Radiotherapy in the Management of Gastrointestinal Stromal Tumors: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Data Management
2.1. Protocol
2.2. Study Design
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Information Sources and Search Strategy
2.5. Study Selection
2.6. Methodological Quality Assessment of Included Studies
2.7. Data Collection Process and Data Items
2.8. Outcomes of Interest
3. Results
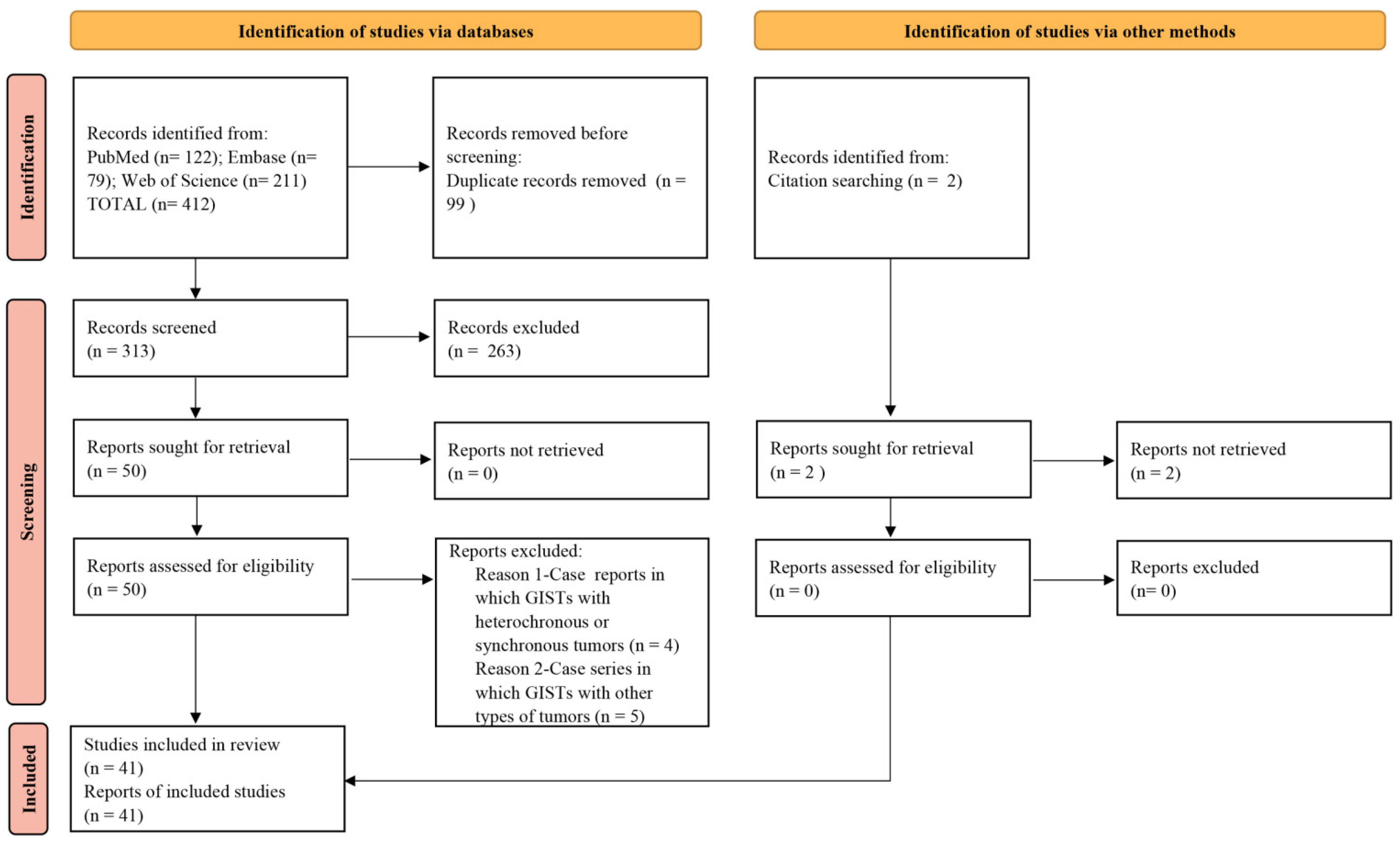
3.1. Study Selection
3.2. Patient Response to Radiation and Follow-Up
3.3. Symptom Palliation
3.4. Adverse Events
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors—Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001, 438, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Chen, X.; Zhang, B.; Cao, D. Apatinib Treatment in Metastatic Gastrointestinal Stromal Tumor. Front. Oncol. 2019, 9, 470. [Google Scholar] [CrossRef]
- Mantese, G. Gastrointestinal stromal tumor: Epidemiology, diagnosis, and treatment. Curr. Opin. Gastroenterol. 2019, 35, 555–559. [Google Scholar] [CrossRef]
- Joensuu, H. Gastrointestinal stromal tumor (GIST). Ann. Oncol. 2006, 17 (Suppl. S10), x280–x286. [Google Scholar] [CrossRef]
- Balachandran, V.P.; DeMatteo, R.P. Gastrointestinal stromal tumors: Who should get imatinib and for how long? Adv. Surg. 2014, 48, 165–183. [Google Scholar] [CrossRef]
- Cai, Z.; Yin, Y.; Shen, C.; Tang, S.; Yin, X.; Chen, Z.; Zhang, B. Role of surgical resection for patients with recurrent or metastatic gastrointestinal stromal tumors: A systematic review and meta-analysis. Int. J. Surg. 2018, 56, 108–114. [Google Scholar] [CrossRef]
- Ahmed, M. Recent advances in the management of gastrointestinal stromal tumor. World J. Clin. Cases 2020, 8, 3142–3155. [Google Scholar] [CrossRef]
- Ahmed, K.A.; Caudell, J.J.; El-Haddad, G.; Berglund, A.E.; Welsh, E.A.; Yue, B.; Hoffe, S.E.; Naghavi, A.O.; Abuodeh, Y.A.; Frakes, J.M.; et al. Radiosensitivity Differences between Liver Metastases Based on Primary Histology Suggest Implications for Clinical Outcomes after Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1399–1404. [Google Scholar] [CrossRef]
- Lolli, C.; Pantaleo, M.A.; Nannini, M.; Saponara, M.; Pallotti, M.C.; Scioscio, V.D.; Barbieri, E.; Mandrioli, A.; Biasco, G. Successful radiotherapy for local control of progressively increasing metastasis of gastrointestinal stromal tumor. Rare Tumors 2011, 3, e49. [Google Scholar] [CrossRef]
- Cuaron, J.J.; Goodman, K.A.; Lee, N.; Wu, A.J. External beam radiation therapy for locally advanced and metastatic gastrointestinal stromal tumors. Radiat. Oncol. 2013, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, C.; Chang, C.; Shen, C.; Yin, Y.; Yin, X.; Jiang, Z.; Zhao, Z.; Mu, M.; Cao, D.; et al. Comparative safety and tolerability of approved PARP inhibitors in cancer: A systematic review and network meta-analysis. Pharmacol. Res. 2021, 172, 105808. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Mu, P.F. Chapter 7: Systematic Reviews of Etiology and Risk; JBI Reviewer’s Manual; The Joanna Briggs Institute: Adelaide, Australia, 2019. [Google Scholar]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, C.A.; Brady, L.W.; Heintzelman, R.C. Radiotherapy in the treatment of gastrointestinal stromal tumor. Rare Tumors 2011, 3, e35. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Gultekin, M.; Yalcin, S.; Tuncel, M.; Gedikoglu, G.; Yildiz, F.; Cengiz, M. Stereotactic ablative radiotherapy for bone metastasis of gastrointestinal stromal tumor: Case report and review of the literature. Rep. Pract. Oncol. Radiother. 2020, 25, 331–335. [Google Scholar] [CrossRef]
