Virtual Reality Rehabilitation Systems for Cancer Survivors: A Narrative Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
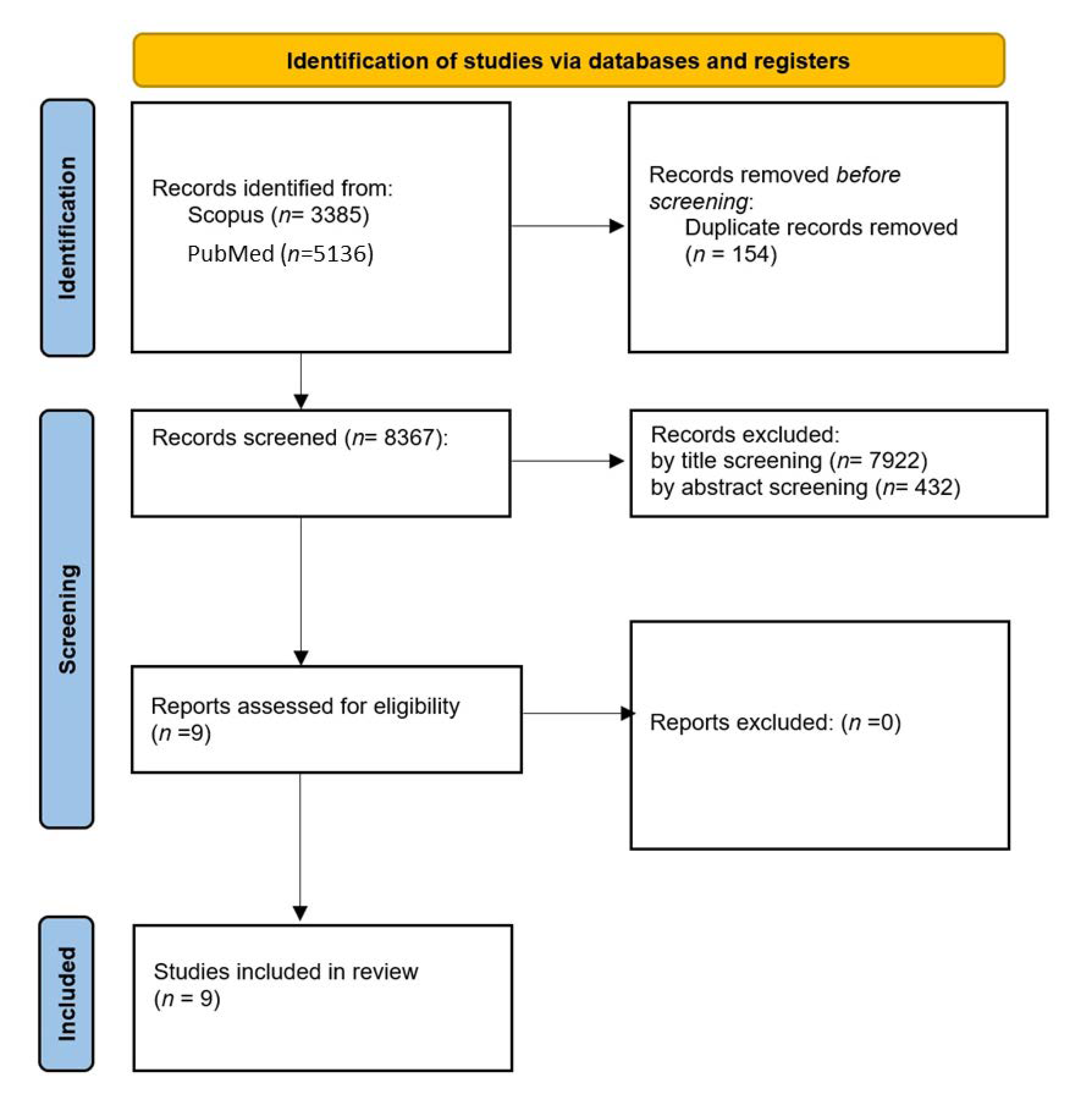
2. Methods
Database Search
3. Results
3.1. Pain
3.2. Fatigue
3.3. Lymphedema
3.4. Cognitive Impairment
3.5. Motor Performance
3.6. Chemotherapy-Induced Peripheral Neuropathy
3.7. Adherence to Rehabilitation Programs
4. Discussion
“With my lack of mobility that’s resulted from my illness, I really enjoyed the VR as it made me feel like I’m not house bound…”
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Boffetta, P.; La Vecchia, C.; Rota, M.; Bertuccio, P.; Malvezzi, M.; Negri, E. The Global Decrease in Cancer Mortality: Trends and Disparities. Ann. Oncol. 2016, 27, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019–2021; American Cancer Society: New York, NY, USA, 2019. [Google Scholar]
- Shapiro, C.L. Cancer Survivorship. N. Engl. J. Med. 2018, 379, 2438–2450. [Google Scholar] [CrossRef]
- Van den Beuken-van Everdingen, M.H.J.; Hochstenbach, L.M.J.; Joosten, E.A.J.; Tjan-Heijnen, V.C.G.; Janssen, D.J.A. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090.e9. [Google Scholar] [CrossRef]
- Bernier Carney, K.; Starkweather, A.; Lucas, R.; Ersig, A.L.; Guite, J.W.; Young, E. Deconstructing Pain Disability through Concept Analysis. Pain Manag. Nurs. 2019, 20, 482–488. [Google Scholar] [CrossRef]
- Bernas, M.; Thiadens, S.R.J.; Smoot, B.; Armer, J.M.; Stewart, P.; Granzow, J. Lymphedema Following Cancer Therapy: Overview and Options. Clin. Exp. Metastasis 2018, 35, 547–551. [Google Scholar] [CrossRef]
- Bernas, M.; Thiadens, S.R.J.; Stewart, P.; Granzow, J. Secondary Lymphedema from Cancer Therapy. Clin. Exp. Metastasis 2021, 39, 239–247. [Google Scholar] [CrossRef]
- Shaitelman, S.F.; Cromwell, K.D.; Rasmussen, J.C.; Stout, N.L.; Armer, J.M.; Lasinski, B.B.; Cormier, J.N. Recent Progress in the Treatment and Prevention of Cancer-Related Lymphedema: Lymphedema Treatment and Prevention. CA Cancer J. Clin. 2015, 65, 55–81. [Google Scholar] [CrossRef]
- Eide, S.; Feng, Z.-P. Doxorubicin Chemotherapy-Induced “Chemo-Brain”: Meta-Analysis. Eur. J. Pharmacol. 2020, 881, 173078. [Google Scholar] [CrossRef] [PubMed]
- Hermelink, K. Chemotherapy and Cognitive Function in Breast Cancer Patients: The So-Called Chemo Brain. JNCI Monogr. 2015, 2015, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-Y.; Mi, W.-L.; Wu, G.-C.; Wang, Y.-Q.; Mao-Ying, Q.-L. Prevention and Treatment for Chemotherapy-Induced Peripheral Neuropathy: Therapies Based on CIPN Mechanisms. Curr. Neuropharmacol. 2019, 17, 184–196. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.K.; Baima, J.; Mayer, R.S. Impairment-Driven Cancer Rehabilitation: An Essential Component of Quality Care and Survivorship: Impairment-Driven Cancer Rehabilitation. CA Cancer J. Clin. 2013, 63, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.R.; Steed, L.; Quirk, H.; Greasley, R.U.; Saxton, J.M.; Taylor, S.J.; Rosario, D.J.; Thaha, M.A.; Bourke, L. Interventions for Promoting Habitual Exercise in People Living with and beyond Cancer. Cochrane Database Syst. Rev. 2018, 9, CD010192. [Google Scholar] [CrossRef]
- Mitchell, L.J.; Bisdounis, L.; Ballesio, A.; Omlin, X.; Kyle, S.D. The Impact of Cognitive Behavioural Therapy for Insomnia on Objective Sleep Parameters: A Meta-Analysis and Systematic Review. Sleep Med. Rev. 2019, 47, 90–102. [Google Scholar] [CrossRef]
- Dimeo, F.C. Effects of Exercise on Cancer-Related Fatigue. Cancer 2001, 92, 1689–1693. [Google Scholar] [CrossRef]
- Cheville, A.L.; Mustian, K.; Winters-Stone, K.; Zucker, D.S.; Gamble, G.L.; Alfano, C.M. Cancer Rehabilitation. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 1–17. [Google Scholar] [CrossRef]
