Robot-Assisted Minimally Invasive Esophagectomy versus Open Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion/Exclusion Criteria
- Participants: Patients of any race, age, or sex undergoing esophagectomy for esophageal cancer.
- Interventions: RAMIE and OE. Studies were included independent of the surgical approach (i.e., transthoracic (Ivor-Lewis or McKeown), or transhiatal). Procedures were identified as RAMIE if the robotic approach was utilized for the thoracic phase of the operation, irrespective of the abdominal phase approach. The procedures were further classified as a) totally robotic (TRAMIE) if the robotic approach was utilized for both the thoracic and the abdominal part of the operation and b) hybrid if the robotic approach was utilized for the thoracic part of the operation or both types of the aforementioned approaches were included in a single sample.
- Comparison: Studies were deemed eligible only if RAMIE was directly compared to OE.
- Outcomes: The primary surgical outcome measures were the rates of overall complications, overall pulmonary complications, anastomotic leakage, 30-day mortality, and 90-day mortality. The primary oncological outcomes were the number of total lymph nodes resected, the margin-negative resection (R0) rate, the overall survival, (OS) and the disease-free survival (DFS). The secondary outcome measures were total operative time, estimated blood loss (EBL), hospital length of stay (LOS), and length of intensive care unit (ICU) stay as well the rates of pneumonia, acute respiratory distress syndrome (ARDS), postoperative hemorrhage, chylothorax, recurrent laryngeal nerve (RLN) palsy, atrial fibrillation, and wound infection.
2.2. Literature Search Strategy
2.3. Data Tabulation and Extraction
2.4. Quality of Evidence Assessment
2.5. Statistical Analysis
2.5.1. Data Pooling
2.5.2. Meta-Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. Study Quality and Publication Bias Assessment
3.3. Primary Surgical Outcomes
3.3.1. Overall Complication Rate
3.3.2. Overall Pulmonary Complication Rate
3.3.3. Anastomotic Leakage Rate
3.3.4. Thirty-Day Mortality Rate
3.3.5. Ninety-Day Mortality Rate
3.4. Primary Oncological Outcomes
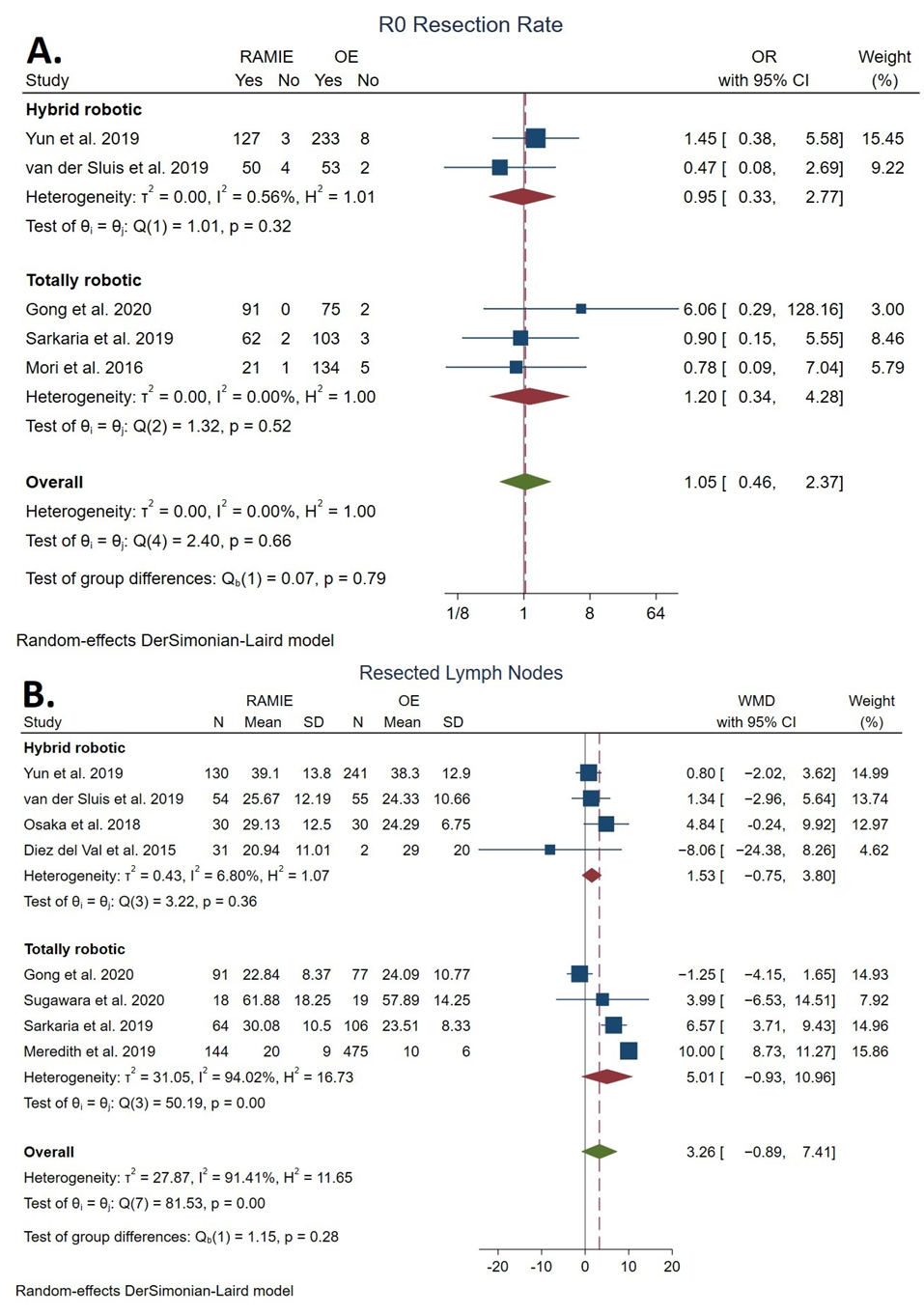
3.4.1. Total Lymph Nodes Resected
3.4.2. R0 Resection Rate
3.4.3. Overall Survival
3.4.4. Disease-Free Survival
3.5. Secondary Outcomes
3.5.1. Operative Time
3.5.2. Estimated Blood Loss
3.5.3. ICU Length of Stay
3.5.4. Length of Hospital Stay
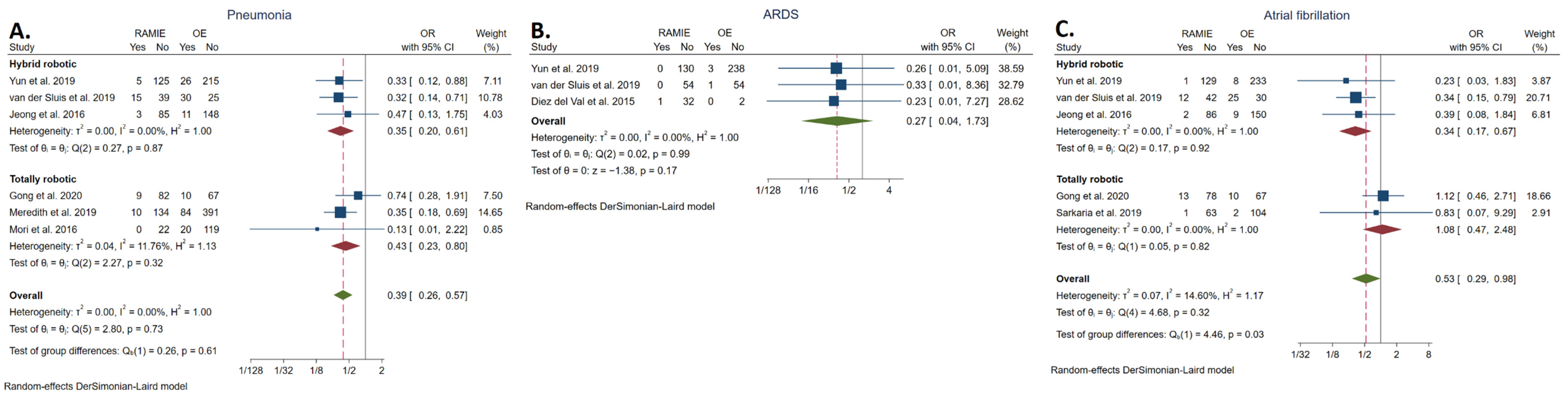
3.5.5. Pneumonia Rate
3.5.6. ARDS Rate
3.5.7. Atrial Fibrillation Rate
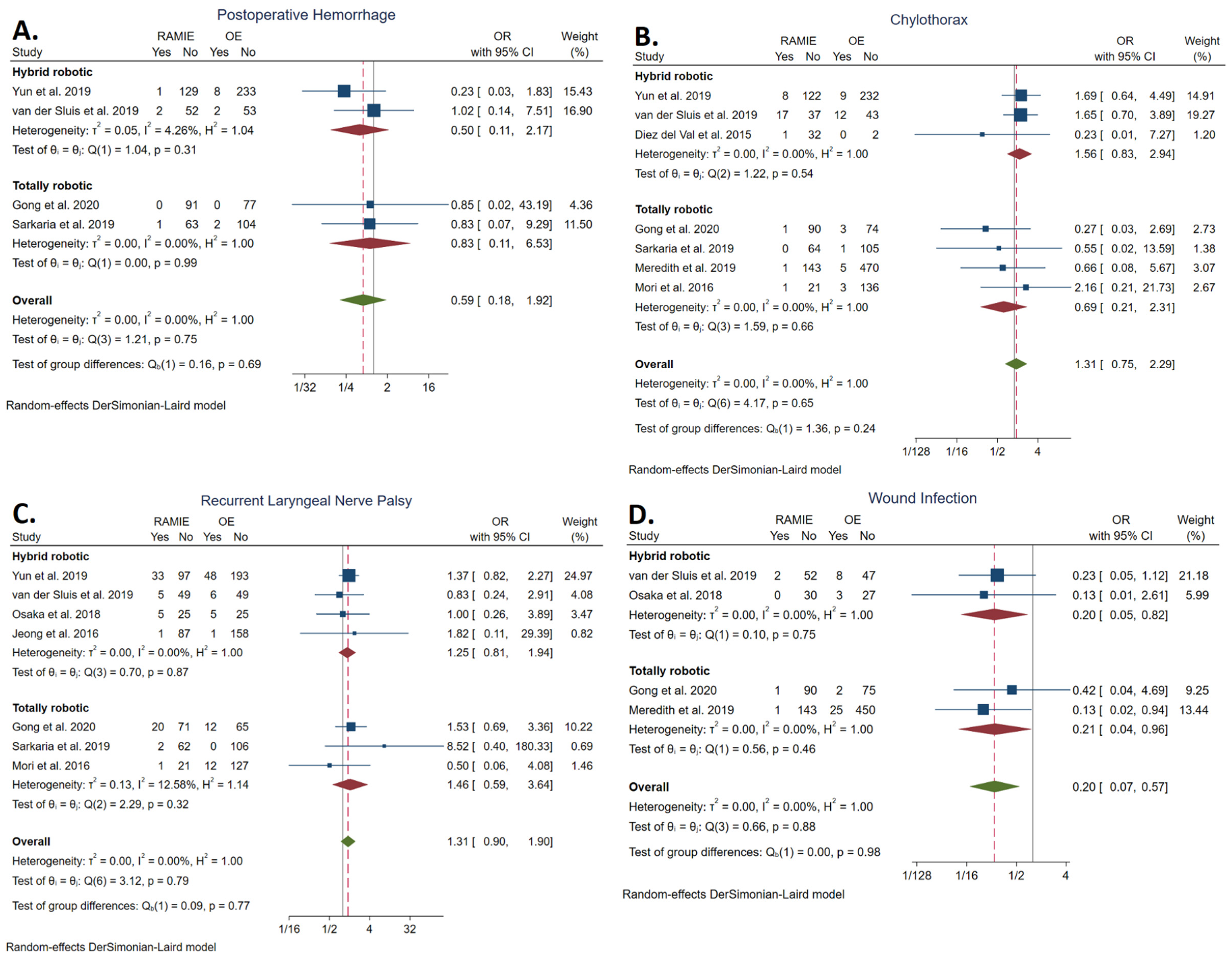
3.5.8. Postoperative Hemorrhage Rate
3.5.9. Chylothorax Rate
3.5.10. Recurrent Laryngeal Nerve Palsy Rate
3.5.11. Wound Infection Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ajani, J.A.; Barthel, J.S.; Bentrem, D.J.; Amico, T.A.D.; Das, P.; Denlinger, C.S.; Fuchs, C.S.; Gerdes, H.; Glasgow, R.E.; Hayman, J.A.; et al. Esophageal and Esophagogastric Junction Cancers Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2011, 9, 830–887. [Google Scholar] [CrossRef] [Green Version]