- Carvalho, J.; Teixeira, M.; Silva, F.T.; Esteves, A.; Ribeiro, C.; Guerra, D. Esophageal Gastrointestinal Stromal Tumor with Rare Intracranial Metastasis. Case Rep. Gastrointest Med. 2020, 2020, 8842006. [Google Scholar] [CrossRef]
- Naoe, H.; Kaku, E.; Ido, Y.; Gushima, R.; Maki, Y.; Saito, H.; Yokote, S.; Gushima, R.; Nonaka, K.; Hoshida, Y.; et al. Brain metastasis from gastrointestinal stromal tumor: A case report and review of the literature. Case Rep. Gastroenterol. 2011, 5, 583–589. [Google Scholar] [CrossRef]
- Maria, O.M.; Alghamdi, O.; Baabdullah, R.; El-Hakim, M.; Al-Halabi, H.; Makhoul, N.M. Gastrointestinal stromal tumor with maxillary metastasis: A case report and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, e1–e5. [Google Scholar] [CrossRef]
- Andruska, N.; Mahapatra, L.; Brenneman, R.; MacArthur, K.M.; Oppelt, P.; Baumann, B.C. False-positive pregnancy test secondary to ectopic expression of human chorionic gonadotropin by a gastrointestinal stromal tumor. J. Surg. Oncol. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Gatto, L.; Nannini, M.; Saponara, M.; Di Scioscio, V.; Beltramo, G.; Frezza, G.P.; Ercolani, G.; Pinna, A.D.; Astolfi, A.; Urbini, M.; et al. Radiotherapy in the management of gist: State of the art and new potential scenarios. Clin. Sarcoma Res. 2017, 7, 1. [Google Scholar] [CrossRef]
- Boruban, C.; Sencan, O.; Akmansu, M.; Atik, E.T.; Ozbek, S. Metastatic gastrointestinal stromal tumor with long-term response after treatment with concomitant radiotherapy and imatinib mesylate. Anticancer Drugs 2007, 18, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bi, W.L.; Dunn, I.F. Metastatic Gastrointestinal Stromal Tumor to the Skull. World Neurosurg. 2016, 89, 725.e11-6. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.; Morgan, D.; Denobile, J.; Williams, J. Adjuvant radiotherapy for gastrointestinal stromal tumor of the rectum. Dig. Dis. Sci. 2001, 46, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Koike, H.; Fujita, T.; Tsujino, H.; Iwamoto, Y. Sunitinib treatment for multiple brain metastases from jejunal gastrointestinal stromal tumor: Case report. Neurol. Med. Chir. 2014, 54, 664–669. [Google Scholar] [CrossRef]
- Hamada, S.; Itami, A.; Watanabe, G.; Nakayama, S.; Tanaka, E.; Hojo, M.; Yoshizawa, A.; Hirota, S.; Sakai, Y. Intracranial metastasis from an esophageal gastrointestinal stromal tumor. Intern. Med. 2010, 49, 781–785. [Google Scholar] [CrossRef]
- Halpern, J.; Kim, Y.J.; Sultana, R.; Villani, G. Effectiveness of radiation therapy in GIST: A case report. J. Gastrointest Oncol. 2012, 3, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Di Scioscio, V.; Greco, L.; Pallotti, M.C.; Pantaleo, M.A.; Maleddu, A.; Nannini, M.; Bazzocchi, A.; Di Battista, M.; Mandrioli, A.; Lolli, C.; et al. Three cases of bone metastases in patients with gastrointestinal stromal tumors. Rare Tumors 2011, 3, e17. [Google Scholar] [CrossRef]
- Tezcan, Y.; Koç, M. Gastrointestinal stromal tumor of the rectum with bone and liver metastasis: A case study. Med. Oncol. 2011, 28 (Suppl. S1), S204–S206. [Google Scholar] [CrossRef]
- Aktan, M.; Koc, M.; Yavuz, B.B.; Kanyilmaz, G. Two cases of gastrointestinal stromal tumor of the small intestine with liver and bone metastasis. Ann. Transl. Med. 2015, 3, 259. [Google Scholar] [CrossRef]
- Katayanagi, S.; Yokoyama, T.; Makuuchi, Y.; Osakabe, H.; Iwamoto, H.; Sumi, T.; Hirano, H.; Katsumata, K.; Tsuchida, A.; Hirota, S.; et al. Long-Term Survival after Multidisciplinary Treatment Including Surgery for Metachronous Metastases of Small Intestinal Gastrointestinal Stromal Tumors after Curative Resection: A Case Report. Am. J. Case Rep. 2019, 20, 1942–1948. [Google Scholar] [CrossRef]