- Hansen, D.G.; Larsen, P.V.; Holm, L.V.; Rottmann, N.; Bergholdt, S.H.; Søndergaard, J. Association between Unmet Needs and Quality of Life of Cancer Patients: A Population-Based Study. Acta Oncol. 2013, 52, 391–399. [Google Scholar] [CrossRef]
- Stensvold, E.; Stadskleiv, K.; Myklebust, T.Å.; Wesenberg, F.; Helseth, E.; Bechensteen, A.G.; Brandal, P. Unmet Rehabilitation Needs in 86% of Norwegian Paediatric Embryonal Brain Tumour Survivors. Acta Paediatr. 2020, 109, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Cheville, A.L.; Kornblith, A.B.; Basford, J.R. An Examination of the Causes for the Underutilization of Rehabilitation Services Among People with Advanced Cancer. Am. J. Phys. Med. Rehabil. 2011, 90, S27–S37. [Google Scholar] [CrossRef] [PubMed]
- Pudkasam, S.; Polman, R.; Pitcher, M.; Fisher, M.; Chinlumprasert, N.; Stojanovska, L.; Apostolopoulos, V. Physical Activity and Breast Cancer Survivors: Importance of Adherence, Motivational Interviewing and Psychological Health. Maturitas 2018, 116, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Nuara, A.; Fabbri-Destro, M.; Scalona, E.; Lenzi, S.E.; Rizzolatti, G.; Avanzini, P. Telerehabilitation in Response to Constrained Physical Distance: An Opportunity to Rethink Neurorehabilitative Routines. J. Neurol. 2021, 269, 627–638. [Google Scholar] [CrossRef]
- Meyding-Lamadé, U.; Bassa, B.; Tibitanzl, P.; Davtyan, A.; Lamadé, E.K.; Craemer, E.M. Telerehabilitation: Von der virtuellen Welt zur Realität—Medizin im 21. Jahrhundert: Videogestützte Therapie in Zeiten von COVID-19. Nervenarzt 2021, 92, 127–136. [Google Scholar] [CrossRef]
- Lambert, G.; Alos, N.; Bernier, P.; Laverdière, C.; Drummond, K.; Dahan-Oliel, N.; Lemay, M.; Veilleux, L.-N.; Kairy, D. Patient and Parent Experiences with Group Telerehabilitation for Child Survivors of Acute Lymphoblastic Leukemia. Int. J. Environ. Res. Public Health 2021, 18, 3610. [Google Scholar] [CrossRef]
- Galiano-Castillo, N.; Cantarero-Villanueva, I.; Fernández-Lao, C.; Ariza-García, A.; Díaz-Rodríguez, L.; Del-Moral-Ávila, R.; Arroyo-Morales, M. Telehealth System: A Randomized Controlled Trial Evaluating the Impact of an Internet-Based Exercise Intervention on Quality of Life, Pain, Muscle Strength, and Fatigue in Breast Cancer Survivors: Telehealth System in Breast Cancer. Cancer 2016, 122, 3166–3174. [Google Scholar] [CrossRef]
- Dennett, A.; Harding, K.E.; Reimert, J.; Morris, R.; Parente, P.; Taylor, N.F. Telerehabilitation Was Safe, Feasible and Increased Exercise Uptake in Cancer Survivors: A Process Evaluation (Preprint). JMIR Cancer 2021, 7, e33130. [Google Scholar] [CrossRef]
- Navarra-Ventura, G.; Gomà, G.; de Haro, C.; Jodar, M.; Sarlabous, L.; Hernando, D.; Bailón, R.; Ochagavía, A.; Blanch, L.; López-Aguilar, J.; et al. Virtual Reality-Based Early Neurocognitive Stimulation in Critically Ill Patients: A Pilot Randomized Clinical Trial. J. Pers. Med. 2021, 11, 1260. [Google Scholar] [CrossRef]
- Villiger, M.; Liviero, J.; Awai, L.; Stoop, R.; Pyk, P.; Clijsen, R.; Curt, A.; Eng, K.; Bolliger, M. Home-Based Virtual Reality-Augmented Training Improves Lower Limb Muscle Strength, Balance, and Functional Mobility Following Chronic Incomplete Spinal Cord Injury. Front. Neurol. 2017, 8, 635. [Google Scholar] [CrossRef]
- Gandolfi, M.; Geroin, C.; Dimitrova, E.; Boldrini, P.; Waldner, A.; Bonadiman, S.; Picelli, A.; Regazzo, S.; Stirbu, E.; Primon, D.; et al. Virtual Reality Telerehabilitation for Postural Instability in Parkinson’s Disease: A Multicenter, Single-Blind, Randomized, Controlled Trial. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lloréns, R.; Noé, E.; Colomer, C.; Alcañiz, M. Effectiveness, Usability, and Cost-Benefit of a Virtual Reality–Based Telerehabilitation Program for Balance Recovery After Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2015, 96, 418–425.e2. [Google Scholar] [CrossRef] [PubMed]
