Reirradiation of Locally Recurrent Prostate Cancer with Cyberknife® System or Volumetric Modulated Arc Therapy (VMAT) and IGRT-Clarity®: Outcomes, Toxicities and Dosimetric Evaluation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. First Irradiation
2.3. Salvage SBRT: Planning and Delivery
2.4. Bladder and Rectal Preparations
2.5. Dosimetric Evaluation
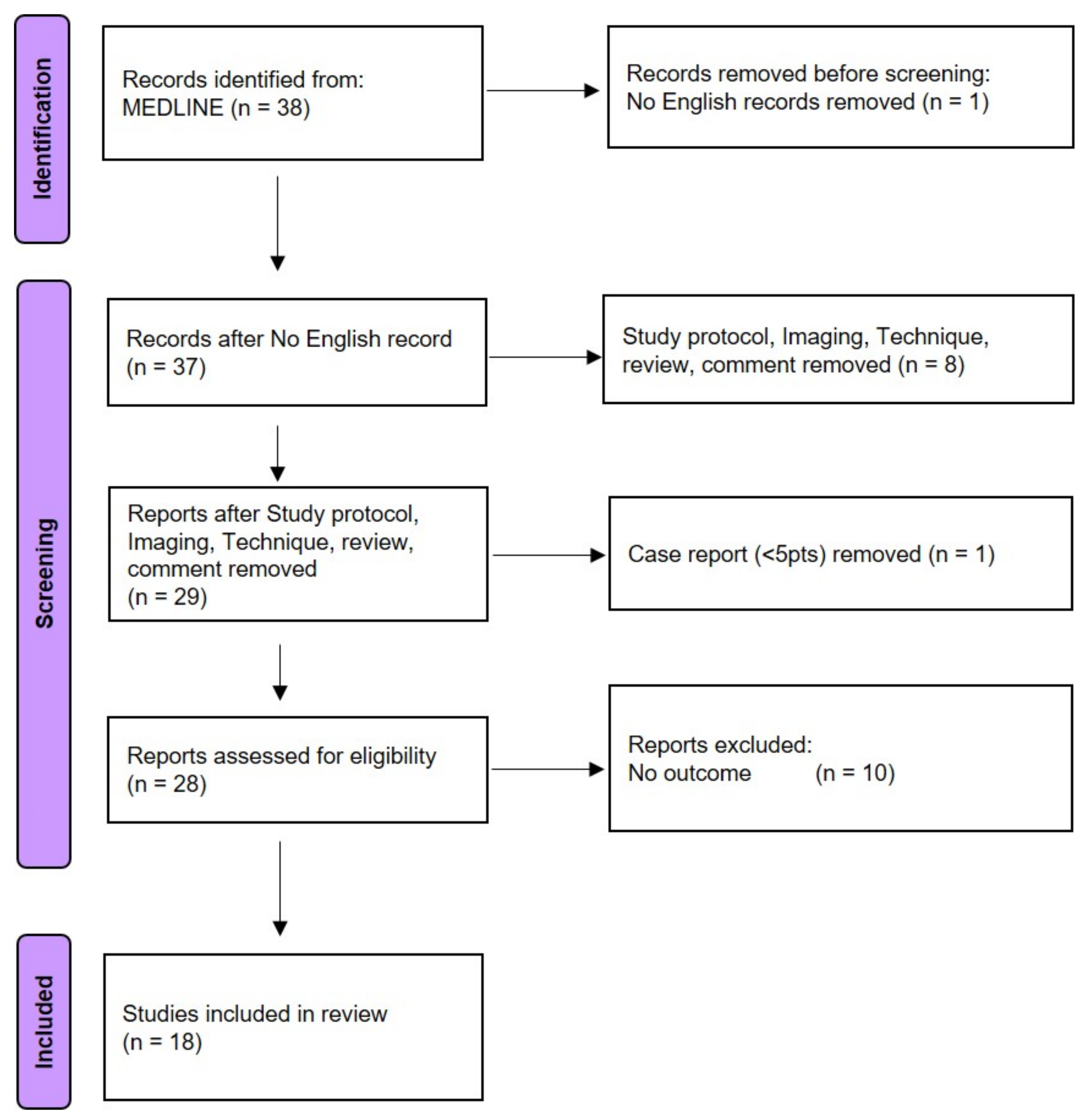
2.6. Literature Review
2.7. Trial Approval
3. Results
3.1. Oncologic Outcome
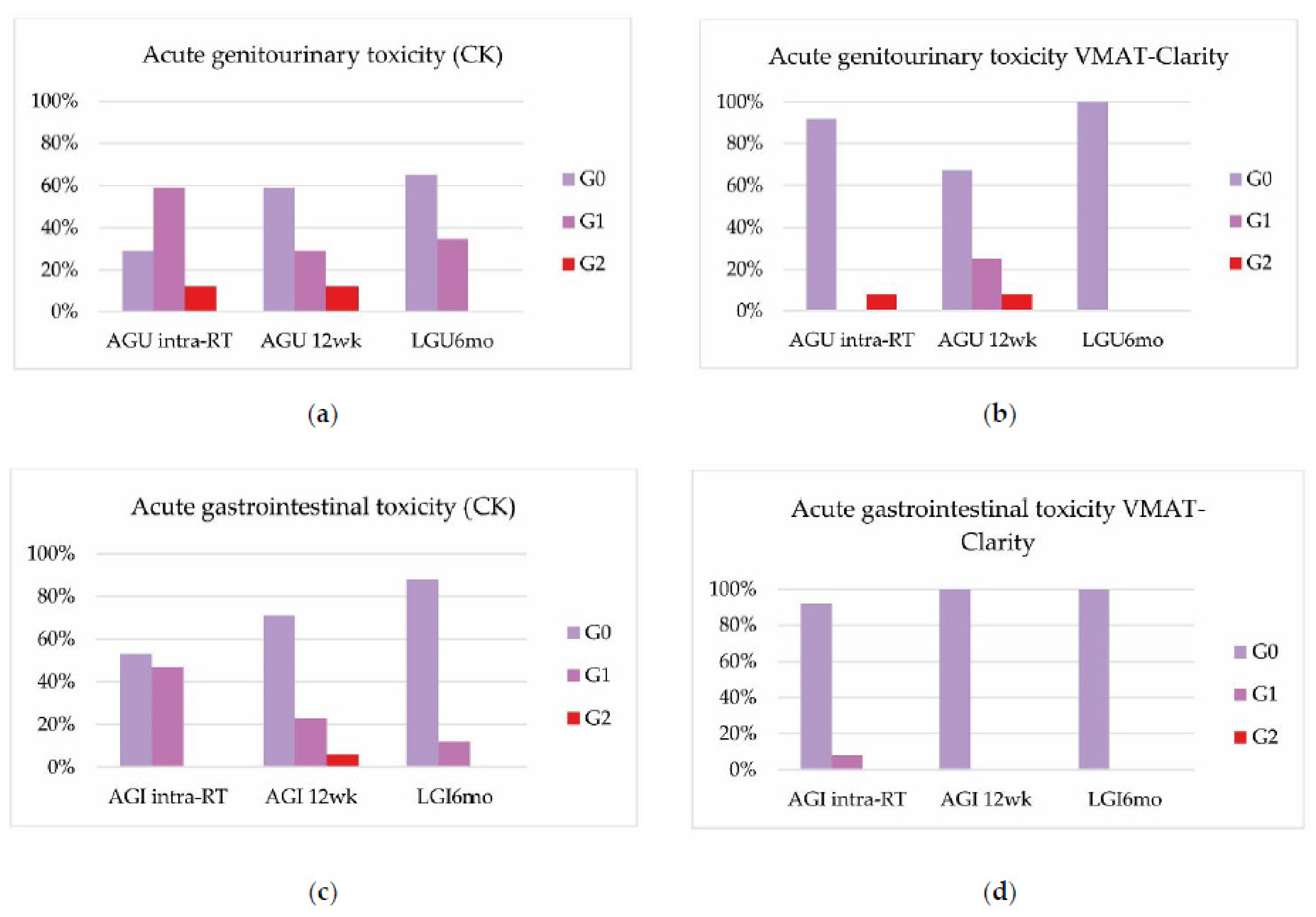
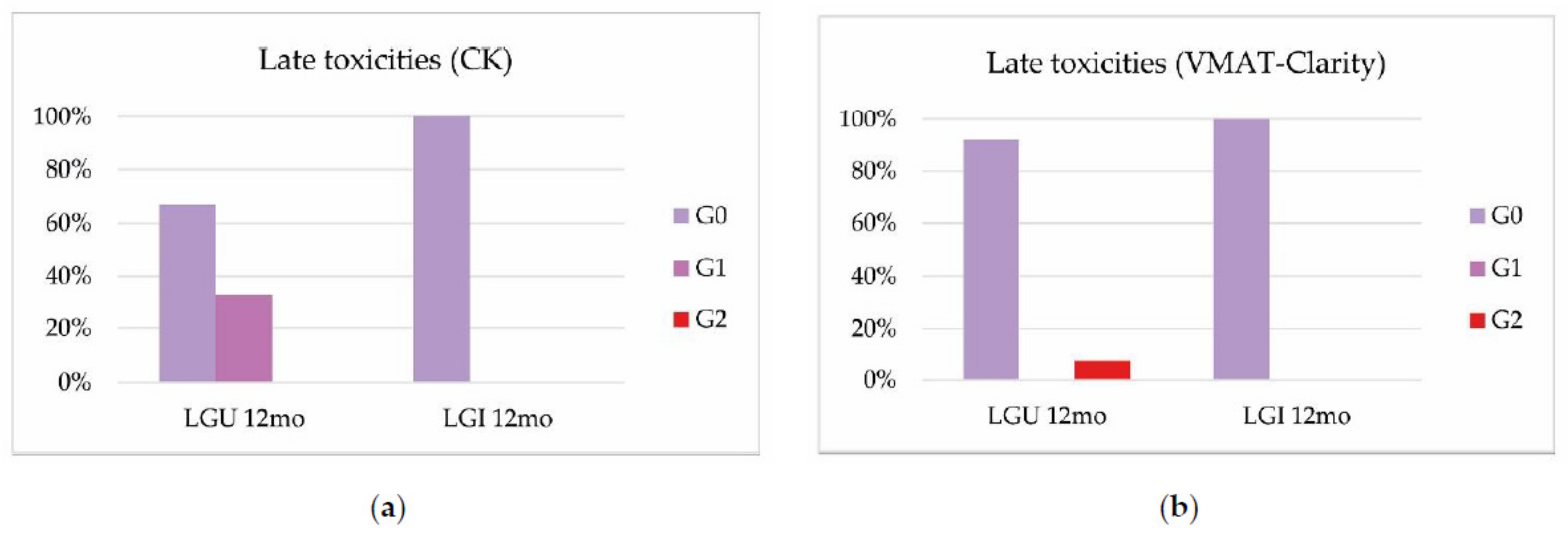
3.2. Toxicities
3.3. Dosimetric Data
3.4. Literature Search
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, P.L.; D’Amico, A.V.; Lee, A.K.; Suh, W.W. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: A systematic review of the literature. Cancer 2007, 110, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; O’Sullivan, M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J. Urol. 2000, 163, 1743–1746. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Sadetsky, N.; Konety, B.R.; Resnick, M.I.; Carroll, P.R. Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer 2008, 112, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Lairson, D.R.; Swartz, M.D.; Du, X.L. Risks of major long-term side effects associated with androgen-deprivation therapy in men with prostate cancer. Pharmacotherapy 2018, 38, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Mechanisms of resist- ance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 2015, 4, 365–380. [Google Scholar] [PubMed]
- Alongi, F.; De Bari, B.; Campostrini, F.; Arcangeli, S.; Matei, D.V.; Lopci, E.; Petralia, G.; Bellomi, M.; Chiti, A.; Magrini, S.M.; et al. Salvage therapy of intraprostatic failure after radical external-beam radiotherapy for prostate cancer: A review. Crit. Rev. Oncol. Hematol. 2013, 88, 550–563. [Google Scholar] [CrossRef]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019, 5, 479–505. [Google Scholar] [CrossRef] [Green Version]
- Zerini, D.; Jereczek-Fossa, B.A.; Fodor, C.; Bazzani, F.; Maucieri, A.; Ronchi, S.; Ferrario, S.; Colangione, S.P.; Gerardi, M.A.; Caputo, M.; et al. Salvage image-guided intensity modulated or stereotactic body reirradiation of local recurrence of prostate cancer. Br. J. Radiol. 2015, 88, 20150197. [Google Scholar] [CrossRef] [Green Version]
- Loi, M.; Di Cataldo, V.; Simontacchi, G.; Detti, B.; Bonomo, P.; Masi, L.; Desideri, I.; Greto, D.; Francolini, G.; Carfora, V.; et al. Robotic Stereotactic Retreatment for Biochemical Control in Previously Irradiated Patients Affected by Recurrent Prostate Cancer. Clin. Oncol. 2018, 30, 93–100. [Google Scholar] [CrossRef]
- Fuller, D.B.; Wurzer, J.; Shirazi, R.; Bridge, S.S.; Law, J.; Mardirossian, G. High-dose-rate stereotactic body radiation therapy for post radiation therapy locally recurrent prostatic carcinoma: Preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract. Radiat. Oncol. 2015, 5, e615–e623. [Google Scholar] [CrossRef]
- Panebianco, V.; Barchetti, F.; Sciarra, A.; Musio, D.; Forte, V.; Gentile, V.; Tombolini, V.; Catalano, C. Prostate cancer recurrence after radical prostatectomy: The role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur. Radiol. 2013, 23, 1745–1752. [Google Scholar] [CrossRef]
- Han, B.; Najafi, M.; Cooper, D.T.; Lachaine, M.; von Eyben, R.; Hancock, S.; Hristov, D. Evaluation of transperineal ultrasound imaging as a potential solution for target tracking during hypofractionated radiotherapy for prostate cancer. Radiat. Oncol. 2018, 13, 151. [Google Scholar] [CrossRef]