- Lo, Y.T.; Mak, D.S.K.; Nolan, C.P. Surgical management of vertebral metastatic gastrointestinal stromal tumor: Case illustration, literature review, and pooled analysis. Surg. Neurol. Int. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Abuzakhm, S.M.; Acre-Lara, C.E.; Zhao, W.; Hitchcock, C.; Mohamed, N.; Arbogast, D.; Shah, M.H. Unusual metastases of gastrointestinal stromal tumor and genotypic correlates: Case report and review of the literature. J. Gastrointest Oncol. 2011, 2, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Feki, J.; Bouzguenda, R.; Ayedi, L.; Bradi, M.; Boudawara, T.; Daoud, J.; Frikha, M. Bone metastases from gastrointestinal stromal tumor: A case report. Case Rep. Oncol. Med. 2012, 2012, 509845. [Google Scholar] [CrossRef] [PubMed]
- Slimack, N.P.; Liu, J.C.; Koski, T.; McClendon, J., Jr.; O’Shaughnessy, B.A. Metastatic gastrointestinal stromal tumor to the thoracic and lumbar spine: First reported case and surgical treatment. Spine J. 2012, 12, e7–e12. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Numaga, J.; Kagaya, F.; Takazawa, Y.; Suzuki, S.; Koseki, N.; Kato, S.; Kaburaki, T.; Kawashima, H. Case of optic nerve involvement in metastasis of a gastrointestinal stromal tumor. Jpn. J. Ophthalmol. 2004, 48, 166–168. [Google Scholar] [CrossRef]
- Wong, C.S.; Chu, Y.C. Intra-cranial metastasis of gastrointestinal stromal tumor. Chin. Med. J. 2011, 124, 3595–3597. [Google Scholar]
- Drazin, D.; Spitler, K.; Jeswani, S.; Shirzadi, A.; Bannykh, S.; Patil, C. Multiple intracranial metastases from a gastric gastrointestinal stromal tumor. J. Clin. Neurosci. 2013, 20, 471–473. [Google Scholar] [CrossRef]
- Sato, K.; Tanaka, T.; Kato, N.; Ishii, T.; Terao, T.; Murayama, Y. Metastatic cerebellar gastrointestinal stromal tumor with obstructive hydrocephalus arising from the small intestine: A case report and review of the literature. Case Rep. Oncol. Med. 2014, 2014, 343178. [Google Scholar] [CrossRef]
- Barrière, J.; Thariat, J.; Vandenbos, F.; Bondiau, P.Y.; Peyrottes, I.; Peyrade, F. Diplopia as the first symptom of an aggressive metastatic rectal stromal tumor. Onkologie 2009, 32, 345–347. [Google Scholar] [CrossRef]
- Badri, M.; Chabaane, M.; Gader, G.; Bahri, K.; Zammel, I. Cerebellar metastasis of gastrointestinal stromal tumor: A case report and review of the literature. Int. J. Surg. Case Rep. 2018, 42, 165–168. [Google Scholar] [CrossRef]
- Jang, S.J.; Kwon, J.H.; Han, Y. A Ruptured Metastatic Hepatic Gastrointestinal Stromal Tumor Treated by Angiographic Embolization. Korean J. Gastroenterol. 2018, 72, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Puri, T.; Gunabushanam, G.; Malik, M.; Goyal, S.; Das, A.K.; Julka, P.K.; Rath, G.K. Mesenteric gastrointestinal stromal tumour presenting as intracranial space occupying lesion. World J. Surg. Oncol. 2006, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kim, N.K.; Lee, C.H.; Lee, K.Y.; Sohn, S.K.; Cho, C.H.; Kim, H.; Pyo, H.R.; Rha, S.Y.; Chung, H.C. Gastrointestinal stromal tumor of the rectum: An analysis of seven cases. Surg. Today 2007, 37, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Bonilla, A.; Bonilla-Reyes, P.A. Somatostatin Receptor Avidity in Gastrointestinal Stromal Tumors: Theranostic Implications of Gallium-68 Scan and Eligibility for Peptide Receptor Radionuclide Therapy. Cureus 2017, 9, e1710. [Google Scholar] [CrossRef]
- Joensuu, H.; Eriksson, M.; Collan, J.; Balk, M.H.; Leyvraz, S.; Montemurro, M. Radiotherapy for GIST progressing during or after tyrosine kinase inhibitor therapy: A prospective study. Radiother. Oncol. 2015, 116, 233–238. [Google Scholar] [CrossRef]