- Berton, A.; Longo, U.G.; Candela, V.; Fioravanti, S.; Giannone, L.; Arcangeli, V.; Alciati, V.; Berton, C.; Facchinetti, G.; Marchetti, A.; et al. Virtual Reality, Augmented Reality, Gamification, and Telerehabilitation: Psychological Impact on Orthopedic Patients’ Rehabilitation. J. Clin. Med. 2020, 9, 2567. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; George, S.; Thomas, S.; Deutsch, J.E.; Crotty, M. Virtual Reality for Stroke Rehabilitation. Cochrane Database Syst. Rev. 2015, 2, CD008349. [Google Scholar] [CrossRef]
- Chi, B.; Chau, B.; Yeo, E.; Ta, P. Virtual Reality for Spinal Cord Injury-Associated Neuropathic Pain: Systematic Review. Ann. Phys. Rehabil. Med. 2019, 62, 49–57. [Google Scholar] [CrossRef]
- Maggio, M.G.; Russo, M.; Cuzzola, M.F.; Destro, M.; La Rosa, G.; Molonia, F.; Bramanti, P.; Lombardo, G.; De Luca, R.; Calabrò, R.S. Virtual Reality in Multiple Sclerosis Rehabilitation: A Review on Cognitive and Motor Outcomes. J. Clin. Neurosci. 2019, 65, 106–111. [Google Scholar] [CrossRef]
- Amirthalingam, J.; Paidi, G.; Alshowaikh, K.; Iroshani Jayarathna, A.; Salibindla, D.B.A.M.R.; Karpinska-Leydier, K.; Ergin, H.E. Virtual Reality Intervention to Help Improve Motor Function in Patients Undergoing Rehabilitation for Cerebral Palsy, Parkinson’s Disease, or Stroke: A Systematic Review of Randomized Controlled Trials. Cureus 2021, 13, e16763. [Google Scholar] [CrossRef]
- Ravi, D.K.; Kumar, N.; Singhi, P. Effectiveness of Virtual Reality Rehabilitation for Children and Adolescents with Cerebral Palsy: An Updated Evidence-Based Systematic Review. Physiotherapy 2017, 103, 245–258. [Google Scholar] [CrossRef]
- Gagliardi, C.; Turconi, A.C.; Biffi, E.; Maghini, C.; Marelli, A.; Cesareo, A.; Diella, E.; Panzeri, D. Immersive Virtual Reality to Improve Walking Abilities in Cerebral Palsy: A Pilot Study. Ann. Biomed. Eng. 2018, 46, 1376–1384. [Google Scholar] [CrossRef]
- Rose, T.; Nam, C.S.; Chen, K.B. Immersion of Virtual Reality for Rehabilitation—Review. Appl. Ergon. 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Perez-Marcos, D. Virtual Reality Experiences, Embodiment, Videogames and Their Dimensions in Neurorehabilitation. J. Neuroeng. Rehabil. 2018, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Oesch, P.; Kool, J.; Fernandez-Luque, L.; Brox, E.; Evertsen, G.; Civit, A.; Hilfiker, R.; Bachmann, S. Exergames versus Self-Regulated Exercises with Instruction Leaflets to Improve Adherence during Geriatric Rehabilitation: A Randomized Controlled Trial. BMC Geriatr. 2017, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, A.; Samad-Soltani, T.; Salahzadeh, Z.; Rezaei-Hachesu, P. Effectiveness of Virtual Reality-Based Exercise Therapy in Rehabilitation: A Scoping Review. Inform. Med. Unlocked 2021, 24, 100562. [Google Scholar] [CrossRef]
- Chirico, A.; Lucidi, F.; De Laurentiis, M.; Milanese, C.; Napoli, A.; Giordano, A. Virtual Reality in Health System: Beyond Entertainment. A Mini-Review on the Efficacy of VR During Cancer Treatment: Efficacy of vr during cancer treatment. J. Cell. Physiol. 2016, 231, 275–287. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, J.-E.; Cheng, A.S.K.; Cheng, H.; Wefel, J.S. Meta-Analysis of the Efficacy of Virtual Reality–Based Interventions in Cancer-Related Symptom Management. Integr. Cancer Ther. 2019, 18, 1534735419871108. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A Comparison Study of Specificity and Sensitivity in Three Search Tools for Qualitative Systematic Reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Atef, D.; Elkeblawy, M.M.; El-Sebaie, A.; Abouelnaga, W.A.I. A Quasi-Randomized Clinical Trial: Virtual Reality versus Proprioceptive Neuromuscular Facilitation for Postmastectomy Lymphedema. J. Egypt. Natl. Cancer Inst. 2020, 32, 29. [Google Scholar] [CrossRef]