- Cury, F.L.; Shenouda, G.; Souhami, L.; Duclos, M.; Faria, S.L.; David, M.; Verhaegen, F.; Corns, R.; Falco, T. Ultrasound-based image guided radiotherapy for prostate cancer: Comparison of crossmodality and intramodality methods for daily localization during external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1562–1567. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Referred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiology. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Caroli, P.; Colangione, S.P.; De Giorgi, U.; Ghigi, G.; Celli, M.; Scarpi, E.; Monti, M.; Di Iorio, V.; Sarnelli, A.; Paganelli, G.; et al. 68Ga-PSMA-11 PET/CT-Guided Stereotactic Body Radiation Therapy Retreatment in Prostate Cancer Patients with PSA Failure after Salvage Radiotherapy. Biomedicines 2020, 8, 536. [Google Scholar] [CrossRef]
- Fuller, D.B.; Wurzer, J.; Shirazi, R.; Bridge, S.; Law, J.; Crabtree, T.; Mardirossian, G. Retreatment for Local Recurrence of Prostatic Carcinoma After Prior Therapeutic Irradiation: Efficacy and Toxicity of HDR-Like SBRT. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Jereczek-Fossa, B.A.; Beltramo, G.; Fariselli, L.; Fodor, C.; Santoro, L.; Vavassori, A.; Zerini, D.; Gherardi, F.; Ascione, C.; Bossi-Zanetti, I.; et al. Robotic Image-Guided Stereotactic Radiotherapy, for Isolated Recurrent Primary, Lymph Node or Metastatic Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 889–897. [Google Scholar] [CrossRef]
- Francolini, G.; Loi, M.; Di Cataldo, V.; Detti, B.; Stocchi, G.; Masi, L.; Doro, R.; Scoccimarro, E.; Bellini, C.; Aquilano, M.; et al. Stereotactic Re-irradiation in Recurrent Prostate Cancer after Previous Postoperative or Definitive Radiotherapy: Long-term Results after a Median Follow-up of 4 Years. Clin. Oncol. 2022, 34, 50–56. [Google Scholar] [CrossRef]
- Janoray, G.; Reynaud-Bougnoux, A.; Ruffier-Loubière, A.; Bernadou, G.; Pointreau, Y.; Calais, G. Stereotactic body re-irradiation therapy for locally recurrent prostate cancer after external-beam radiation therapy: Initial report. Cancer Radiother. 2016, 20, 275–281. [Google Scholar] [CrossRef]
- Mbeutcha, A.; Chauveinc, L.; Bondiau, P.-Y.; Chand, M.-E.; Durand, M.; Chevallier, D.; Amiel, J.; Kee, D.L.C.; Hannoun-Lévi, J.M. Salvage prostate re-irradiation using high-dose-rate brachytherapy or focal stereotactic body radiotherapy for local recurrence after definitive radiation therapy. Radiat. Oncol. 2017, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Zerini, D.; Jereczek-Fossa, B.A.; Ciabattoni, A.; Mirri, A.; Bertoni, F.; Fersino, S.; D’Agostino, G.; Lohr, F.; Mortellaro, G.; Triggiani, L.; et al. PROLAPSE: Survey about local prostate cancer relapse salvage treatment with external beam re-irradiation: Results of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). J. Cancer Res. Clin. Oncol. 2020, 146, 2311–2317. [Google Scholar] [CrossRef]
- Leroy, T.; Lacornerie, T.; Bogart, E.; Nickers, P.; Lartigau, E.; Pasquier, D. Salvage robotic SBRT for local prostate cancer recurrence after radiotherapy: Preliminary results of the Oscar Lambret Center. Radiat. Oncol. 2017, 12, 95. [Google Scholar] [CrossRef]
- Miszczyk, L.; Stąpor-Fudzinska, M.; Miszczyk, M.; Maciejewski, B.; Tukiendorf, A. Salvage CyberKnife-Based Reirradiation of Patients with Recurrent Prostate Cancer: The Single-Center Experience. Technol. Cancer Res. Treat. 2018, 17, 1533033818785496. [Google Scholar] [CrossRef]
- Detti, B.; Bonomo, P.; Masi, L.; Doro, R.; Cipressi, S.; Iermano, C.; Bonucci, I.; Franceschini, D.; Di Brina, L.; Baki, M.; et al. CyberKnife stereotactic radiotherapy for isolated recurrence in the prostatic bed. World J. Urol. 2016, 34, 311–317. [Google Scholar] [CrossRef]
- Bergamin, S.; Eade, T.; Kneebone, A.; Booth, J.; Hsiao, E.; Schembri, G.P.; Szymura, K.; Le, A.; Kwong, C.; Brown, C.; et al. Interim results of a prospective PSMA-directed focal stereotactic re-irradiation trial for locally recurrent prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1172–1178. [Google Scholar] [CrossRef]
