Nasopharyngeal Carcinoma Radiomic Evaluation with Serial PET/CT: Exploring Features Predictive of Survival in Patients with Long-Term Follow-Up
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Image Acquisition
2.3. Radiomics and Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients and Tumors
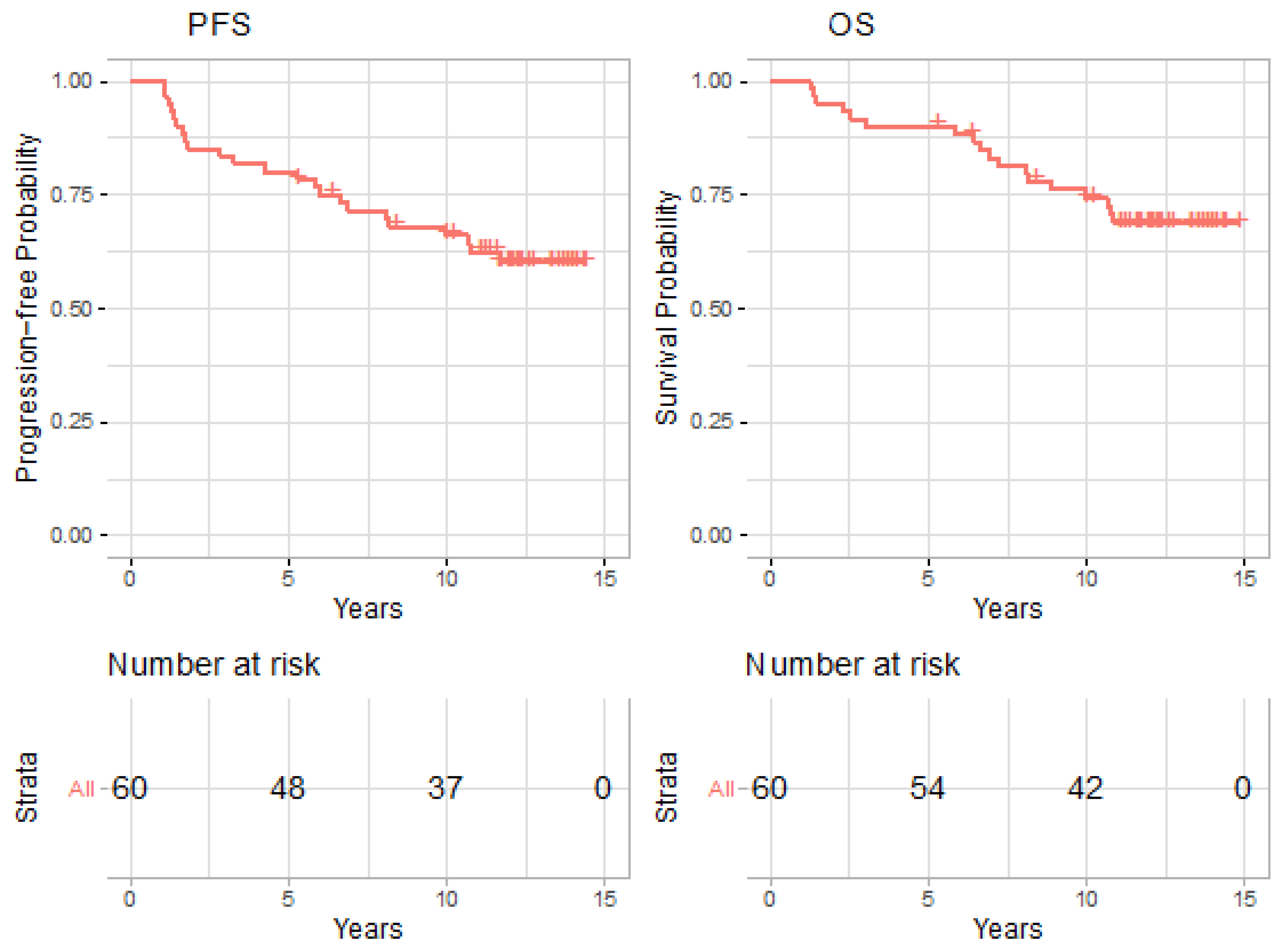
3.2. Treatment Outcomes
3.3. Time-Dependent vs. Baseline Cox Model
3.4. Landmark Analyses
4. Discussion
4.1. Nasopharyngeal Carcinoma
4.2. Overall and Progression-Free Survival
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heron, D.E.; Andrade, R.S.; Beriwal, S.; Smith, R.P. PET-CT in radiation oncology: The impact on diagnosis, treatment planning, and assessment of treatment response. Am. J. Clin. Oncol. 2008, 31, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, G.J.; Champ, C.; Fogh, S.; Wang, F.; Reddy, E.; Intenzo, C.; Dusing, R.W.; Machtay, M. FDG-PET staging and importance of lymph node SUV in head and neck cancer. Head Neck Oncol. 2010, 2, 19. [Google Scholar] [CrossRef][Green Version]
- MacManus, M.; Nestle, U.; Rosenzweig, K.E.; Carrio, I.; Messa, C.; Belohlavek, O.; Danna, M.; Inoue, T.; Deniaud-Alexandre, E.; Schipani, S.; et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006–2007. Radiother. Oncol. 2009, 91, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Nestle, U.; Weber, W.; Hentschel, M.; Grosu, A.L. Biological imaging in radiation therapy: Role of positron emission tomography. Phys. Med. Biol. 2009, 54, R1–R25. [Google Scholar] [CrossRef]
- Ong, S.C.; Schöder, H.; Lee, N.Y.; Patel, S.G.; Carlson, D.; Fury, M.; Pfister, D.G.; Shah, J.P.; Larson, S.M.; Kraus, D.H. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for Locoregional advanced head and neck cancer. J. Nucl. Med. 2008, 49, 532–540. [Google Scholar] [CrossRef]
- Schoder, H.; Fury, M.; Lee, N.; Kraus, D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J. Nucl. Med. 2009, 50 (Suppl. 1), 74S–88S. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Hartill, C.E.; Baker, S.; Woods, E.; Convery, D.J.; Greener, A.G. Specific recommendations for accurate and direct use of PET-CT in PET guided radiotherapy for head and neck sites. Med. Phys. 2014, 41, 041710. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.; Cheng, W.; Zhu, C.; Chen, L.; Xia, F.; Wang, M.; Yang, F.; Ma, X. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Medicine 2017, 96, e8084. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xie, G.; Liao, G.; Wang, B.; Yan, M.; Li, H.; Yuan, Y. Prognostic value of 18F-FDG-PET/CT in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Oncotarget 2017, 8, 33884–33896. [Google Scholar] [CrossRef]