- Omari, J.; Drewes, R.; Matthias, M.; Mohnike, K.; Seidensticker, M.; Seidensticker, R.; Streitparth, T.; Ricke, J.; Powerski, M.; Pech, M. Treatment of metastatic, imatinib refractory, gastrointestinal stroma tumor with image-guided high-dose-rate interstitial brachytherapy. Brachytherapy 2019, 18, 63–70. [Google Scholar] [CrossRef]
- Rathmann, N.; Diehl, S.J.; Dinter, D.; Schütte, J.; Pink, D.; Schoenberg, S.O.; Hohenberger, P. Radioembolization in patients with progressive gastrointestinal stromal tumor liver metastases undergoing treatment with tyrosine kinase inhibitors. J. Vasc. Interv. Radiol. 2015, 26, 231–238. [Google Scholar] [CrossRef]
- Shioyama, Y.; Yakeishi, Y.; Watanabe, T.; Nakamura, K.; Kunitake, N.; Kimura, M.; Sasaki, M.; Honda, H.; Terashima, H.; Masuda, K. Long-term control for a retroperitoneal metastasis of malignant gastrointestinal stromal tumor after chemoradiotherapy and immunotherapy. Acta Oncol. 2001, 40, 102–104. [Google Scholar] [CrossRef]
- Yang, P.C.; Guo, J.C. Radiotherapy as salvage treatment after failure of tyrosine kinase inhibitors for a patient with advanced gastrointestinal stromal tumor. J. Cancer Res. Pract. 2018, 5, 156–160. [Google Scholar] [CrossRef]
- Ciresa, M.; D’Angelillo, R.M.; Ramella, S.; Cellini, F.; Gaudino, D.; Stimato, G.; Fiore, M.; Greco, C.; Nudo, R.; Trodella, L. Molecularly targeted therapy and radiotherapy in the management of localized gastrointestinal stromal tumor (GIST) of the rectum: A case report. Tumori 2009, 95, 236–239. [Google Scholar] [CrossRef]
- Patterson, T.; Li, H.; Chai, J.; Debruyns, A.; Simmons, C.; Hart, J.; Pollock, P.; Holloway, C.L.; Truong, P.T.; Feng, X. Locoregional Treatments for Metastatic Gastrointestinal Stromal Tumor in British Columbia: A Retrospective Cohort Study from January 2008 to December 2017. Cancers 2022, 14, 1477. [Google Scholar] [CrossRef] [PubMed]
- Al-Jarani, B.; Soon Chan, W.; Dulu, A.; Nahass, T.; Pastores, S. Radiation-induced pericardial cutaneous fistula. Crit. Care Med. 2022, 50, 163. [Google Scholar] [CrossRef]
- Kelly, C.M.; Gutierrez Sainz, L.; Chi, P. The management of metastatic GIST: Current standard and investigational therapeutics. J. Hematol. Oncol. 2021, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Blay, J.Y.; Hirota, S.; Kitagawa, Y.; Kang, Y.K. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016, 19, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef]
- Cavnar, M.J.; Seier, K.; Curtin, C.; Balachandran, V.P.; Coit, D.G.; Yoon, S.S.; Crago, A.M.; Strong, V.E.; Tap, W.D.; Gönen, M.; et al. Outcome of 1000 Patients with Gastrointestinal Stromal Tumor (GIST) Treated by Surgery in the Pre- and Post-imatinib Eras. Ann. Surg. 2021, 273, 128–138. [Google Scholar] [CrossRef]
- Joensuu, H.; Eriksson, M.; Sundby Hall, K.; Hartmann, J.T.; Pink, D.; Schütte, J.; Ramadori, G.; Hohenberger, P.; Duyster, J.; Al-Batran, S.E.; et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA 2012, 307, 1265–1272. [Google Scholar] [CrossRef]
- Wu, C.E.; Tzen, C.Y.; Wang, S.Y.; Yeh, C.N. Clinical Diagnosis of Gastrointestinal Stromal Tumor (GIST): From the Molecular Genetic Point of View. Cancers 2019, 11, 679. [Google Scholar] [CrossRef]
- Grunewald, S.; Klug, L.R.; Mühlenberg, T.; Lategahn, J.; Falkenhorst, J.; Town, A.; Ehrt, C.; Wardelmann, E.; Hartmann, W.; Schildhaus, H.U.; et al. Resistance to Avapritinib in PDGFRA-Driven GIST Is Caused by Secondary Mutations in the PDGFRA Kinase Domain. Cancer Discov. 2021, 11, 108–125. [Google Scholar] [CrossRef]
- von Mehren, M.; Joensuu, H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018, 36, 136–143. [Google Scholar] [CrossRef]