- Ando, N.; Ozawa, S.; Kitagawa, Y.; Shinozawa, Y.; Kitajima, M. Improvement in the Results of Surgical Treatment of Advanced Squamous Esophageal Carcinoma During 15 Consecutive Years. Ann. Surg. 2000, 232, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Miyata, H.; Gotoh, M.; Kitagawa, Y.; Baba, H.; Kimura, W.; Tomita, N.; Nakagoe, T.; Shimada, M.; Sugihara, K.; et al. A Risk Model for Esophagectomy Using Data of 5354 Patients Included in a Japanese Nationwide Web-Based Database. Ann. Surg. 2014, 260, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Lerut, T.; Moons, J.; Coosemans, W.; Van Raemdonck, D.; De Leyn, P.; Decaluwé, H.; Decker, G.; Nafteux, P. Postoperative Complications after Transthoracic Esophagectomy for Cancer of the Esophagus and Gastroesophageal Junction Are Correlated with Early Cancer Recurrence: Role of Systematic Grading of Complications Using the Modified Clavien Classification. Ann. Surg. 2009, 250, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Liu, Y.; Ward, K.C.; Force, S.D.; Pickens, A.; Sancheti, M.S.; Javidfar, J.; Fernandez, F.G.; Khullar, O.V. Excess Cost and Predictive Factors of Esophagectomy Complications in the SEER-Medicare Database. Ann. Thorac. Surg. 2018, 106, 1484–1491. [Google Scholar] [CrossRef] [Green Version]
- Luketich, J.D.; Alvelo-Rivera, M.; Buenaventura, P.O.; Christie, N.A.; McCaughan, J.S.; Litle, V.R.; Schauer, P.R.; Close, J.M.; Fernando, H.C.; Zinner, M.J. Minimally Invasive Esophagectomy: Outcomes in 222 Patients. Ann. Surg. 2003, 238, 486–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanivelu, C.; Prakash, A.; Senthilkumar, R.; Senthilnathan, P.; Parthasarathi, R.; Rajan, P.S.; Venkatachlam, S. Minimally Invasive Esophagectomy: Thoracoscopic Mobilization of the Esophagus and Mediastinal Lymphadenectomy in Prone Position—Experience of 130 Patients. J. Am. Coll. Surg. 2006, 203, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Biere, S.S.A.Y.; van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.G.; Hollmann, M.W.; de Lange, E.S.; et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef]
- Van Der Sluis, P.C.; Schizas, D.; Liakakos, T.; van Hillegersberg, R. Minimally Invasive Esophagectomy. Dig. Surg. 2020, 37, 93–100. [Google Scholar] [CrossRef]
- Gottlieb-Vedi, E.; Kauppila, J.H.; Malietzis, G.; Nilsson, M.; Markar, S.R.; Lagergren, J. Long-term Survival in Esophageal Cancer After Minimally Invasive Compared to Open Esophagectomy: A Systematic Review and Meta-Analysis. Ann. Surg. 2019, 270, 1005–1017. [Google Scholar] [CrossRef]
- Markar, S.R.; Ni, M.; Gisbertz, S.S.; van der Werf, L.; Straatman, J.; van der Peet, D.; Cuesta, M.A.; Hanna, G.B.; van Berge Henegouwen, M.I. Implementation of Minimally Invasive Esophagectomy From a Randomized Controlled Trial Setting to National Practice. J. Clin. Oncol. 2020, 38, 2130. [Google Scholar] [CrossRef]
- Decker, G.; Coosemans, W.; De Leyn, P.; Decaluwé, H.; Nafteux, P.; Van Raemdonck, D.; Lerut, T. Minimally Invasive Esophagectomy for Cancer. Eur. J. Cardio-Thorac. Surg. 2009, 35, 13–21. [Google Scholar] [CrossRef]
- Van Hillegersberg, R.; Boone, J.; Draaisma, W.A.; Broeders, I.A.M.J.; Giezeman, M.J.M.M.; Rinkes, I.H.M.B. First Experience with Robot-Assisted Thoracoscopic Esophagolymphadenectomy for Esophageal Cancer. Surg. Endosc. Other Interv. Tech. 2006, 20, 1435–1439. [Google Scholar] [CrossRef]