- Axenie, C.; Kurz, D. Role of Kinematics Assessment and Multimodal Sensorimotor Training for Motion Deficits in Breast Cancer Chemotherapy-Induced Polyneuropathy: A Perspective on Virtual Reality Avatars. Front. Oncol. 2020, 10, 1419. [Google Scholar] [CrossRef]
- Basha, M.A.; Aboelnour, N.H.; Alsharidah, A.S.; Kamel, F.H. Effect of Exercise Mode on Physical Function and Quality of Life in Breast Cancer–Related Lymphedema: A Randomized Trial. Support. Care Cancer 2021, 3, 2101–2110. [Google Scholar] [CrossRef]
- Feyzioğlu, Ö.; Dinçer, S.; Akan, A.; Algun, Z.C. Is Xbox 360 Kinect-Based Virtual Reality Training as Effective as Standard Physiotherapy in Patients Undergoing Breast Cancer Surgery? Support. Care Cancer 2020, 28, 4295–4303. [Google Scholar] [CrossRef]
- Hoffman, A.J.; Brintnall, R.A.; Brown, J.K.; von Eye, A.; Jones, L.W.; Alderink, G.; Ritz-Holland, D.; Enter, M.; Patzelt, L.H.; VanOtteren, G.M. Virtual Reality Bringing a New Reality to Postthoracotomy Lung Cancer Patients Via a Home-Based Exercise Intervention Targeting Fatigue While Undergoing Adjuvant Treatment. Cancer Nurs. 2014, 37, 23–33. [Google Scholar] [CrossRef] [PubMed]
- House, G.; Burdea, G.; Grampurohit, N.; Polistico, K.; Roll, D.; Damiani, F.; Hundal, J.; Demesmin, D. A Feasibility Study to Determine the Benefits of Upper Extremity Virtual Rehabilitation Therapy for Coping with Chronic Pain Post-Cancer Surgery. Br. J. Pain 2016, 10, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.M.; Cavadino, A.; Chin, S.; Little, Z.; Akroyd, A.; Tennant, G.; Dobson, R.; Broom, R.; Gautier, A. The Benefits and Acceptability of Virtual Reality Interventions for Women with Metastatic Breast Cancer in Their Homes; a Pilot Randomised Trial. BMC Cancer 2022, 22, 360. [Google Scholar] [CrossRef]
- Schwenk, M.; Grewal, G.S.; Holloway, D.; Muchna, A.; Garland, L.; Najafi, B. Interactive Sensor-Based Balance Training in Older Cancer Patients with Chemotherapy-Induced Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2016, 62, 553–563. [Google Scholar] [CrossRef]
- Tsuda, K.; Sudo, K.; Goto, G.; Takai, M.; Itokawa, T.; Isshiki, T.; Takei, N.; Tanimoto, T.; Komatsu, T. A Feasibility Study of Virtual Reality Exercise in Elderly Patients with Hematologic Malignancies Receiving Chemotherapy. Intern. Med. 2016, 55, 347–352. [Google Scholar] [CrossRef]
- Goudman, L.; Jansen, J.; Billot, M.; Vets, N.; De Smedt, A.; Roulaud, M.; Rigoard, P.; Moens, M. Virtual Reality Applications in Chronic Pain Management: Systematic Review and Meta-Analysis. JMIR Serious Games 2022, 10, e34402. [Google Scholar] [CrossRef] [PubMed]
- Alemanno, F.; Houdayer, E.; Emedoli, D.; Locatelli, M.; Mortini, P.; Mandelli, C.; Raggi, A.; Iannaccone, S. Efficacy of Virtual Reality to Reduce Chronic Low Back Pain: Proof-of-Concept of a Non-Pharmacological Approach on Pain, Quality of Life, Neuropsychological and Functional Outcome. PLoS ONE 2019, 14, e0216858. [Google Scholar] [CrossRef] [PubMed]
- Brea-Gómez, B.; Torres-Sánchez, I.; Ortiz-Rubio, A.; Calvache-Mateo, A.; Cabrera-Martos, I.; López-López, L.; Valenza, M.C. Virtual Reality in the Treatment of Adults with Chronic Low Back Pain: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 11806. [Google Scholar] [CrossRef]