- Fletcher, J.A.; Rubin, B.P. KIT mutations in GIST. Curr. Opin. Genet Dev. 2007, 17, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Saponara, M.; Pantaleo, M.A.; Nannini, M.; Biasco, G. Treatments for gastrointestinal stromal tumors that are resistant to standard therapies. Future Oncol. 2014, 10, 2045–2059. [Google Scholar] [CrossRef] [PubMed]
- Raut, C.P.; Posner, M.; Desai, J.; Morgan, J.A.; George, S.; Zahrieh, D.; Fletcher, C.D.; Demetri, G.D.; Bertagnolli, M.M. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J. Clin. Oncol. 2006, 24, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; McCall, J.; Adam, A.; O’Donnell, D.; Ashley, S.; Al-Muderis, O.; Thway, K.; Fisher, C.; Judson, I.R. Radiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcoma. Eur. J. Surg. Oncol. 2010, 36, 477–482. [Google Scholar] [CrossRef]
- Joensuu, H.; Eriksson, M.; Sundby Hall, K.; Reichardt, A.; Hermes, B.; Schütte, J.; Cameron, S.; Hohenberger, P.; Jost, P.J.; Al-Batran, S.E.; et al. Survival Outcomes Associated with 3 Years vs. 1 Year of Adjuvant Imatinib for Patients with High-Risk Gastrointestinal Stromal Tumors: An Analysis of a Randomized Clinical Trial after 10-Year Follow-Up. JAMA Oncol. 2020, 6, 1241–1246. [Google Scholar] [CrossRef]
- Casali, P.G.; Zalcberg, J.; Le Cesne, A.; Reichardt, P.; Blay, J.Y.; Lindner, L.H.; Judson, I.R.; Schöffski, P.; Leyvraz, S.; Italiano, A.; et al. Ten-Year Progression-Free and Overall Survival in Patients with Unresectable or Metastatic GI Stromal Tumors: Long-Term Analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III Randomized Trial on Imatinib at Two Dose Levels. J. Clin. Oncol. 2017, 35, 1713–1720. [Google Scholar] [CrossRef]
- Mehren, M.v.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastrointestinal Stromal Tumors (GISTs), Version 1. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf (accessed on 21 January 2022).
- Rutkowski, P.; Bylina, E.; Lugowska, I.; Teterycz, P.; Klimczak, A.; Streb, J.; Czarnecka, A.M.; Osuch, C. Treatment outcomes in older patients with advanced gastrointestinal stromal tumor (GIST). J. Geriatr. Oncol. 2018, 9, 520–525. [Google Scholar] [CrossRef]
- Shirao, K.; Nishida, T.; Doi, T.; Komatsu, Y.; Muro, K.; Li, Y.; Ueda, E.; Ohtsu, A. Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. Investig. New Drugs 2010, 28, 866–875. [Google Scholar] [CrossRef]
- Komatsu, Y.; Doi, T.; Sawaki, A.; Kanda, T.; Yamada, Y.; Kuss, I.; Demetri, G.D.; Nishida, T. Regorafenib for advanced gastrointestinal stromal tumors following imatinib and sunitinib treatment: A subgroup analysis evaluating Japanese patients in the phase III GRID trial. Int. J. Clin. Oncol. 2015, 20, 905–912. [Google Scholar] [CrossRef]
- Sym, S.J.; Ryu, M.H.; Lee, J.L.; Chang, H.M.; Kim, T.W.; Kim, H.C.; Kim, K.H.; Yook, J.H.; Kim, B.S.; Kang, Y.K. Surgical intervention following imatinib treatment in patients with advanced gastrointestinal stromal tumors (GISTs). J. Surg. Oncol. 2008, 98, 27–33. [Google Scholar] [CrossRef]
- Yeh, C.N.; Hu, C.H.; Wang, S.Y.; Wu, C.E.; Chen, J.S.; Tsai, C.Y.; Hsu, J.T.; Yeh, T.S. Cytoreductive Surgery may be beneficial for highly selected patients with Metastatic Gastrointestinal Stromal Tumors receiving Regorafenib facing Local Progression: A Case Controlled Study. J. Cancer 2021, 12, 3335–3343. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, M.; Balachandran, V.P.; Li, G.Z.; Bertagnolli, M.M.; Antonescu, C.; Tap, W.; Singer, S.; DeMatteo, R.P.; Raut, C.P. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated with Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann. Surg. 2018, 268, 296–302. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv267. [Google Scholar] [CrossRef] [PubMed]
- Pierie, J.P.; Choudry, U.; Muzikansky, A.; Yeap, B.Y.; Souba, W.W.; Ott, M.J. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch. Surg. 2001, 136, 383–389. [Google Scholar] [CrossRef]