- van Workum, F.; Stenstra, M.H.B.C.; Berkelmans, G.H.K.; Slaman, A.E.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; van den Wildenberg, F.J.H.; Polat, F.; Irino, T.; Nilsson, M.; et al. Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann. Surg. 2019, 269, 88–94. [Google Scholar] [CrossRef]
- van der Sluis, P.C.; Tagkalos, E.; Hadzijusufovic, E.; Babic, B.; Uzun, E.; van Hillegersberg, R.; Lang, H.; Grimminger, P.P. Robot-Assisted Minimally Invasive Esophagectomy with Intrathoracic Anastomosis (Ivor Lewis): Promising Results in 100 Consecutive Patients (the European Experience). J. Gastrointest. Surg. 2020, 25, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kim, D.J.; Yu, W.S.; Jung, H.S. Robot-assisted thoracoscopic esophagectomy with extensive mediastinal lymphadenectomy: Experience with 114 consecutive patients with intrathoracic esophageal cancer. Dis. Esophagus 2016, 29, 326–332. [Google Scholar] [CrossRef]
- van der Sluis, P.C.; Ruurda, J.P.; Verhage, R.J.J.; van der Horst, S.; Haverkamp, L.; Siersema, P.D.; Borel Rinkes, I.H.M.; ten Kate, F.J.W.; van Hillegersberg, R. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann. Surg. Oncol. 2015, 22, 1350–1356. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Su, Y.; Yang, Y.; Sun, Y.; Ye, B.; Guo, X.; Mao, T.; Hua, R.; Li, Z. Robot assisted esophagectomy for esophageal squamous cell carcinoma. J. Thorac. Dis. 2018, 10, 3767–3775. [Google Scholar] [CrossRef]
- Jin, D.; Yao, L.; Yu, J.; Liu, R.; Guo, T.; Yang, K.; Gou, Y. Robotic-assisted minimally invasive esophagectomy versus the conventional minimally invasive one: A meta-analysis and systematic review. Int. J. Med. Robot. Comput. Assist. Surg. 2019, 15, e1988. [Google Scholar] [CrossRef]
- Haverkamp, L.; Seesing, M.F.J.; Ruurda, J.P.; Boone, J.; van Hillegersberg, R. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis. Esophagus 2017, 30, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Van der Sluis, P.C.; van der Horst, S.; May, A.M.; Schippers, C.; Brosens, L.A.A.; Joore, H.C.A.; Kroese, C.C.; Mohammad, N.H.; Mook, S.; Vleggaar, F.P.; et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann. Surg. 2019, 269, 621–630. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.K.; Chong, B.K.; Kim, H.J.; Lee, I.-S.; Gong, C.-S.; Kim, B.S.; Lee, G.D.; Choi, S.; Kim, H.R.; Kim, D.K.; et al. Comparative Outcomes of Robot-Assisted Minimally Invasive versus Open Esophagectomy in Patients with Esophageal Squamous Cell Carcinoma: A Propensity Score-Weighted Analysis. Dis. Esophagus 2020, 33, doz071. [Google Scholar] [CrossRef]
- Yun, J.K.; Lee, I.-S.; Gong, C.-S.; Kim, B.S.; Kim, H.R.; Kim, D.K.; Park, S.-I.; Kim, Y.-H. Clinical utility of robot-assisted transthoracic esophagectomy in advanced esophageal cancer after neoadjuvant chemoradiation therapy. J. Thorac. Dis. 2019, 11, 2913–2923. [Google Scholar] [CrossRef]
- Wohlin, C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. In Proceedings of the the 18th International Conference on Evaluation and Assessment in Software Engineering, ACM, New York, NY, USA, 13 May 2014; pp. 1–10. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0 (Updated March 2011); The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Haldane, J.B.S. The estimation and significance of the logarithm of a ratio of frequencies. Ann. Hum. Genet. 1956, 20, 309–311. [Google Scholar] [CrossRef]