- D’Agostino, G.R.; Di Brina, L.; Mancosu, P.; Franzese, C.; Iftode, C.; Franceschini, D.; Clerici, E.; Tozzi, A.; Navarria, P.; Scorsetti, M. Reirradiation of Locally Recurrent Prostate Cancer with Volumetric Modulated Arc Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 614–621. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Rojas, D.P.; Zerini, D.; Fodor, C.; Viola, A.; Fanetti, G.; Volpe, S.; Luraschi, R.; Bazani, A.; Rondi, E.; et al. Reirradiation for isolated local recurrence of prostate cancer: Mono-institutional series of 64 patients treated with salvage stereotactic body radiotherapy (SBRT). Br. J. Radiol. 2019, 92, 20180494. [Google Scholar] [CrossRef] [Green Version]
- Ozyigit, G.; Hurmuz, P.; Akinci, D.; Esen, S.C.B.; Yilmaz, M.T.; Akdogan, B.; Akyol, F.H. Hyaluronic acid spacer in focal prostate reirradiation: A single centre experience. Cancer Radiother. 2020, 24, 805–811. [Google Scholar] [CrossRef]
- Lewin, R.; Amit, U.; Laufer, M.; Berger, R.; Dotan, Z.; Domachevsky, L.; Davidson, T.; Portnoy, O.; Tsvang, L.; Ben-Ayun, M.; et al. Salvage re-irradiation using stereotactic body radiation therapy for locally recurrent prostate cancer: The impact of castration sensitivity on treatment outcomes. Radiat. Oncol. 2021, 16, 114. [Google Scholar] [CrossRef]
- Vavassori, A.; Jereczek-Fossa, B.A.; Beltramo, G.; De Cicco, L.; Fariselli, L.; Bianchi, L.C.; Possanzini, M.; Bergantin, A.; De Cobelli, O.; Orecchia, R. Image-guided robotic radiosurgery as salvage therapy for locally recurrent prostate cancer after external beam irradiation: Retrospective feasibility study on six cases. Tumori 2010, 96, 71–75. [Google Scholar] [CrossRef]
- Olivier, J.; Basson, L.; Puech, P.; Lacornerie, T.; Villers, A.; Wallet, J.; Lartigau, E.; Pasquier, D. Stereotactic re-irradiation for local recurrence in the prostatic bed after prostatectomy: Preliminary results. Front. Oncol. 2019, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Ametrano, G.; Borzillo, V.; Quarto, M.; Muto, M.; Di Franco, R.; Federica, S.; Loffredo, F.; Paolo, M. Dosimetric comparison among cyberknife, helical tomotherapy and VMAT for hypofractionated treatment in localized prostate cancer. Medicine 2020, 99, e23574. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; De Martino, F.; Savino, F.; D’Alesio, V.; Arrichiello, C.; Quarto, M.; Loffredo, F.; Di Franco, R.; Borzillo, V.; Muto, M.; et al. SBRT for Localized Prostate Cancer: CyberKnife vs. VMAT-FFF, a Dosimetric Study. Life 2022, 12, 711. [Google Scholar] [CrossRef] [PubMed]

| Gleason Score | No. | T Stage | No. |
|---|---|---|---|
| 6(3 + 3) | 10 | 1c | 5 |
| 7(3 + 4) | 4 | 2a | 2 |
| 7(4 + 3) | 5 | 2b | 6 |
| 8(3 + 5) | 1 | 2c | 6 |
| 8(4 + 4) | 5 | 3a | 4 |
| 9(4 + 5) | 1 | 3b | 3 |
| 9(5 + 4) | 2 | 4 | 1 |
| Unknown | 1 | Unknown | 2 |
| PSA at diagnosis (ng/mL) | No. | Age at diagnosis (ys) | No. |
| <10 | |||
| 10–20 | 13 | 50–60 | 7 |
| >20 | 9 | 61–70 | 16 |
| Mean (38.83) | 7 | 71–80 | 6 |
| Median (11) | >80 | 0 | |
| Range (3.53–345) | |||
| Radiotherapy (Total Normalized Dose) | No. | Radiotherapy (Fraction Dose) | No. |
| 64–84 Gy | 25 | 1.8–2.0 Gy | 25 |
| 35 Gy | 3 | 5 Gy | 3 |
| 38 Gy | 1 | 9.5 Gy | 1 |
| Technique of Radiotherapy | No. | Time between 1st RT and 1st relapse (ys) | No. |
| 3DCRT | 18 | 6 | |
| IMRT | 3 | <4 | 13 |
| VMAT | 4 | 4–8 | 4 |
| SBRT | 3 | 9–10 | 6 |
| BT | 1 | >10 |
| OAR | Dose Limit | OAR | Dose Limit | OAR | Dose Limit |
|---|---|---|---|---|---|
| Bladder | V30 Gy < 10% V15 Gy < 40% D30%< 3.94 Gy | Rectum | V30 Gy < 5% V27 Gy < 10% V24 Gy < 20% V15 Gy < 50% V21 Gy < 25 cc D30% < 8.4 Gy D60% < 4.08 Gy | Penile Bulb | V29.5 Gy < 50% V24 Gy < 50% |
| Age at Recurrence (y) | No. | PSA at Re-RT (ng/mL) | No. | T-Restaging | No. | Restaging Imaging | No. |
|---|---|---|---|---|---|---|---|
| <1 | 6 | 2a | 4 | MRI | 1 | ||