- Crosby, J.A.; Catton, C.N.; Davis, A.; Couture, J.; O’Sullivan, B.; Kandel, R.; Swallow, C.J. Malignant gastrointestinal stromal tumors of the small intestine: A review of 50 cases from a prospective database. Ann. Surg. Oncol. 2001, 8, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wu, C.E.; Lai, C.C.; Chen, J.S.; Tsai, C.Y.; Cheng, C.T.; Yeh, T.S.; Yeh, C.N. Prospective Evaluation of Neoadjuvant Imatinib Use in Locally Advanced Gastrointestinal Stromal Tumors: Emphasis on the Optimal Duration of Neoadjuvant Imatinib Use, Safety, and Oncological Outcome. Cancers 2019, 11, 424. [Google Scholar] [CrossRef]
- Wright, C.L.; Werner, J.D.; Tran, J.M.; Gates, V.L.; Rikabi, A.A.; Shah, M.H.; Salem, R. Radiation pneumonitis following yttrium-90 radioembolization: Case report and literature review. J. Vasc. Interv. Radiol. 2012, 23, 669–674. [Google Scholar] [CrossRef]
- Tejwani, A.; Wu, S.; Jia, Y.; Agulnik, M.; Millender, L.; Lacouture, M.E. Increased risk of high-grade dermatologic toxicities with radiation plus epidermal growth factor receptor inhibitor therapy. Cancer 2009, 115, 1286–1299. [Google Scholar] [CrossRef]
- Tong, C.C.; Ko, E.C.; Sung, M.W.; Cesaretti, J.A.; Stock, R.G.; Packer, S.H.; Forsythe, K.; Genden, E.M.; Schwartz, M.; Lau, K.H.; et al. Phase II trial of concurrent sunitinib and image-guided radiotherapy for oligometastases. PLoS ONE 2012, 7, e36979. [Google Scholar] [CrossRef][Green Version]
- Kao, J.; Packer, S.; Vu, H.L.; Schwartz, M.E.; Sung, M.W.; Stock, R.G.; Lo, Y.C.; Huang, D.; Chen, S.H.; Cesaretti, J.A. Phase 1 study of concurrent sunitinib and image-guided radiotherapy followed by maintenance sunitinib for patients with oligometastases: Acute toxicity and preliminary response. Cancer 2009, 115, 3571–3580. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Lin, L.C.; Kuo, Y.C.; Liang, J.A.; Kuo, C.C.; Chiou, J.F. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.L.; Tseng, C.L.; Soliman, H.; Weiss, Y.; Sahgal, A.; Myrehaug, S. Stereotactic Body Radiotherapy (SBRT) for Oligometastatic Spine Metastases: An Overview. Front. Oncol. 2019, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- De Rose, F.; Franceschini, D.; Reggiori, G.; Stravato, A.; Navarria, P.; Ascolese, A.M.; Tomatis, S.; Mancosu, P.; Scorsetti, M. Organs at risk in lung SBRT. Phys. Med. 2017, 44, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, L.; Tseng, C.L.; Sahgal, A. Emerging technologies in stereotactic body radiotherapy. Chin. Clin. Oncol. 2017, 6, S12. [Google Scholar] [CrossRef]

| References | Age/Gender | Location | Previous TKIs | Means/Total Dose*Fractions # | Concomitant TKIs | Palliation | Response | Side Effects | Follow-Up (Recurrence or Progression/TTR or TTP (mo)/A or D/OS (mo) |
|---|---|---|---|---|---|---|---|---|---|
| Shioyama et al., 2001 [50] | 75/female | retroperitoneum | None | R + C + I/51Gy*34 | None | Yes | PR | NA | No/72/A/72 |
| Pollock et al., 2001 [25] | 77/female | rectum | None | S + R/50.4Gy*NA | None | Yes | - | desquamation of perineum/grade 2 | No/18/A/18 |
| Akiyama et al., 2004 [37] | 60/male | around the left optic nerve | None | R/54Gy*18 | None | Yes | NA | NA | NA/NA/D/4.5 |
| Puri et al., 2006 [44] | 42/male | right parietal lobe | None | S + R + C/60Gy*NA | None | Yes | - | NA | No/20/D/20 |
| Boruban et al., 2007 [23] | 55/male | pelvic | None | S(I) + R + T/54Gy*27 | imatinib | Yes | CR | NA | No/37/A/37 |
| Barrière et al., 2009 [41] | 57/male | clivus/lumbar spine | imatinib/sunitinib (R) | R + T/NA | sunitinib/nilotinib | No | NA | NA | NA/NA/D/5 |