- Anscombe, F.J. On Estimating Binomial Response Relations. Biometrika 1956, 43, 461. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Gong, L.; Jiang, H.; Yue, J.; Duan, X.; Tang, P.; Ren, P.; Zhao, X.; Liu, X.; Zhang, X.; Yu, Z. Comparison of the short-term outcomes of robot-assisted minimally invasive, video-assisted minimally invasive, and open esophagectomy. J. Thorac. Dis. 2020, 12, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, I.S.; Rizk, N.P.; Goldman, D.A.; Sima, C.; Tan, K.S.; Bains, M.S.; Adusumilli, P.S.; Molena, D.; Bott, M.; Atkinson, T.; et al. Early Quality of Life Outcomes After Robotic-Assisted Minimally Invasive and Open Esophagectomy. Ann. Thorac. Surg. 2019, 108, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.M.; Kim, J.A.; Ahn, H.J.; Yang, M.; Heo, B.Y.; Lee, S.H. Decreased Incidence of Postoperative Delirium in Robot-assisted Thoracoscopic Esophagectomy Compared with Open Transthoracic Esophagectomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 516–522. [Google Scholar] [CrossRef]
- Meredith, K.L.; Maramara, T.; Blinn, P.; Lee, D.; Huston, J.; Shridhar, R. Comparative Perioperative Outcomes by Esophagectomy Surgical Technique. J. Gastrointest. Surg. 2019, 24, 1261–1268. [Google Scholar] [CrossRef]
- Osaka, Y.; Tachibana, S.; Ota, Y.; Suda, T.; Makuuti, Y.; Watanabe, T.; Iwasaki, K.; Katsumata, K.; Tsuchida, A. Usefulness of robot-assisted thoracoscopic esophagectomy. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 225–231. [Google Scholar] [CrossRef]
- Mori, K.; Yamagata, Y.; Aikou, S.; Nishida, M.; Kiyokawa, T.; Yagi, K.; Yamashita, H.; Nomura, S.; Seto, Y. Short-term outcomes of robotic radical esophagectomy for esophageal cancer by a nontransthoracic approach compared with conventional transthoracic surgery. Dis. Esophagus 2016, 29, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, K.; Yoshimura, S.; Yagi, K.; Nishida, M.; Aikou, S.; Yamagata, Y.; Mori, K.; Yamashita, H.; Seto, Y. Long-term health-related quality of life following robot-assisted radical transmediastinal esophagectomy. Surg. Endosc. 2019, 34, 1602–1611. [Google Scholar] [CrossRef]
- del Val, I.D.; Loureiro, C.; McCulloch, P. The IDEAL prospective development study format for reporting surgical innovations. An illustrative case study of robotic oesophagectomy. Int. J. Surg. 2015, 19, 104–111. [Google Scholar] [CrossRef]
- Weksler, B.; Sullivan, J.L. Survival After Esophagectomy: A Propensity-Matched Study of Different Surgical Approaches. Ann. Thorac. Surg. 2017, 104, 1138–1146. [Google Scholar] [CrossRef] [Green Version]
- Espinoza-Mercado, F.; Imai, T.A.; Borgella, J.D.; Sarkissian, A.; Serna-Gallegos, D.; Alban, R.F.; Soukiasian, H.J. Does the Approach Matter? Comparing Survival in Robotic, Minimally Invasive, and Open Esophagectomies. Ann. Thorac. Surg. 2019, 107, 378–385. [Google Scholar] [CrossRef]
- Yerokun, B.A.; Sun, Z.; Jeffrey Yang, C.F.; Gulack, B.C.; Speicher, P.J.; Adam, M.A.; D’Amico, T.A.; Onaitis, M.W.; Harpole, D.H.; Berry, M.F.; et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann. Thorac. Surg. 2016, 102, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Schieman, C.; Wigle, D.A.; Deschamps, C.; NIcholsii, F.C.; Cassivi, S.D.; Shen, K.R.; Allen, M.S. Patterns of operative mortality following esophagectomy. Dis. Esophagus 2012, 25, 645–651. [Google Scholar] [CrossRef]