- Cortés-Pérez, I.; Sánchez-Alcalá, M.; Nieto-Escámez, F.A.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis. Sensors 2021, 21, 7389. [Google Scholar] [CrossRef]
- Ioannou, A.; Papastavrou, E.; Avraamides, M.N.; Charalambous, A. Virtual Reality and Symptoms Management of Anxiety, Depression, Fatigue, and Pain: A Systematic Review. SAGE Open Nurs. 2020, 6, 2377960820936163. [Google Scholar] [CrossRef]
- Coons, M.J.; Roehrig, M.; Spring, B. The Potential of Virtual Reality Technologies to Improve Adherence to Weight Loss Behaviors. J. Diabetes Sci. Technol. 2011, 5, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.S.; Sayeed, R.; Schwenk, M.; Bharara, M.; Menzies, R.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Balance Rehabilitation: Promoting the Role of Virtual Reality in Patients with Diabetic Peripheral Neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Iruthayarajah, J.; McIntyre, A.; Cotoi, A.; Macaluso, S.; Teasell, R. The Use of Virtual Reality for Balance among Individuals with Chronic Stroke: A Systematic Review and Meta-Analysis. Top. Stroke Rehabil. 2017, 24, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Donath, L.; Rössler, R.; Faude, O. Effects of Virtual Reality Training (Exergaming) Compared to Alternative Exercise Training and Passive Control on Standing Balance and Functional Mobility in Healthy Community-Dwelling Seniors: A Meta-Analytical Review. Sports Med. 2016, 46, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Phu, S.; Vogrin, S.; Al Saedi, A.; Duque, G. Balance Training Using Virtual Reality Improves Balance and Physical Performance in Older Adults at High Risk of Falls. Clin. Interv. Aging 2019, 14, 1567–1577. [Google Scholar] [CrossRef]
- Cho, H.; Sohng, K.-Y. The Effect of a Virtual Reality Exercise Program on Physical Fitness, Body Composition, and Fatigue in Hemodialysis Patients. J. Phys. Ther. Sci. 2014, 26, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual Reality in Cognitive and Motor Rehabilitation: Facts, Fiction and Fallacies. Expert Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef]
- Faria, A.L.; Andrade, A.; Soares, L.; Badia, S.B.I. Benefits of Virtual Reality Based Cognitive Rehabilitation through Simulated Activities of Daily Living: A Randomized Controlled Trial with Stroke Patients. J. Neuroeng. Rehabil. 2016, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-N. Combined Effects of Virtual Reality and Computer Game-Based Cognitive Therapy on the Development of Visual-Motor Integration in Children with Intellectual Disabilities: A Pilot Study. Occup. Ther. Int. 2021, 2021, 6696779. [Google Scholar] [CrossRef]
- Aminov, A.; Rogers, J.M.; Middleton, S.; Caeyenberghs, K.; Wilson, P.H. What Do Randomized Controlled Trials Say about Virtual Rehabilitation in Stroke? A Systematic Literature Review and Meta-Analysis of Upper-Limb and Cognitive Outcomes. J. Neuroeng. Rehabil. 2018, 15, 29. [Google Scholar] [CrossRef]
- Carelli, L.; Morganti, F.; Poletti, B.; Corra, B.; Weiss, P.L.T.; Kizony, R.; Silani, V.; Riva, G. A NeuroVR Based Tool for Cognitive Assessment and Rehabilitation of Post-Stroke Patients: Two Case Studies. Stud. Health Technol. Inform. 2009, 144, 243–247. [Google Scholar] [PubMed]
- Sleight, A.G.; Lyons, K.D.; Vigen, C.; Macdonald, H.; Clark, F. The Association of Health-Related Quality of Life with Unmet Supportive Care Needs and Sociodemographic Factors in Low-Income Latina Breast Cancer Survivors: A Single-Centre Pilot Study. Disabil. Rehabil. 2019, 41, 3151–3156. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; González, P.; López-Jaquero, V.; Montero, F.; Molina, J.P.; Romero-Ayuso, D. Adaptive, Multisensorial, Physiological and Social: The Next Generation of Telerehabilitation Systems. Front. Neuroinform. 2018, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.; Staal, J.B.; van Goor, H. Could Virtual Reality Play a Role in the Rehabilitation after COVID-19 Infection? BMJ Open Sport Exerc. Med. 2020, 6, e000943. [Google Scholar] [CrossRef]