| Ciresa et al., 2009 [52] | 54/male | rectum | None | R + T/37.8Gy*21 | imatinib | Yes | PR | neutropenia/grade 3/proctitis/grade 2 | NA/NA/NA/NA |
| Hamada et al., 2010 [27] | 54/female | left frontal lobe | imatinib(W) | S + R/NA | None | NA | - | NA | No/6/A/6 |
| Tezcan et al., 2011 [30] | 83/male | right femur head | None | R + T/30Gy*10 | imatinib | Yes | NA | NA | NA/NA/A/NA |
| Knowlton et al., 2011 [16] | 37/male | stomach | None | S + R/36Gy*24 | None | Yes | - | No | No/240/D/240 |
| Naoe et al., 2011 [19] | 77/female | right cerebral peduncle/left occipital lobe | None | R + T/NA/S + R + T/NA | Imatinib (I) | NA | NA/- | NA | No/2/D/2 |
| Lolli et al., 2011 [10] | 48/female | left supraclavicular | imatinib/sunitinib/nilotinib/sorafenib (R) | R + T/50Gy*25 | sorafenib | Yes | SD | well tolerated | No/NA/A/NA |
| Di Scioscio et al., 2011 [29] | 62/male | spine | None | R + T/30Gy*NA | imatinib | Yes | NA | NA | Yes/24/D/34 |
| Abuzakhm et al., 2011 [34] | 57/female | left humerus | imatinib/sunitinib (R) | R + T/NA | sunitinib | NA | NA | NA | NA/NA/D/2 |
| Wong et al., 2011 [38] | 26/male | left frontal temporal | imatinib/sunitinib (R) | S + R/NA | None | NA | - | NA | No/4/A/4 |
| Slimack et al., 2012 [36] | 37/male | spine | imatinib (R) | S + R + C/NA | None | Yes | - | NA | No/24/A//24 |
| Halpern et al., 2012 [28] | 62/male | right upper quadrant/retroperitoneum | imatinib (I) | R/63.4Gy*NA | None | Yes | PR | well tolerated | No/3/A/3 |
| Feki et al., 2012 [35] | 58/male | sternoclavicular joint | None | R + T/30Gy*NA | imatinib | Yes | PR | NA | No/10/A/19 |
| Drazin et al., 2013 [39] | 60/male | left frontal lobe/left cerebellum | None | R/18Gy*1/S + R/NA | None | Yes | NA/- | NA | No/15/A/15 |
| Takeuchi et al., 2014 [26] | 74/male | right lateral ventricle | imatinib/sunitinib (R) | R + T/NA | sunitinib | - | CR | NA | No/4/A/4 |
| Sato et al., 2014 [40] | 80/male | vermis | None | S + R/22Gy*11 | None | Yes | - | NA | Yes/1/D/3 |
| Aktan et al., 2015 [31] | 56/male | right femur/L1–3 vertebrae | Imatinib (R) | R + T/30Gy*10 | imatinib | Yes | NA | NA | NA/NA/D/2 |
| 70/male | L2 vertebra | Imatinib (R) | R + T/30Gy*NA | imatinib | Yes | NA | NA | NA/NA/D/1.5 | |
| Gupta et al., 2016 [24] | 64/female | right frontal skull | Imatinib (R) | S + R + T/35Gy*14 | imatinib/sunitinib | Yes | - | NA | Yes/21/A/24 |
| Gatto et al., 2017 [22] | 62/male | paracaval lesion | imatinib/sunitinib (R) | R + T/35Gy*14 | regorafenib | Yes | PR | No | No/36/A/36 |
| 44/male | pararenal/supraclavicular | imatinib/sunitinib (R) | R/85Gy*9/R + T/32Gy*5 | sunitinib | Yes | SD | nausea/NA | No/5/A/5 | |
| Loaiza-Bonilla et al., 2017 [46] | 35/male | liver/right retropharyngeal | imatinib (R) | R + T/NA | regorafenib | - | SD | NA | NA/NA/A/3 |
| Badri et al., 2018 [42] | 66/male | right cerebellum | None | S + R/NA | NA | NA | - | NA | No/12/A/12 |
| Jang et al., 2018 [43] | 70/male | liver | Imatinib (R) | E + R + T/40Gy*16 | Imatinib | Yes | NA | NA | No/6/A/6 |
| Yang et al., 2018 [51] | 74/male | duodenal bulb | imatinib/sunitinib (R) | R/32.5Gy*13 | None | Yes | PR | NA | Yes/9/D/16 |
| Katayanagi et al., 2019 [32] | 56/male | T8 vertebra/right ilium | imatinib/sunitinib (R) | R + T/37.5Gy*15 | sunitinib/imatinib | NA | NA | NA | NA/NA/D/19 |
| Yilmaz et al., 2020 [17] | 31/male | right iliac bone | imatinib (R) | R + T/24Gy*3 | sunitinib | Yes | CR | No | No/16/A/16 |
| Carvalho et al., 2020 [18] | 76/female | left frontal lobe/right cerebellar | imatinib (R) | S + R + T/NA/R + T/NA | imatinib | No | -/NA | NA | NA/NA/D/6 |
| Andruska et al., 2020 [21] | 29/female | caudate lobe of liver | imatinib/sunitinib/sorafenib/regorafenib (R) | R + T/30Gy*10 | regorafenib/sunitinib | - | NA | NA | NA/NA/D/NA |