- Chicklore, S.; Goh, V.; Siddique, M.; Roy, A.; Marsden, P.K.; Cook, G.J. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 133–140. [Google Scholar] [CrossRef]
- El Naqa, I.; Grigsby, P.; Apte, A.; Kidd, E.; Donnelly, E.; Khullar, D.; Chaudhari, S.; Yang, D.; Schmitt, M.; Laforest, R.; et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009, 42, 1162–1171. [Google Scholar] [CrossRef]
- Tixier, F.; Le Rest, C.C.; Hatt, M.; Albarghach, N.; Pradier, O.; Metges, J.-P.; Corcos, L.; Visvikis, D. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J. Nucl. Med. 2011, 52, 369–378. [Google Scholar] [CrossRef]
- Chan, S.-C.; Chang, K.-P.; Fang, Y.-H.D.; Tsang, N.-M.; Ng, S.-H.; Hsu, C.-L.; Liao, C.-T.; Yen, T.-C. Tumor heterogeneity measured on F-18 fluorodeoxyglucose positron emission tomography/computed tomography combined with plasma Epstein-Barr Virus load predicts prognosis in patients with primary nasopharyngeal carcinoma. Laryngoscope 2017, 127, E22–E28. [Google Scholar] [CrossRef]
- Peng, L.; Hong, X.; Yuan, Q.; Lu, L.; Wang, Q.; Chen, W. Prediction of local recurrence and distant metastasis using radiomics analysis of pretreatment nasopharyngeal [18F]FDG PET/CT images. Ann. Nucl. Med. 2021, 35, 458–468. [Google Scholar] [CrossRef] [PubMed]
- De Bernardi, E.; Buda, A.; Guerra, L.; Vicini, D.; Elisei, F.; Landoni, C.; Fruscio, R.; Messa, C.; Crivellaro, C. Radiomics of the primary tumour as a tool to improve (18)F-FDG-PET sensitivity in detecting nodal metastases in endometrial cancer. EJNMMI Res. 2018, 8, 86. [Google Scholar] [CrossRef]
- Reuzé, S.; Orlhac, F.; Chargari, C.; Nioche, C.; Limkin, E.; Riet, F.; Escande, A.; Haie-Meder, C.; Dercle, L.; Gouy, S.; et al. Prediction of cervical cancer recurrence using textural features extracted from 18F-FDG PET images acquired with different scanners. Oncotarget 2017, 8, 43169–43179. [Google Scholar] [CrossRef]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reason. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative approach. Nat. Commun. 2014, 3, 4006. [Google Scholar] [CrossRef]