- Annesi, J.J.; Mazas, J. Effects of Virtual Reality-Enhanced Exercise Equipment on Adherence and Exercise-Induced Feeling States. Percept. Mot. Skills 1997, 85, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.C. A Meta-Analysis and Systematic Literature Review of Virtual Reality Rehabilitation Programs. Comput. Hum. Behav. 2017, 70, 317–327. [Google Scholar] [CrossRef]
- Edwards, E.A.; Lumsden, J.; Rivas, C.; Steed, L.; Edwards, L.A.; Thiyagarajan, A.; Sohanpal, R.; Caton, H.; Griffiths, C.J.; Munafò, M.R.; et al. Gamification for Health Promotion: Systematic Review of Behaviour Change Techniques in Smartphone Apps. BMJ Open 2016, 6, e012447. [Google Scholar] [CrossRef]
- Johnson, D.; Deterding, S.; Kuhn, K.-A.; Staneva, A.; Stoyanov, S.; Hides, L. Gamification for Health and Wellbeing: A Systematic Review of the Literature. Internet Interv. 2016, 6, 89–106. [Google Scholar] [CrossRef]
- Mitchell, R.; Schuster, L.; Jin, H.S. Playing Alone: Can Game Design Elements Satisfy User Needs in Gamified MHealth Services? Health Promot. Int. 2021, 37, daab168. [Google Scholar] [CrossRef]
- Goršič, M.; Cikajlo, I.; Novak, D. Competitive and Cooperative Arm Rehabilitation Games Played by a Patient and Unimpaired Person: Effects on Motivation and Exercise Intensity. J. Neuroeng. Rehabil. 2017, 14, 23. [Google Scholar] [CrossRef]
- Vang, M.H.; Fox, J. Race in Virtual Environments: Competitive versus Cooperative Games with Black or White Avatars. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 235–240. [Google Scholar] [CrossRef]
- Navarro, M.D.; Llorens, R.; Borrego, A.; Alcañiz, M.; Noé, E.; Ferri, J. Competition Enhances the Effectiveness and Motivation of Attention Rehabilitation After Stroke. A Randomized Controlled Trial. Front. Hum. Neurosci. 2020, 14, 575403. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-S.; Son, K.H.; Kim, H.J. Effects of Virtual Reality Training Using Nintendo Wii and Treadmill Walking Exercise on Balance and Walking for Stroke Patients. J. Phys. Ther. Sci. 2016, 28, 3112–3115. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, E.M.J.; Mirelman, A.; Alcock, L.; Rochester, L.; Nieuwhof, F.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; Cereatti, A.; Della Croce, U.; et al. Do Patients with Parkinson’s Disease With Freezing of Gait Respond Differently Than Those Without to Treadmill Training Augmented by Virtual Reality? Neurorehabil. Neural Repair 2020, 34, 440–449. [Google Scholar] [CrossRef]
- Cho, K.H.; Lee, W.H. Effect of Treadmill Training Based Real-World Video Recording on Balance and Gait in Chronic Stroke Patients: A Randomized Controlled Trial. Gait Posture 2014, 39, 523–528. [Google Scholar] [CrossRef]
- Mirelman, A.; Rochester, L.; Reelick, M.; Nieuwhof, F.; Pelosin, E.; Abbruzzese, G.; Dockx, K.; Nieuwboer, A.; Hausdorff, J.M. V-TIME: A Treadmill Training Program Augmented by Virtual Reality to Decrease Fall Risk in Older Adults: Study Design of a Randomized Controlled Trial. BMC Neurol. 2013, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a Non-Immersive Virtual Reality Component to Treadmill Training to Reduce Fall Risk in Older Adults (V-TIME): A Randomised Controlled Trial. Lancet Lond. Engl. 2016, 388, 1170–1182. [Google Scholar] [CrossRef]