- Morita, M.; Nakanoko, T.; Fujinaka, Y.; Kubo, N.; Yamashita, N.; Yoshinaga, K.; Saeki, H.; Emi, Y.; Kakeji, Y.; Shirabe, K.; et al. In-Hospital Mortality After a Surgical Resection for Esophageal Cancer: Analyses of the Associated Factors and Historical Changes. Ann. Surg. Oncol. 2011, 18, 1757–1765. [Google Scholar] [CrossRef]
- Mertens, A.C.; Kalff, M.C.; Eshuis, W.J.; Van Gulik, T.M.; Henegouwen, M.I.V.B.; Gisbertz, S.S. Transthoracic Versus Transhiatal Esophagectomy for Esophageal Cancer: A Nationwide Propensity Score-Matched Cohort Analysis. Ann. Surg. Oncol. 2020, 28, 175–183. [Google Scholar] [CrossRef]
- Bailey, S.H.; Bull, D.A.; Harpole, D.H.; Rentz, J.J.; Neumayer, L.A.; Pappas, T.N.; Daley, J.; Henderson, W.G.; Krasnicka, B.; Khuri, S.F. Outcomes after esophagectomy: A ten-year prospective cohort. Ann. Thorac. Surg. 2003, 75, 217–222. [Google Scholar] [CrossRef]
- Atkins, B.Z.; Shah, A.S.; Hutcheson, K.A.; Mangum, J.H.; Pappas, T.N.; Harpole, D.H.; D’Amico, T.A. Reducing Hospital Morbidity and Mortality Following Esophagectomy. Ann. Thorac. Surg. 2004, 78, 1170–1176. [Google Scholar] [CrossRef]
- Kataoka, K.; Takeuchi, H.; Mizusawa, J.; Igaki, H.; Ozawa, S.; Abe, T.; Nakamura, K.; Kato, K.; Ando, N.; Kitagawa, Y. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer. Ann. Surg. 2017, 265, 1152–1157. [Google Scholar] [CrossRef]
- Murthy, R.A.; Clarke, N.S.; Kernstine, K.H. Minimally Invasive and Robotic Esophagectomy: A Review. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2018, 13, 391–403. [Google Scholar] [CrossRef]
- Lv, L.; Hu, W.; Ren, Y.; Wei, X. Minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: A meta-analysis. OncoTargets Ther. 2016, 9, 6751–6762. [Google Scholar] [CrossRef] [Green Version]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef]
- Kassis, E.S.; Kosinski, A.S.; Ross, P.; Koppes, K.E.; Donahue, J.M.; Daniel, V.C. Predictors of Anastomotic Leak After Esophagectomy: An Analysis of The Society of Thoracic Surgeons General Thoracic Database. Ann. Thorac. Surg. 2013, 96, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Su, Q.; Ren, Z.; Wen, J.; Xue, Z.; Zhang, L.; Chu, X. Comparison of short-term outcomes between minimally invasive McKeown and Ivor Lewis esophagectomy for esophageal or junctional cancer: A systematic review and meta-analysis. OncoTargets Ther. 2018, 11, 6057–6069. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Ma, G.; Li, X.; Li, J.; Yan, Y.; Liu, P.; He, J.; Ren, Y. Is minimally invasive esophagectomy effective for preventing anastomotic leakages after esophagectomy for cancer? A systematic review and meta-analysis. World J. Surg. Oncol. 2015, 13, 269. [Google Scholar] [CrossRef] [Green Version]
- Seesing, M.F.J.; Borggreve, A.S.; Ruurda, J.P.; van Hillegersberg, R. New-onset atrial fibrillation after esophagectomy for cancer. J. Thorac. Dis. 2019, 11, S831–S834. [Google Scholar] [CrossRef]
- Schizas, D.; Kosmopoulos, M.; Giannopoulos, S.; Kokkinidis, D.G.; Karampetsou, N.; Papanastasiou, C.; Rouvelas, I.; Liakakos, T. Meta-analysis of risk factors and complications associated with atrial fibrillation after oesophagectomy. Br. J. Surg. 2019, 106, 534–547. [Google Scholar] [CrossRef]
