostic Value of 2-[18F]FDG PET-CT in Metastatic Melanoma Patients Receiving Immunotherapy. Eur. J. Radiol. 2022, 146, 110107. [Google Scholar] [CrossRef]
- Iravani, A.; Osman, M.M.; Weppler, A.M.; Wallace, R.; Galligan, A.; Lasocki, A.; Hunter, M.O.; Akhurst, T.; Hofman, M.S.; Lau, P.K.H.; et al. FDG PET/CT for Tumoral and Systemic Immune Response Monitoring of Advanced Melanoma during First-Line Combination Ipilimumab and Nivolumab Treatment. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Seban, R.-D.; Nemer, J.S.; Marabelle, A.; Yeh, R.; Deutsch, E.; Ammari, S.; Moya-Plana, A.; Mokrane, F.-Z.; Gartrell, R.D.; Finkel, G.; et al. Prognostic and Theranostic 18F-FDG PET Biomarkers for Anti-PD1 Immunotherapy in Metastatic Melanoma: Association with Outcome and Transcriptomics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2298–2310. [Google Scholar] [CrossRef]
- Basler, L.; Gabryś, H.S.; Hogan, S.A.; Pavic, M.; Bogowicz, M.; Vuong, D.; Tanadini-Lang, S.; Förster, R.; Kudura, K.; Huellner, M.W.; et al. Radiomics, Tumor Volume, and Blood Biomarkers for Early Prediction of Pseudoprogression in Patients with Metastatic Melanoma Treated with Immune Checkpoint Inhibition. Clin. Cancer Res. 2020, 26, 4414–4425. [Google Scholar] [CrossRef] [Green Version]
- Queirolo, P.; Spagnolo, F. Atypical Responses in Patients with Advanced Melanoma, Lung Cancer, Renal-Cell Carcinoma and Other Solid Tumors Treated with Anti-PD-1 Drugs: A Systematic Review. Cancer Treat. Rev. 2017, 59, 71–78. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Kopp-Schneider, A.; Hakim-Meibodi, L.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. 18F-FDG PET/CT Longitudinal Studies in Patients with Advanced Metastatic Melanoma for Response Evaluation of Combination Treatment with Vemurafenib and Ipilimumab. Melanoma Res. 2019, 29, 178–186. [Google Scholar] [CrossRef]
- Humbert, O.; Cadour, N.; Paquet, M.; Schiappa, R.; Poudenx, M.; Chardin, D.; Borchiellini, D.; Benisvy, D.; Ouvrier, M.J.; Zwarthoed, C.; et al. 18FDG PET/CT in the Early Assessment of Non-Small Cell Lung Cancer Response to Immunotherapy: Frequency and Clinical Significance of Atypical Evolutive Patterns. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1158–1167. [Google Scholar] [CrossRef]
- Wong, S.Q.; Raleigh, J.M.; Callahan, J.; Vergara, I.A.; Ftouni, S.; Hatzimihalis, A.; Colebatch, A.J.; Li, J.; Semple, T.; Doig, K.; et al. Circulating Tumor DNA Analysis and Functional Imaging Provide Complementary Approaches for Comprehensive Disease Monitoring in Metastatic Melanoma. JCO Precis. Oncol. 2017, 1–14. [Google Scholar] [CrossRef]




| Patient Characteristics | Patients nb (Total = 29) |
|---|---|
| Gender | |
| Female | 14 (48%) |
| Male | 15 (52%) |
| Type of treatment | |
| pembrolizumab | 15 (52%) |
| nivolumab | 9 (31%) |
| nivolumab + ipilimumab | 5 (17%) |
| Breslow (mm) | |
| ≤1 | 4 |
| 1, 1–4 | 9 |
| >4 | 13 |
| Missing data | 3 |
| Mutation status | |
| BRAF WT/M/NA | 22/7/0 |
| NRAS WT/M/NA | 19/9/1 |
| Ckit WT/M/NA | 10/2/17 |
| ECOG | |
| 0 | 17 |
| 1 | 10 |
| 2 | 2 |
| LDH level | |
| normal | 19 |
| high | 8 |
| Missing data | 2 |
| Type of primary melanoma | |
| cutaneous mucosal | 23 4 |
| unknown | 2 |
| imPERCIST 5 | |||||
|---|---|---|---|---|---|
| PERCIST 5 | CR (n) | PR (n) | SD (n) | PD (n) | Total (n) |
| CR | 3 | 0 | 0 | 0 | 3 |
| PR | 0 | 8 | 0 | 0 | 8 |
| SD | 0 | 0 | 4 | 0 | 4 |
| PD | 0 | 0 | 6 | 8 | 14 |
| Total | 3 | 8 | 10 | 8 | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas, A.; Delyon, J.; Martineau, A.; Blanc, E.; Allayous, C.; Da Meda, L.; Merlet, P.; Lebbé, C.; Baroudjian, B.; Vercellino, L. 18FDG PET Assessment of Therapeutic Response in Patients with Advanced or Metastatic Melanoma Treated with First-Line Immune Checkpoint Inhibitors. Cancers 2022, 14, 3190. https://doi.org/10.3390/cancers14133190
Rivas A, Delyon J, Martineau A, Blanc E, Allayous C, Da Meda L, Merlet P, Lebbé C, Baroudjian B, Vercellino L. 18FDG PET Assessment of Therapeutic Response in Patients with Advanced or Metastatic Melanoma Treated with First-Line Immune Checkpoint Inhibitors. Cancers. 2022; 14(13):3190. https://doi.org/10.3390/cancers14133190
Chicago/Turabian StyleRivas, Alexia, Julie Delyon, Antoine Martineau, Estelle Blanc, Clara Allayous, Laetitia Da Meda, Pascal Merlet, Céleste Lebbé, Barouyr Baroudjian, and Laetitia Vercellino. 2022. "18FDG PET Assessment of Therapeutic Response in Patients with Advanced or Metastatic Melanoma Treated with First-Line Immune Checkpoint Inhibitors" Cancers 14, no. 13: 3190. https://doi.org/10.3390/cancers14133190
APA StyleRivas, A., Delyon, J., Martineau, A., Blanc, E., Allayous, C., Da Meda, L., Merlet, P., Lebbé, C., Baroudjian, B., & Vercellino, L. (2022). 18FDG PET Assessment of Therapeutic Response in Patients with Advanced or Metastatic Melanoma Treated with First-Line Immune Checkpoint Inhibitors. Cancers, 14(13), 3190. https://doi.org/10.3390/cancers14133190