| Lo et al., 2020 [33] | 63/male | T9 vertebra | imatinib/sunitinib/regorafenib/dasatinib (R) | S + R/30Gy*10 | None | NA | - | NA | NA/NA/D/2 |
| Maria et al., 2022 [20] | 77/male | left maxillary | imatinib/2 additional lines (R) | R/35Gy*10 | None | Yes | PR | mucositis/grade2/dermatitis/grade1 | NA/NA/D/8 |
| Al-Jarani et al., 2022 [54] | 52/female | liver/xiphoid | NA | NA | NA | - | NA | change in skin, dermatitis, sclerosis, fistula/NA | NA/NA/A/48 |
| References | Sex, Total No. (Male/Female) | Age, Median (Range),y | Sites | Previous TKIs, Patients No. | Means/ Dose Range | Concomitant TKIs, Patients No. | Symptom Palliation, Patients No. | Response | Follow-Up, Range (mo)/Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Baik et al., 2007 [45] | 4 (1/3) | 53 (41–68) | Rectum | None | R/45–54 Gy | None | NA | - | 21–75/No recurrence and all alive |
| Cuaron et al., 2013 [11] | 15 (8/7) | 68 (41–86) | Bone/Abdomen/Pelvis, | 11 | R/15–50 Gy | 5 | 12 | PR in 5 patients, SD in 9 | 1.4–28.3/12 deaths |
| Joensuu et al., 2015 [47] | 25 (17/8) | 61.4 (19.7–86.5) | Abdomen | 25 | R/30–40 Gy | 19 | NA | PR in 2 patients, SD in 20 | 2–74/20 patients progressed and 18 deaths |
| Rathmann et al., 2015 [49] | 9 (7/2) | 55 (34–74) | Liver | 9 | RE/0.55–1.88 Gbq | 9 | NA | CR in 3 patients, PR in 5, SD in 1 | 10–72/8 progressed and 4 deaths |
| Omari et al., 2019 [48] | 10 (9/1) | 58.5 (37–68) | Liver/Peritoneum | 10 | iBT/6.7–22.0 Gy | 7 | NA | LTC 97.5% | 2.3–92.9/one relapse and 6 deaths |
| Patterson et al., 2022 [53] | 12 (7/5) | 69 (36–79) | NA | NA | R/20-50 Gy | 12 | 9 | SD in 1 patient, PD in 1 | NA |
| Response | R | R + nT | R + rT | R + sT |
|---|---|---|---|---|
| CR | 0 | 1 | 1 | 1 |
| PR | 6 | 1 | 4 | 2 |
| SD | 6 | 3 | 5 | 0 |
| PD | 2 | 0 | 0 | 0 |
| NA | 5 | 1 | 12 | 3 |
| N | 19 | 6 | 22 | 6 |
| Response | Brain | Neck | Chest | Abdomen | Pelvis | Bone and Joint | N |
|---|---|---|---|---|---|---|---|
| CR | 1 | 1 | 1 | 3 | |||
| PR | 1 | 6 | 1 | 5 | 13 | ||
| SD | 3 | 1 | 5 | 1 | 4 | 14 | |
| PD | 2 | 2 | |||||
| NA | 4 | 3 | 14 | 21 | |||
| N | 5 | 3 | 2 | 14 | 3 | 26 | 53 |
| Continued TKIs | Resistant to 0 TKIs | Resistant to 1 TKI | Resistant to 2 TKIs | Resistant to ≥3TKIs |
|---|---|---|---|---|
| None | 4 | 1 | 1 | |
| rTKI | - | 4 | 4 | |
| nTKI | - | 2 | 2 | |
| sTKI | 4 | - | - | - |
| NA | ||||
| N | 8 | 6 | 7 | 1 |
| Continued TKIs | Resistant to 0 TKIs | Resistant to 1 TKI | Resistant to 2 TKIs | Resistant to ≥3 TKIs |
|---|---|---|---|---|
| None | 6 | 1 | 1 | 1 |
| rTKI | - | 2 | ||
| nTKI | - | |||
| sTKI | 1 | - | - | - |
| NA | 1 | |||
| N | 8 | 3 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Jiang, T.; Mu, M.; Zhao, Z.; Yin, X.; Cai, Z.; Zhang, B.; Yin, Y. Radiotherapy in the Management of Gastrointestinal Stromal Tumors: A Systematic Review. Cancers 2022, 14, 3169. https://doi.org/10.3390/cancers14133169
Zhang H, Jiang T, Mu M, Zhao Z, Yin X, Cai Z, Zhang B, Yin Y. Radiotherapy in the Management of Gastrointestinal Stromal Tumors: A Systematic Review. Cancers. 2022; 14(13):3169. https://doi.org/10.3390/cancers14133169
Chicago/Turabian StyleZhang, Haidong, Tianxiang Jiang, Mingchun Mu, Zhou Zhao, Xiaonan Yin, Zhaolun Cai, Bo Zhang, and Yuan Yin. 2022. "Radiotherapy in the Management of Gastrointestinal Stromal Tumors: A Systematic Review" Cancers 14, no. 13: 3169. https://doi.org/10.3390/cancers14133169
APA StyleZhang, H., Jiang, T., Mu, M., Zhao, Z., Yin, X., Cai, Z., Zhang, B., & Yin, Y. (2022). Radiotherapy in the Management of Gastrointestinal Stromal Tumors: A Systematic Review. Cancers, 14(13), 3169. https://doi.org/10.3390/cancers14133169