- Boone, J.; Schipper, M.E.I.; Moojen, W.A.; Borel Rinkes, I.H.M.; Cromheecke, G.J.E.; van Hillegersberg, R. Robot-assisted thoracoscopic oesophagectomy for cancer. Br. J. Surg. 2009, 96, 878–886. [Google Scholar] [CrossRef]
- Fabian, T.; Martin, J.; Katigbak, M.; McKelvey, A.A.; Federico, J.A. Thoracoscopic esophageal mobilization during minimally invasive esophagectomy: A head-to-head comparison of prone versus decubitus positions. Surg. Endosc. 2008, 22, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Noshiro, H.; Iwasaki, H.; Kobayashi, K.; Uchiyama, A.; Miyasaka, Y.; Masatsugu, T.; Koike, K.; Miyazaki, K. Lymphadenectomy along the left recurrent laryngeal nerve by a minimally invasive esophagectomy in the prone position for thoracic esophageal cancer. Surg. Endosc. 2010, 24, 2965–2973. [Google Scholar] [CrossRef]
- Ruurda, J.P.; van Vroonhoven, T.J.M.V.; Broeders, I.A.M.J. Robot-assisted surgical systems: A new era in laparoscopic surgery. Ann. R. Coll. Surg. Engl. 2002, 84, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Boshier, P.R.; Ziff, C.; Adam, M.E.; Fehervari, M.; Markar, S.R.; Hanna, G.B. Effect of perioperative blood transfusion on the long-term survival of patients undergoing esophagectomy for esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2017, 31, dox134. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Sodergren, M.H.; Purkayastha, S.; Mayer, E.K.; James, D.; Athanasiou, T.; Yang, G.-Z.; Darzi, A. The role of robotic assisted laparoscopy for oesophagogastric oncological resection; an appraisal of the literature. Dis. Esophagus 2010, 24, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluis, P.C.; Ruurda, J.P.; Van Der Horst, S.; Goense, L.; Van Hillegersberg, R. Learning Curve for Robot-Assisted Minimally Invasive Thoracoscopic Esophagectomy: Results From 312 Cases. Ann. Thorac. Surg. 2018, 106, 264–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, E.; Markar, S.R.; Ruurda, J.P.; Hanna, G.B.; van Hillegersberg, R. Prognostic Value of Lymph Node Yield on Overall Survival in Esophageal Cancer Patients: A Systematic Review and Meta-Analysis. Ann. Surg. 2019, 269, 261–268. [Google Scholar] [CrossRef]
- Samson, P.; Puri, V.; Broderick, S.; Patterson, G.A.; Meyers, B.; Crabtree, T. Extent of Lymphadenectomy Is Associated with Improved Overall Survival After Esophagectomy with or without Induction Therapy. Ann. Thorac. Surg. 2016, 103, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Markar, S.R.; Gronnier, C.; Duhamel, A.; Pasquer, A.; Théreaux, J.; du Rieu, M.C.; Lefevre, J.H.; Turner, K.; Luc, G.; Mariette, C. Significance of Microscopically Incomplete Resection Margin After Esophagectomy for Esophageal Cancer. Ann. Surg. 2016, 263, 712–718. [Google Scholar] [CrossRef]
- Park, S.; Hyun, K.; Lee, H.J.; Park, I.K.; Kim, Y.T.; Kang, C.H. A study of the learning curve for robotic oesophagectomy for oesophageal cancer. Eur. J. Cardio-Thoracic Surg. 2017, 53, 862–870. [Google Scholar] [CrossRef]



| Single-Center Studies | |||||||
| Author | Year | Country | Study Period | Type of Study | RAMIE | OE | RAMIE Technique |
| Gong et al. | 2020 | China | Jan 2016 to Dec 2018 | Retrospective cohort | 77 | 91 | Robotic thoracic + abdominal phase; cervical anastomosis |