| 50–60 | 0 | 1–2 | 9 | 2b | 10 | PET-CH | 5 |
| 61–70 | 10 | 2.1–3 | 4 | 2c | 4 | PET-CH/MRI | 9 |
| 71–80 | 15 | 3.1–4 | 4 | 3a | 4 | PET-CH/BS | 1 |
| >80 | 4 | 4.1–5 | 2 | 3b | 3 | PET-PSMA | 2 |
| 5.1–10 | 2 | 4 | 4 | PET-PSMA/MRI | 10 | ||
| >10 | 2 | PET-FDG/MRI | 1 | ||||
| Time between 1st RT and re-RT (y) | No. | Re-RT technique | No. | Dose/n.fx | No. | ADT at first RT | No. |
| No | 7 | ||||||
| <4 | 5 | Cyberknife® | 30 Gy/5 fx | 27 | Yes | 22 | |
| 4 | 4 | VMAT-Clarity® | 17 | 35 Gy/5 fx | 1 | Duration (mo) | |
| 5–10 | 16 | 12 | 30 Gy/3 fx | 1 | ≤12 | 8 | |
| 11–17 | 4 | 13–36 | 7 | ||||
| >36 | 7 |
| Follow-Up (Months)—Patients (pts)—Percentage (%) | ||||
|---|---|---|---|---|
| Patients at Risk at: | 3 Months | 6 Months | 9 Months | 12 Months |
| Freedom from Local Recurrence | ||||
| CK® pts | 16/17 (94%) | 15/17 (88%) | 14/16 (86%) | 13/15 (87%) |
| VMAT-Clarity® pts | 11/12 (92%) | 11/12 (92%) | 11/12 (92%) | 11/12 (92%) |
| Tot pts | 27/29 (93%) | 26/29 (90%) | 25/28 (89%) | 24/27 (89%) |
| Freedom from Distant Metastases | ||||
| CK® pts | 15/17 (88%) | 15/17 (88%) | 14/16 (86%) | 13/15 (87%) |
| VMAT-Clarity® pts | 12/12 (100%) | 12/12 (100%) | 10/12 (83%) | 10/12 (83%) |
| Tot pts | 27/29 (93%) | 27/29 (93%) | 24/28 (86%) | 23/27 (85%) |
| Freedom from Androgen Deprivation Therapy (ADT) | ||||
| CK® pts | 9/17 (53%) | 9/17 (53%) | 8/16 (50%) | 8/15 (53%) |
| VMAT-Clarity® pts | 8/12 (67%) | 8/12 (67%) | 5/12 (42%) | 5/12 (42%) |
| Tot pts | 17/29 (59%) | 17/29 (59%) | 13/28 (46%) | 13/27 (48%) |
| Freedom from Biochemical Relapse | ||||
| CK® pts | 16/17 (94%) | 15/17 (88%) | 14/16 (88%) | 13/15 87%) |
| VMAT-Clarity® pts | 10/12 (83%) | 10/12 (83%) | 10/12 (83%) | 8/12 (67%) |
| Tot pts | 26/29 (90%) | 25/29 (86%) | 24/28 (86%) | 22/27 (81%) |
| Prostatic Gland | GTV cm3 | PTV cm3 | Dmax PTV | Rectum D30% (<8.4 Gy) | Rectum D60% (<4.08 Gy) | Bladder D30% (<3.94 Gy) | Penis Bulb cm3 | Penis Bulb (Dmax) | Penis Bulb V29.5 Gy (<50%) | Penis Bulb V24 Gy (<50%) | Bladder V15 Gy (<40%) | Bladder V30 Gy (<10%) | Rectum V15 Gy (<50%) | Rectum V21 Gy (<25 cc) | Rectum V24 Gy (<20%) | Rectum V27 Gy (<10%) | Rectum V30 Gy (<5%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK 1 | 27.74 | 54.07 | 36.59 | 11.60 | 3.78 | 11.88 | 5.38 | 22.54 | 0.00 | 0.00 | 21.40 | 4.90 | 22.00 | 6.48 | 7.00 | 4.00 | 1.30 |
| CK 2 | 34.40 | 69.68 | 36.59 | 12.62 | 7.23 | 16.38 | 15.85 | 31.47 | 1.10 | 8.70 | 34.10 | 6.90 | 22.10 | 5.85 | 5.30 | 2.40 | 0.40 |
| CK 3 | 39.74 | 73.25 | 36.59 | 8.79 | 5.48 | 11.14 | 20.23 | 18.02 | 0.00 | 0.00 | 18.00 | 2.60 | 12.40 | 6.30 | 3.70 | 2.00 | 0.50 |
| CK 4 | 65.52 | 119.63 | 38.46 | 16.17 | 8.32 | 14.40 | 5.00 | 28.63 | 0.00 | 12.30 | 27.80 | 4.20 | 33.80 | 15.5 | 11.70 | 7.20 | 3.70 |
| CK 5 | 64.60 | 110.25 | 37.04 | 10.36 | 4.45 | 9.94 | 7.43 | 23.75 | 0.00 | 0.00 | 10.90 | 2.50 | 16.10 | 6.89 | 4.80 | 2.60 | 0.70 |
| CK 6 | 40.24 | 77.00 | 38.96 | 13.77 | 8.16 | 18.73 | 8.12 | 30.34 | 0.90 | 14.10 | 42.50 | 7.40 | 25.90 | 7.05 | 9.80 | 6.00 | 2.20 |
| CK 7 | 23.74 | 50.62 | 37.50 | 15.71 | 5.90 | 11.16 | 7.32 | 16.74 | 0.00 | 0.00 | 19.30 | 3.00 | 31.50 | 13.86 | 14.00 | 9.10 | 4.40 |
| CK 8 | 39.72 | 78.60 | 37.50 | 10.71 | 6.84 | 14.00 | 11.83 | 33.26 | 5.10 | 14.50 | 27.70 | 8.00 | 17.10 | 6.32 | 6.20 | 4.10 | 2.10 |
| CK 9 | 25.69 | 56.22 | 37.04 | 12.33 | 6.10 | 6.40 | 9.40 | 19.75 | 0.00 | 0.00 | 3.00 | 0.00 | 20.00 | 5.07 | 4.90 | 2.80 | 1.10 |
| CK 10 | 22.67 | 47.86 | 37.50 | 11.85 | 6.91 | 9.63 | 7.24 | 29.08 | 0.00 | 11.90 | 15.70 | 3.70 | 20.20 | 5.35 | 6.50 | 3.80 | 1.30 |
| CK 11 | 24.89 | 54.93 | 37.50 | 12.69 | 7.22 | 8.47 | 1.42 | 30.99 | 11.90 | 86.00 | 13.30 | 4.90 | 22.00 | 6.29 | 4.80 | 5.20 | 2.70 |
| CK 12 | 50.31 | 90.12 | 37.50 | 8.80 | 3.34 | 6.83 | 13.01 | 28.38 | 0.00 | 6.70 | 11.00 | 1.90 | 15.10 | 7.03 | 5.00 | 2.40 | 0.50 |