- Proulx, C.E.; Beaulac, M.; David, M.; Deguire, C.; Haché, C.; Klug, F.; Kupnik, M.; Higgins, J.; Gagnon, D.H. Review of the Effects of Soft Robotic Gloves for Activity-Based Rehabilitation in Individuals with Reduced Hand Function and Manual Dexterity Following a Neurological Event. J. Rehabil. Assist. Technol. Eng. 2020, 7, 2055668320918130. [Google Scholar] [CrossRef]
- Demolder, C.; Molina, A.; Hammond, F.L.; Yeo, W.-H. Recent Advances in Wearable Biosensing Gloves and Sensory Feedback Biosystems for Enhancing Rehabilitation, Prostheses, Healthcare, and Virtual Reality. Biosens. Bioelectron. 2021, 190, 113443. [Google Scholar] [CrossRef]
- Wang, Q.; Kang, B.; Kristensson, P.O. Supporting Physical and Mental Health Rehabilitation at Home with Virtual Reality Headsets and Force Feedback Gloves. In Proceedings of the 2021 IEEE Conference on Virtual Reality and 3D User Interfaces Abstracts and Workshops (VRW), Lisbon, Portugal, 27 March–1 April 2021; pp. 685–686. [Google Scholar]
- Abd El-Kafy, E.M.; Alshehri, M.A.; El-Fiky, A.A.-R.; Guermazi, M.A.; Mahmoud, H.M. The Effect of Robot-Mediated Virtual Reality Gaming on Upper Limb Spasticity Poststroke: A Randomized-Controlled Trial. Games Health J. 2022, 11, 93–103. [Google Scholar] [CrossRef]
- Baur, K.; Schättin, A.; de Bruin, E.D.; Riener, R.; Duarte, J.E.; Wolf, P. Trends in Robot-Assisted and Virtual Reality-Assisted Neuromuscular Therapy: A Systematic Review of Health-Related Multiplayer Games. J. Neuroeng. Rehabil. 2018, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Burdea, G.C.; Cioi, D.; Kale, A.; Janes, W.E.; Ross, S.A.; Engsberg, J.R. Robotics and Gaming to Improve Ankle Strength, Motor Control, and Function in Children with Cerebral Palsy—A Case Study Series. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic Gait Rehabilitation and Substitution Devices in Neurological Disorders: Where Are We Now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef] [PubMed]
- De Mauro, A.; Carrasco, E.; Oyarzun, D.; Ardanza, A.; Frizera Neto, A.; Torricelli, D.; Pons, J.L.; Gil, A.; Florez, J. Virtual Reality System in Conjunction with Neurorobotics and Neuroprosthetics for Rehabilitation of Motor Disorders. Stud. Health Technol. Inform. 2011, 163, 163–165. [Google Scholar] [PubMed]
| Included Study | Study Design | VRR System | Considered Impairment | Outcome | Conclusions |
|---|---|---|---|---|---|
| Atef et al., 2020 [48] | Comparative study | Nintendo Wii games | Post-mastectomy lymphedema | Upper limb function (quickDASH); arm volume | VR training was not inferior to regular proprioceptive neuromuscular facilitation in improving functioning and reducing volume. |
| Axenie et al., 2020 [49] | Perspective study | Virtual reality avatar-based kinematics assessment and sensorimotor training | Chemotherapy-induced polyneuropathy | Not applicable | Virtual reality software allowed for simultaneous kinematics assessment and multimodal sensorimotor stimulation. In addition, it may facilitate motion training through the use of avatars. |
| Basha et al., 2021 [50] | Comparative study | Xbox Kinect with games involving upper limb movement | Breast cancer-related lymphedema | Pain (VAS), upper limb function (DASH), shoulder and elbow ROM, hand grip strength, quality of life | VR training was superior to resistance exercises for pain, upper limb function, and shoulder ROM outcomes. |
| Feyzioğlu et al., 2019 [51] | Comparative study | Xbox Kinect | Post-mastectomy arm and shoulder impairment | Pain (VAS), grip strength, functionality (disabilities of the arm, shoulder, and hand questionnaire), muscle strength, ROM and fear of movement (TKS) | Both standardized therapy and VRR resulted in significant changes in pain, ROM, muscle strength, grip strength, functionality, and TKS scores, without any significant differences between groups. Fear of movement was significantly improved in the VRR group but the standard physiotherapy group displayed more improvement in functionality. |