| Sugawara et al. | 2020 | Japan | Apr 2015 to Jan 2017 | Prospective cohort | 18 | 19 | Robotic transhiatal combined with a video-assisted cervical approach; cervical anastomosis |
| Sarkaria et al. | 2019 | USA | Mar 2012 to Aug 2014 | Prospective cohort | 64 | 106 | Robotic thoracic + abdominal phase; cervical or intrathoracic anastomosis |
| Yun et al. | 2019 | South Korea | Jan 2012 to Dec 2016 | Retrospective cohort | 130 | 241 | Robotic thoracic phase; robotic abdominal or laparoscopic phase; cervical or intrathoracic anastomosis |
| Meredith et al. | 2019 | USA | 1999 to 2016 | Retrospective cohort | 144 | 475 | Robotic thoracic + abdominal phase; intrathoracic anastomosis |
| van der Sluis et al. | 2019 | The Netherlands | Jan 2012 to Aug 2016 | Randomized controlled trial | 54 | 55 | Robotic thoracic phase; laparoscopic abdominal phase; cervical anastomosis |
| Osaka et al. | 2018 | Japan | Jun 2010 to Dec 2013 (RAMIE), 2006–2010 (OE) | Retrospective cohort | 30 | 30 | Robotic thoracic phase |
| Jeong et al. | 2016 | South Korea | Dec 2012 to Apr 2015 | Retrospective cohort | 88 | 159 | Robotic thoracic phase; open abdominal phase; cervical anastomosis |
| Mori et al. | 2016 | Japan | Nov 2012 to Jul 2014 (RAMIE), May 2008 to Jul 2014 (OE) | Prospective cohort | 22 | 139 | Robotic transhiatal combined with a video-assisted cervical approach; cervical anastomosis |
| Diez del Val et al. | 2015 | Spain | Dec 2009 to N/A | Prospective cohort | 33 | 2 | Robotic thoracic or transhiatal phase; intrathoracic or cervical anastomosis (respectively) |
| Total | 1999 to 2019 | 674 | 1303 | ||||
| National Registry Studies | |||||||
| Author | Year | Country | Study Period | Type of Study | RAMIE | Open | |
| Weksler et al. | 2017 | USA | 2010 to 2013 | Retrospective cohort | 569 | 569 | N/A |
| RAMIE (n = 674) | OE (n = 1303) | ||
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 63.5 ± 8.5 | 62.7 ± 9.27 | |
| Male/Female | 545 (85.0)/96 (15.0) | 1133 (87.1)/168 (12.9)) | |
| BMI (kg/m2) | 25.8 ± 6.8 | 25.7 ± 5.4 | |
| Preoperative characteristics | |||
| ASA physical status | |||
| 1–2 | 195 (55.7) | 427 (60.7) | |
| 3–4 | 155 (44.3) | 276 (39.3) | |
| Charlson–Deyo score | |||
| 0 | 18 (16.5) | 17 (17.7) | |
| 1 | 31 (28.4) | 33 (34.4) | |
| 2 | 42 (38.5) | 35 (36.5) | |
| 3 | 14 (12.8) | 11 (11.5) | |
| 4 | 4 (3.7) | 0 (0.0) | |
| Tumor characteristics | |||
| Tumor location | |||
| Proximal 1/3 | 57 (13.9) | 94 (14.1) | |
| Middle 1/3 | 136 (33.3) | 246 (36.9) | |
| Distal 1/3 + GEJ | 216 (52.8) | 327 (49.0) | |
| Tumor histology | |||
| Squamous cell carcinoma | 271 (72.7) | 472 (74.4) | |
| Adenocarcinoma | 100 (26.8) | 154 (24.3) | |
| Other | 2 (0.5) | 8 (1.3) | |
| Tumor differentiation | |||
| G1 (well-differentiated) | 13 (14.3) | 11 (14.3) | |
| G2 (moderately differentiated) | 54 (59.3) | 51 (66.2) | |
| G3 (poorly differentiated) | 24 (26.3) | 15 (19.5) | |
| Clinical stage (according to TNM) | |||
| Stage 0 | 1 (0.2) | 2 (0.2) | |
| Stage I | 243 (39.9) | 318(30.3) | |
| Stage II | 181 (29.7) | 333 (31.7) | |
| Stage III | 178 (29.2) | 375 (35.7) | |
| Stage IV | 6 (1.0) | 23 (2.2) | |
| Tumor size (cm) | 5.0 ± 2.1 | 4.4 ± 1.8 | |
| Neoadjuvant treatment | 292 (51.8) | 596 (59.3) | |