| CK 13 | 31.10 | 57.05 | 38.46 | 13.54 | 5.24 | 11.18 | 7.27 | 13.86 | 0.00 | 0.00 | 25.10 | 6.70 | 26.50 | 10.23 | 10.30 | 6.40 | 2.90 |
| Median (range) | 34.40 (22.67–65.52) | 69.68 (47.86–119.63) | 37.5 (36.59–38.96) | 12.33 (8.79–16.17) | 6.1 (3.34–8.32) | 11.16 (6.4–18.73) | 7.43 (1.42–20.23) | 28.38 (13.86–33.26) | 0 (0–11.9) | 6.7 (0–86) | 19.3 (3–42.5) | 4.2 (0.1–8.0) | 22 (12.4–33.8) | 6.48 (5.07–15.5) | 6.2 (3.7–14) | 4 (2–9.1) | 1.3 (0.4–4.4) |
| Clarity 1 | 81.78 | 136.33 | 30.19 | 18.03 | 13.07 | 16.17 | 8.20 | 30.26 | 22.85 | 70.84 | 33.27 | 1.84 | 46.87 | 12.03 | 13.1 | 7.98 | 0.00 |
| Clarity 2 | 31.68 | 66.16 | 30.31 | 14.31 | 9.11 | 8.89 | 3.38 | 21.96 | 0.00 | 0.00 | 22.79 | 0.5 | 27.04 | 8 | 9.23 | 6.21 | 0.00 |
| Clarity 3 | 52.39 | 95.22 | 30.25 | 14.89 | 10.34 | 9.42 | 1.98 | 30.12 | 7.45 | 31.44 | 16.43 | 1.29 | 29.79 | 9.67 | 10.69 | 6.2 | 0.64 |
| Clarity 4 | 23.75 | 51.40 | 30.23 | 16.91 | 13.73 | 19.32 | 3.74 | 29.96 | 31.50 | 76.27 | 34.46 | 0.14 | 52.53 | 7.51 | 4.96 | 2.17 | 0.00 |
| Clarity 5 | 50.51 | 95.31 | 30.21 | 18.12 | 13.36 | 26.93 | 4.50 | 29.9 | 14.68 | 44.6 | 70.27 | 5.07 | 46.11 | 10.44 | 15.49 | 10.26 | 0.00 |
| Clarity 6 | 33.59 | 65.18 | 30.20 | 14.36 | 10.69 | 10.81 | 3.97 | 30.17 | 17.11 | 42.11 | 23.65 | 2.25 | 27.08 | 7.46 | 10.13 | 6.33 | 0.33 |
| Clarity 7 | 27.71 | 60.38 | 30.25 | 17.15 | 9.20 | 17.55 | 2.78 | 30.02 | 23.86 | 63.61 | 38.13 | 2.15 | 37.64 | 12.49 | 14.19 | 8.89 | 0.84 |
| Clarity 8 | 19.7 | 42.73 | 30.04 | 12.92 | 2.35 | 1.66 | 4.96 | 29.58 | 12.41 | 68.17 | 7 | 0 | 22.75 | 9.64 | 5.33 | 2.94 | 0.00 |
| Clarity 9 | 25.44 | 51.72 | 30.16 | 19.46 | 8.36 | 9.57 | 6.39 | 29.40 | 0.59 | 43.46 | 23.41 | 3.24 | 45.24 | 16.79 | 15.95 | 9.07 | 0.23 |
| Clarity 10 | 19.91 | 51.05 | 30.46 | 14.97 | 5.07 | 0.99 | 4.91 | 30.00 | 13.84 | 45.96 | 4.64 | 0.47 | 29.9 | 8.12 | 6.93 | 3.94 | 0.28 |
| Median (range) | 29.69 (19.7–81.78) | 62.78 (42.73–136.33) | 30.22 (30.04–30.46) | 15.94 (12.92–19.46) | 9.77 (2.35–13.73) | 10.19 (0.99–23.93) | 4.23 (1.98–8.2) | 29.98 (21.96–30.26) | 14.26 (0–31.5) | 45.28 (0–76.27) | 23.53 (4.64–70.27) | 1.56 (0–5.07) | 33.77 (22.75–52.53) | 9.65 (7.46–16.79) | 10.41 (4.96–15.95) | 6.27 (2.17–10.26) | 0.11 (0–0.84) |
| FFF-Clarity 1 | 81.78 | 136.33 | 37.94 | 6.72 | 3.82 | 13.13 | 8.20 | 34.76 | 25.41 | 47.89 | 26.18 | 4.43 | 8.54 | 2.56 | 2.55 | 1.21 | 0.23 |
| FFF-Clarity 2 | 31.68 | 66.16 | 39.31 | 5.89 | 2.80 | 4.65 | 3.38 | 13.76 | 0.00 | 0.00 | 17.68 | 4.44 | 10.29 | 3.17 | 2.91 | 1.10 | 0.10 |
| FFF-Clarity 3 | 52.39 | 95.22 | 37.02 | 5.45 | 2.55 | 3.62 | 1.98 | 31.18 | 1.45 | 15.51 | 13.14 | 3.43 | 13.49 | 5.43 | 6.47 | 4.25 | 2.01 |
| FFF-Clarity 4 | 23.75 | 51.40 | 37.38 | 5.65 | 2.61 | 10.13 | 3.74 | 34.00 | 21.93 | 47.20 | 25.17 | 9.06 | 5.53 | 1.63 | 1.16 | 0.38 | 0.01 |
| FFF-Clarity 5 | 50.51 | 95.31 | 37.37 | 6.29 | 3.83 | 26.24 | 4.50 | 32.87 | 11.61 | 28.66 | 67.01 | 13.95 | 10.70 | 2.76 | 3.52 | 1.66 | 0.25 |
| FFF-Clarity 6 | 33.59 | 65.18 | 38.36 | 5.27 | 2.80 | 9.34 | 3.97 | 34.25 | 14.50 | 32.48 | 22.07 | 3.63 | 12.02 | 4.01 | 5.26 | 3.35 | 1.59 |
| FFF-Clarity 7 | 27.71 | 60.38 | 39.16 | 6.26 | 2.52 | 21.44 | 2.78 | 31.75 | 18.46 | 53.49 | 50.11 | 6.32 | 12.91 | 4.79 | 5.29 | 3.03 | 1.05 |
| FFF-Clarity 8 | 19.70 | 42.73 | 36.97 | 3.88 | 1.01 | 1.05 | 4.96 | 31.88 | 10.10 | 37.34 | 5.28 | 0.76 | 8.04 | 4.30 | 2.07 | 0.67 | 0.02 |
| FFF-Clarity 9 | 25.44 | 51.72 | 39.21 | 5.87 | 1.65 | 4.26 | 6.39 | 28.68 | 0.00 | 10.33 | 16.09 | 5.85 | 12.18 | 4.80 | 4.82 | 2.63 | 0.63 |
| FFF-Clarity 10 | 19.91 | 51.05 | 38.17 | 5.92 | 1.64 | 0.89 | 4.91 | 33.37 | 19.77 | 51.39 | 4.78 | 1.39 | 12.76 | 5.29 | 5.31 | 2.93 | 0.88 |