| Hoffman et al., 2014 [52] | Randomized non-controlled trial | Nintendo Wii Fit Plus | Post-thoracotomy cancer-related fatigue | Levels of adherence (days of training), exercise performance, cancer-related fatigue (0–10 scale), perceived self-efficacy for fatigue self-management (0–10 scale), perceived self-efficacy for walking 30 min (%) | Non-immersive virtual reality improved both CRF and perceived self-efficacy. |
| House et al., 2016 [53] | Feasibility study | BrightArm Duo: robotic rehabilitation table, computerized forearm supports, and display | Post-mastectomy arm impairment, depression in cancer survivors | Pain (NRS); arm function (FMA, upper extremity section); bimanual function (CAHAI-9); hand function (JHFT); upper arm autonomy in ADL (UEFI-20); depression (BDI-II); cognitive function (NAB, HVLT-R, BVM-T, TMT); | VR rehabilitation significantly improved 10/11 cognitive parameters and depression scores. In addition, it improved arm function as well. |
| Reynolds et al., 2022 [54] | Randomized non-controlled trial | Immersive VR headset (Pico Goblin) | Pain, fatigue, depression, anxiety, and stress in metastatic breast cancer patients | Pain (BPI), quality of life (EQ-5D-5L scale), fatigue (FACIT-Fatigue), depression, anxiety, and stress levels, (DASS-SF) | VRR scenarios had significant effects on all considered outcomes. VRR scenarios did not significantly differ in any outcome |
| Schwenk et al., 2015 [55] | Randomized controlled trial | Non-immersive Virtual Reality software connected to triaxial accelerometers, gyroscopes, and magnetometers | Chemotherapy-induced polyneuropathy | Balance (sway of hip, sway of ankle, center of mass movement), gait speed, fear of falling (FES-I score) | Virtual reality improved balance through patient-tailored, sensor-based exercise but did not improve gait speed and fear of falling |
| Tsuda et al., 2016 [56] | Randomized non-controlled trial | Nintendo Wii Fit | Physical performance worsening related to chemotherapy and hematological malignancies | Levels of adherence, physical performance (Barthel index), muscle strength, emotive state (hospital anxiety and depression scale) | Virtual reality exercise programs showed good adherence rates (66.5%) and helped maintain physical performance in hospitalized patients. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melillo, A.; Chirico, A.; De Pietro, G.; Gallo, L.; Caggianese, G.; Barone, D.; De Laurentiis, M.; Giordano, A. Virtual Reality Rehabilitation Systems for Cancer Survivors: A Narrative Review of the Literature. Cancers 2022, 14, 3163. https://doi.org/10.3390/cancers14133163
Melillo A, Chirico A, De Pietro G, Gallo L, Caggianese G, Barone D, De Laurentiis M, Giordano A. Virtual Reality Rehabilitation Systems for Cancer Survivors: A Narrative Review of the Literature. Cancers. 2022; 14(13):3163. https://doi.org/10.3390/cancers14133163
Chicago/Turabian StyleMelillo, Antonio, Andrea Chirico, Giuseppe De Pietro, Luigi Gallo, Giuseppe Caggianese, Daniela Barone, Michelino De Laurentiis, and Antonio Giordano. 2022. "Virtual Reality Rehabilitation Systems for Cancer Survivors: A Narrative Review of the Literature" Cancers 14, no. 13: 3163. https://doi.org/10.3390/cancers14133163
APA StyleMelillo, A., Chirico, A., De Pietro, G., Gallo, L., Caggianese, G., Barone, D., De Laurentiis, M., & Giordano, A. (2022). Virtual Reality Rehabilitation Systems for Cancer Survivors: A Narrative Review of the Literature. Cancers, 14(13), 3163. https://doi.org/10.3390/cancers14133163