| Outcomes | Sum RAMIE | Sum Open | OR/WMD | 95% CI | p Value | I2 (%) | Result | |
|---|---|---|---|---|---|---|---|---|
| Primary surgical | ||||||||
| Overall complications * | 109/391 | 303/893 | 0.66 | [0.42, 1.05] | 0.08 | 50.17 | NS | |
| Overall pulmonary complications * | 49/343 | 174/687 | 0.38 | [0.26, 0.56] | <0.001 | 0.00 | Favors RAMIE | |
| Anastomotic leakage | 46/674 | 79/1303 | 0.93 | [0.60, 1.44] | 0.76 | 0.00 | NS | |
| 30-day mortality | 2/248 | 6/402 | 0.75 | [0.15, 3.78] | 0.73 | 0.00 | NS | |
| 90-day mortality | 10/421 | 18/808 | 0.80 | [0.31, 2.05] | 0.64 | 0.00 | NS | |
| Primary oncological | ||||||||
| Lymph nodes resected | 28.45 ± 14.71 | 21.44 ± 15.53 | 3.26 | [−0.89, 7.41] | 0.12 | 91.41 | NS | |
| R0 resection | 495/649 | 1047/1092 | 1.34 | [0.56, 3.17] | 0.33 | 13.82 | NS | |
| Secondary | ||||||||
| Operative time † | 360.39 ± 115.44 | 306.21 ± 94.58 | 69.45 | [34.39, 104.42] | < 0.001 | 96.58 | Favors OE | |
| EBL ‡ | 209.59 ± 169.02 | 374.38 ± 415.34 | −187.08 | [−283.81, −90.35] | < 0.001 | 95.50 | Favors RAMIE | |
| ICU length of stay § | 1.37 ± 0.56 | 1.59 ± 1.55 | −0.13 | [−0.28, 0.02] | 0.09 | 27.67 | NS | |
| LOS § | 17.10 ± 9.39 | 30.68 ± 23.88 | −9.22 | [−14.39, −4.06] | <0.001 | 96.13 | Favors RAMIE | |
| Pneumonia | 42/529 | 181/1146 | 0.39 | [0.26, 0.57] | <0.001 | 0.00 | Favors RAMIE | |
| ARDS | 1/217 | 4/298 | 0.27 | [0.04, 1.73] | 0.17 | 0.00 | NS | |
| Atrial fibrillation | 29/427 | 54/638 | 0.53 | [0.29, 0.98] | 0.04 | 14.60 | Favors RAMIE | |
| Postoperative hemorrhage | 4/339 | 12/479 | 0.59 | [0.18, 1.92] | 0.38 | 0.00 | NS | |
| Chylothorax | 29/538 | 33/1095 | 1.31 | [0.75, 2.29] | 0.35 | 0.00 | NS | |
| RLN palsy | 67/479 | 84/807 | 1.31 | [0.90, 1.90] | 0.16 | 0.00 | NS | |
| Wound infection | 4/319 | 38/637 | 0.20 | [0.07, 0.57] | <0.001 | 0.00 | Favors RAMIE | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esagian, S.M.; Ziogas, I.A.; Skarentzos, K.; Katsaros, I.; Tsoulfas, G.; Molena, D.; Karamouzis, M.V.; Rouvelas, I.; Nilsson, M.; Schizas, D. Robot-Assisted Minimally Invasive Esophagectomy versus Open Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3177. https://doi.org/10.3390/cancers14133177
Esagian SM, Ziogas IA, Skarentzos K, Katsaros I, Tsoulfas G, Molena D, Karamouzis MV, Rouvelas I, Nilsson M, Schizas D. Robot-Assisted Minimally Invasive Esophagectomy versus Open Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(13):3177. https://doi.org/10.3390/cancers14133177
Chicago/Turabian StyleEsagian, Stepan M., Ioannis A. Ziogas, Konstantinos Skarentzos, Ioannis Katsaros, Georgios Tsoulfas, Daniela Molena, Michalis V. Karamouzis, Ioannis Rouvelas, Magnus Nilsson, and Dimitrios Schizas. 2022. "Robot-Assisted Minimally Invasive Esophagectomy versus Open Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-Analysis" Cancers 14, no. 13: 3177. https://doi.org/10.3390/cancers14133177
APA StyleEsagian, S. M., Ziogas, I. A., Skarentzos, K., Katsaros, I., Tsoulfas, G., Molena, D., Karamouzis, M. V., Rouvelas, I., Nilsson, M., & Schizas, D. (2022). Robot-Assisted Minimally Invasive Esophagectomy versus Open Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-Analysis. Cancers, 14(13), 3177. https://doi.org/10.3390/cancers14133177