| Median (range) | 29.69 (19.7–81.78) | 62.78 (42.73–136.33) | 38.05 (36.97–39.31) | 5.88 (3.88–6.72) | 2.58 (1.01–3.83) | 6.99 (0.89–26.24) | 4.23 (1.98–8.2) | 32.37 (13.76–34.76) | 13.05 (0–25.41) | 34.91 (0–53.49) | 19.87 (4.78–67.01) | 4.43 (0.76–13.95) | 11.36 (5.53–13.49) | 4.15 (1.63–5.43) | 4.17 (1.16–6.47) | 2.14 (0.38–4.25) | 0.44 (0.01–2.01) |
| Author Year (n.pt) | Age Year (Range) | Type of Study | First Treatment Modality | Relaps Time Months (Median) | FU Median Months (Range) | PSA Pre-SBRT Median (Range) | SBRT | RT (Total Dose/n.f) (Daily Fx) | Target | ADT at Reirradiation |
|---|---|---|---|---|---|---|---|---|---|---|
| Caroli et al. [15], 2020 (p. 38) | 75 (71–80) | R | RT (11) RP + RT (27) | - | 27 (4–35) | 1.10 (0.82–2.59) | Tomotherapy | 18 Gy/3 (6) | Prostatic bed | - |
| Fuller et al. [16], 2019 (p. 50) | 74 (50–89) | P | EBRT (3DCRT-IMRT-PT) | 98 (32–241) | 44 (3–110) | 3.97 (0.1–48.6) | CK | 34 Gy/5 (6.8) | Prostate gland | 7(+) |
| Fuller et al. [10], 2015 (p. 29) | 73 (50–89) | P | EBRT (27) BT (1) SBRT (1) | 88 (32–200) | 24 (3–60) | 3.1 (0.1–48.2) | CK | 34 Gy/5 (6.8) | Prostate gland | 7(+) |
| Jereczek-Fossa et al. [17], 2012 (p. 15) | 68.3 (57–82) | R | EBRT (88%) BT (3%) | 66 (24–180) | 9.5 (3–28.9) | 3.51 (1.69–22.9) | CK | 30 Gy/5 (6) | Prostate gland | 5(+) |
| Loi et al. [9], 2018 (p. 50) Francolini et al. [18], 2022 (long-term results) | 76 (62–86) | R | EBRT (28) RP (22) | 76 (9–205) | 48.2 (6.4–86.3) | 10 (3.1–160) | CK | 30 Gy/5 (6) | Dominant intraprostatic lesion Prostate bed | 11(+) |
| Janoray et al. [19], 2016 (p. 21) | 73 (59–85) 80 (91–68) | R | EBRT (11) RP (10) | 98.4 (37.9–246) 98.03 (43.4–398) | 11.7 (2.5–46.5) | 3.43 (1.65–24.1) 3 (0.42–14.5) | CK | 3 pz 35 Gy/5 (7) 15 pz 36.25 Gy/5 (7.25) 3 pz 36 Gy/6 (6) | Prostate gland Locoregional recurrence | 1(+) 1(+) |
| Mbeutcha et al. [20], 2017 (p. 28) | 69 (65–77) 69 (64–75) | R | BT-HDR (16) EBRT (12) | 69 (55–85) 49 (37–70) | 22.5 (8–42) 14.5 (7–23) | 4.37 (2.01–4.76) 4.5 (3.0–6.3) | BRT CK | 35 Gy/5 (7) | Prostate gland | 2(+) 10(+) |
| Zerini et al. [21], 2015 (p. 32) | 73 (60–83) | R | EBRT 29 BT 3 | 99.7 (23–208.4) | 21.3 (2–53) | 3.9 (0.8–16.9) 2.3 (0.7–51.8) | VMAT | 25–30 Gy/5–10 (3–6) 15–25 Gy/3–5 (5) | Prostate gland 22 Prostate bed 10 | 8(+) 3(+) |
| Leroy et al. [22], 2017 (p. 23) | - | R | EBRT 19 (83%) BT 4 (17%) | 65 (28–150) | 22 (6–40) | 2.5 (0–11.7) | CK | 36 Gy/6 (6) | Whole prostate 19 Hemi-prostate 1 Focal treatment 3 | 14(+) |
| Miszczyk et al. [23], 2018 (p. 38) | 71.6 (59–89) | R | RP + EBRT (3) EBRT (31) RP + EBRT + BT (1) EBRT + BT (2) BT (1) | 101 (22–179) | 14.4 (1.6–46.4) | 3.26 (0.12–48.83) | CK | 36.25 Gy/5 (7.25) 36 Gy/6 (6); 30 Gy/5 (6) 30 Gy/2 (10) + 10 boost 30 Gy/3 (10); 18 Gy/3 (6) 20 Gy/2 (10); 22.5 Gy/3 (7.5) 27.5 Gy/5 (5.5) | Prostate gland 1 lobe Local relapse | 21(+) |
| Detti et al. [24], 2015 (p. 16) | 65 (52–78) | R | RP + EBRT (8) RP (8) | 126 (42–256) | 10 (2–21) | 4.1 (0.5–11.09) | CK | 30 Gy/5 (8) 35 Gy/5 (8) | Prostatic bed | 0(+) |
| Bergamin et al. [25], 2020 (p. 25) | 72 (62–83) | R | EBRT (21) EBRT + BT HDR (2) BT LDR (2) | 99 (54–163) | 25 (16–46) | 4.1 (1.1–16.6) | IMRT/VMAT | 36 Gy/6 (6) 38 Gy/6 (6.3) | Prostate gland | 0(+) |
| D’Agostino et al. [26], 2019 (p. 23) | 78 (69–85) | R | RP + RT (8) RT (15) | 90 (26–138) | 33(6–58) | 3.2 (1.2–13.5) | VMAT | 30 Gy/5 (6)25 Gy/5 (5) | Prostate gland (13) Prostatic bed (8) Prostate and local recurrence (2) | 8(+) |
| Jereczek-Fossa et al. [27], 2019 (p. 64) | 73.2 (52.6–81.7) | R | EBRT (59) EBRT + BT (1) BRT-LDR (4) | 99.7 (23–208.4) | 26.1 (3.1–82.4) | 3.89 (0.17–51.8) | IMRT(VERO) (54) IMRT(Trilogy) (7) CK (3) | 30 Gy (20–30)/5 (2–10) | Prostate gland (40) PPI (4) Prostate gland + boost (1) Prostatic surgical bed (19) | 16(+) |
| Ozyigit et al. [28], 2020 (p. 11) | 71 (59–86) | R | EBRT | 63 (23–178) | 19 | 2.33 | CK VMAT (Novalis Versa-HD) | 30 Gy/5 (6) | Focal reirradiation | - |
| Lewin et al. [29], 2021 (p. 30) | 62 (52–75) | R | EBRT (25) BT (5) | 72 (18–176) | 28 | 3.63 (0.05–77) | VMAT | 32.5 Gy/5 (6.5) | Prostate gland (18) Prostate + SV (10) SV (2) | 11(+) |
| Vavassori et al. [30], 2010 (p. 6) | 68 (63–74) | R | EBRT (6) | 13.5 (2.7–38.4) | 11.3 (9.6–18.6) | 3.65 (2.1–14.1) | CK | 30 Gy/5 (6) | Prostate gland (6) | 5(+) |
| Oliver et al. [31], 2019 (p. 12) | 58 | R | RP + EBRT | 6.5 (1–116) | 77.6 (21.4–160.8) | 1.13 (0.57–5.71) | CK | 36 Gy/6 (6) | Prostatic bed (12) | 2(+) |
| Our study, 2022 (p. 29) | 73 (61–86) | R | EBRT (25) SBRT (3) BT (1) | 72 (12–204) | 27 (6–60) | 1.9 (0.2–17) | CK (17) VMAT-IGRT-Clarity (12) | 30 Gy/5 (6) | Prostate gland (23) Prostatic bed (4) Intraprostatic lesion (2) | 12(+) |
| Author, Year (n. pts) | Toxicity | Toxicity (Criteria) | Local Control/Failure | Constraints |
|---|---|---|---|---|
| Caroli et al. [15], 2020 (p. 38) | Acute GU G1: 31.6% | CTCAE v.4.0 | 15mo 95% | - |
| Fuller et al. [16], 2019 (p. 50) | Acute GU G1–G2: 2% Late GU G2 17%; G3 8% | CTCAE v.3.0 | 2y 76%; 5y 60% LRF 94%; DRF 89% | Urethra: Dmax < 120%; D50 < 105%; Rectum: Dmax < 75%; Rectal wall; Dmax < 100% Bladder wall: Dmax < 100% |
| Fuller et al. [10], 2015 (p. 29) | Acute GU G3: 3% Late GU G2: 10%; G3: 3%; G4: 3% | CTCAE v.3.0 | BDFS 2y 82% CDFS 2y 100% | Urethra: Dmax < 120%; D50 < 105% Bladder wall: Dmax < 100%; Rectal wall; Dmax < 100% |
| Jereczek-Fossa et al. [17], 2012 (p. 15) | Acute GU: G1 2: 13%; G2: 13%; G3: 7% Late GU: G1 7%; G2 7%; G3 7% | RTOG | PFS 30mo: 42.6% DP: 5pz | Bladder: Dmax < 120%; Rectum: Dmax < 100%; Small bowel: V21 Gy < 1 cc |
| Loi et al. [9], 2018 (p. 50) Francolini et al. [18], 2022 (long-term results) | Acute GU: G1 18%; G2 2%; G3 2%; Acute GI: G1 8% Late GU: G1 18%; G2 6%; G3 2%; Late GI: G1 2%; G2 4%; G3 2% | CTCAE v.4.03 | BRFS 1y 80%; DMFS 1y 92% BRFS 2y 50%; DMFS 2y 82% | Bladder:Dmax < 120%; Rectum: Dmax < 100% Small bowel V21 Gy < 1 cc |
| Janoray et al. [19], 2016 (p. 21) | Acute: GU: G1 14%; G2 5% Acute GI: G1 10% Late: GU: G1 5% | CTCAE v.4.0 IPSS | BRFS 1y 83.3–85.7% Local Failure 1y 4% | Rectum: V18.1 Gy < 50%; V29 Gy < 20%; V36 Gy < 1 cc; Bladder: V18.1 Gy < 40%; V37 Gy < 10 cc Femoral head: V14.5 Gy < 5% |
| Mbeutcha et al. [20], 2017 (p. 28) | BT Acute GU: G1 30%; G2 40%; Late GU: G1 50%; G2 10% CK Acute GU: G1 27.8%; G2 11.1%; Acute GI: G1 5.6%; G2 11.1% CK Late GU: G1 22%; G2 5.6%; CK Late GI: G2 5.6% | CTCAE v.4.03 | BT: BFFS 44.4% CK: BFFS 44.4%; 33.3% | Urethra: V115 < 1% Rectum: V80 < 1% |
| Zerini et al. [21], 2015 (p. 32) | Prostate Acute: GU: G1 14%; G2 6%; GI: G1 9%; Late: GU: G1 19%; GI: G1 12% Prostatic bed Acute: GU: G1 3%; GI: G2 3%; Late: GU: G2 3%; GU: G1 3% | RTOG/EORTC | BF 1y 9% CF 1y 37% DFS 2y 40.6% OS (21.3mo): 93.7% | Prostate reirradiation Rectum: D30% < 13.8 Gy; D60% < 6.69 Gy; Bladder: D30% < 10.58 Gy Prostatic bed irradiation Rectum: D30% < 8.4 Gy; D60% < 4.08 Gy; Bladder: D30% < 3.94 Gy |
| Leroy et al. [22], 2017 (p. 23) | Acute GU: G1 47%; G2 30%; G3 9% Acute GI: G1 8.7%; G2 8.7% | CTCAE v.4.0 | 2y DFS 54%; OS 100% | Rectum: V27 Gy < 2 cc; V12 Gy < 20%; Bladder: V27 Gy < 5 cc; V12 Gy < 15% Intra-prostatic urethra: V24 Gy < 30%; V36 Gy < 1 cc |
| Miszczyk et al. [23], 2018 (p. 38) | Acute GU: G1 31.8%; G2 13%; G3 3.7%; Acute GI: G1 7.4% Late GU: G1 22.2%; G2 16.7%; G3 12.5%; Late GI G1 11.1%; G2 4.8% | RTOG/EORTC | BF: 13.2% BFFS 86.8% | - |
| Detti et al. [24], 2015 (p. 16) | Acute GU: G2 6% Acute GI: G2 6% | CTCAE v.4.0 | BRR 88% | Rectum: D30% < 18.8 Gy; D60% < 10 Gy; Bladder: D40% < 18.1 Gy; D 50% < 16.6 Gy Urethra: Dmax < 33.7 Gy; Dmean < 31 Gy; Femoral heads: V14.5 Gy < 5%; Penile bulb: V29.5 Gy < 50% |
| Bergamin et al. [25], 2020 (p. 25) | Acute GU: G1 24%; G2 4% Acute GI: G1 8%; G3 4% Late GU: G1 28%; G2 4% GI: G1 8% | CTCAE v.4.03 RTOG/EORTC | BFFF 2y 80% 5y 35–82% | Rectum: D0.1 cc < 33 Gy; D0.5 cc ≤ 28 Gy; D1.0 cc < 24 Gy D2.0 cc ≤ 18 Gy Bladder: D0.1 cc ≤ 33 Gy; D 0.5 cc ≤ 28 Gy; D 1.0 cc ≤ 24 Gy; D 2.0 cc ≤ 18 Gy Urethra: Dmax < 33 Gy; Urethra PRV: Dmax < 36 Gy |
| D’Agostino et al. [26], 2019 (p. 23) | Acute GU: G1 43%; G2 13%; G3 4% Late GU: G1 17%; G3 4% | CTCAE v.4.03 | BRFS 1y 81.6%; 2y 41.7% LC 1y 95%; 2y 61.1% PFS 1y 85.9%; 2y 63.6% | Rectum: V10 Gy < 40%; V18 Gy < 20% Bladder: V10 Gy < 25%; V18 Gy < 15% Femoral heads: V24 Gy < 10%; Penile bulb: V24 Gy < 50% Small intestine: V18 Gy < 5 cm3 |
| Jereczek-Fossa et al. [27], 2019 (p. 64) | Acute GU: G1 20%; G2 5%; G3 1.5%; Acute GI: G1 8%; G2 2% Late GU: G1 28%; G2 9%; G3 1.5%; Late GI: G1 6%; G2 1.5% | RTOG/EORTC | 2y LC: 75%; BFFS: 40%; CFS: 53% | Rectum: D30% < 13.5 Gy; D60% < 6.7 Gy Bladder: D30% < 10.6 Gy |
| Ozyigit et al. [28], 2020 (p. 11) | Acute GI: G3 9% Late GU: G2 9% | CTCAE v.4.0 RTOG/EORTC | BFFS 1y 89%; 2y 48% | Bladder: Dmax < 120%; Rectum: Dmax < 100%; Small bowel: V21 Gy < 1 cc |
| Lewin et al. [29], 2021 (p. 30) | Acute GU: G2 27%; G3 3% Late GU: G2 30%; G3: 3%; Late GI: G2 3% | CTCAE v.4.0 | BF 2y 53%; CF 33% RFS 2y 60%; RFS 3y 53% | Rectal wall V100% < 5%; V90% < 15%; V80% < 20%; V38 Gy < 2 cc |
| Vavassori et al. [30], 2010 (p. 6) | Acute GI: G1 33%; Late GU: G1 33% | RTOG/EORTC | BF 1y 66%; CF 1y 50% | Urethra Dmax < 125%; Rectum Dmax < 75% |
| Oliver et al. [31], 2019 (p. 12) | Acute GU: G1–2 25%; Acute GI: G1–2 8% Late GU: G1-2 12.5% | CTCAE v.4.0 | BRFS 1y 79%; BRFS 2y 56% | Rectum V12 Gy < 20%; V27 Gy < 2 cc; Bladder V12 Gy < 15%; V27 Gy < 5 cc |
| Our study, 2022 (p. 29) | CK Acute GU: G2 23%; Acute GI: G2 6% VMAT-Clarity Acute GU: G2 17%; VMAT-Clarity Late GU: G2 8% | RTOG/EORTC | LRF 1y 89%; DRF 1y 85% BRFS 2y 81% | Rectum: V30 Gy < 5%; V27 Gy < 10%; V24 Gy < 20%; V15 Gy < 50%; V21 Gy < 25 cc; D30% < 8.4 Gy; D60%< 4.08 Gy Bladder: V15 Gy < 40%; V30 Gy < 10%; D30% < 3.94 Gy; Penis bulb V29.5 Gy < 50%; V24 Gy < 50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Franco, R.; Borzillo, V.; Scipilliti, E.; Ametrano, G.; Serra, M.; Arrichiello, C.; Savino, F.; De Martino, F.; D’Alesio, V.; Cammarota, F.; et al. Reirradiation of Locally Recurrent Prostate Cancer with Cyberknife® System or Volumetric Modulated Arc Therapy (VMAT) and IGRT-Clarity®: Outcomes, Toxicities and Dosimetric Evaluation. Cancers 2022, 14, 3187. https://doi.org/10.3390/cancers14133187
Di Franco R, Borzillo V, Scipilliti E, Ametrano G, Serra M, Arrichiello C, Savino F, De Martino F, D’Alesio V, Cammarota F, et al. Reirradiation of Locally Recurrent Prostate Cancer with Cyberknife® System or Volumetric Modulated Arc Therapy (VMAT) and IGRT-Clarity®: Outcomes, Toxicities and Dosimetric Evaluation. Cancers. 2022; 14(13):3187. https://doi.org/10.3390/cancers14133187
Chicago/Turabian StyleDi Franco, Rossella, Valentina Borzillo, Esmeralda Scipilliti, Gianluca Ametrano, Marcello Serra, Cecilia Arrichiello, Federica Savino, Fortuna De Martino, Valentina D’Alesio, Fabrizio Cammarota, and et al. 2022. "Reirradiation of Locally Recurrent Prostate Cancer with Cyberknife® System or Volumetric Modulated Arc Therapy (VMAT) and IGRT-Clarity®: Outcomes, Toxicities and Dosimetric Evaluation" Cancers 14, no. 13: 3187. https://doi.org/10.3390/cancers14133187
APA StyleDi Franco, R., Borzillo, V., Scipilliti, E., Ametrano, G., Serra, M., Arrichiello, C., Savino, F., De Martino, F., D’Alesio, V., Cammarota, F., Crispo, A., Pignata, S., Rossetti, S., Quarto, G., & Muto, P. (2022). Reirradiation of Locally Recurrent Prostate Cancer with Cyberknife® System or Volumetric Modulated Arc Therapy (VMAT) and IGRT-Clarity®: Outcomes, Toxicities and Dosimetric Evaluation. Cancers, 14(13), 3187. https://doi.org/10.3390/cancers14133187