- van Rossum, P.S.; Zhang, L.; Hofstetter, W.L.; van Vulpen, M.; Meijer, G.J.; Court, L.E.; Lin, S.H. The incremental value of subjective and quantitative assessment of 18F-FDG PET for the prediction of pathologic complete response to preoperative chemoradiotherapy in esophageal cancer. J. Nucl. Med. 2016, 57, 691–700. [Google Scholar] [CrossRef]
- Leijenaar, R.T.; Hoebers, F.J.; Aerts, H.J.; van Elmpt, W.J.; Huang, S.H.; Chan, B.; Waldron, J.N.; O’sullivan, B.; Lambin, P. External validation of a prognostic CT-based radiomic signature in oropharyngeal squamous cell carcinoma. Acta Oncol. 2015, 54, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Q.; Liang, C.-H.; He, L.; Tian, J.; Liang, C.-S.; Chen, X.; Ma, Z.-L.; Liu, Z.-Y. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, P.; Dunn, W.D., Jr.; Holder, C.A.; Aerts, H.J. Imaging-genomics reveals driving pathways of MRI derived volumetric tumor phenotype features in glioblastoma. BMC Cancer 2016, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; He, L.; Chen, X.; Pan, D.; Ma, Z.; Liang, C.; Tian, J.; Liang, C. Radiomics signature: A potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology 2016, 281, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Song, J.; Pollom, E.; Alagappan, M.; Shirato, H.; Chang, D.T.; Koong, A.C.; Li, R. Quantitative analysis of 18F-fluorodeoxyglucose positron emission tomography identifies novel prognostic imaging biomarkers in locally advanced pancreatic cancer patients treated with stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Burnside, E.S.; Drukker, K.; Hoadley, K.; Fan, C.; Conzen, S.D.; Whitman, G.J.; Sutton, E.J.; Net, J.M.; et al. MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays. Radiology 2016, 281, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Coroller, T.P.; Grossmann, P.; Hou, Y.; Rios Velazquez, E.; Leijenaar, R.T.H.; Hermann, G.; Lambin, P.; Haibe-Kains, B.; Mak, R.H.; Aerts, H.J.W.L. CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother. Oncol. 2015, 114, 345–350. [Google Scholar] [CrossRef]
- Cunliffe, A.; Armato, S.G., 3rd; Castillo, R.; Pham, N.; Guerrero, T.; Al-Hallaq, H.A. Lung texture in serial thoracic computed tomography scans: Correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 1048–1056. [Google Scholar] [CrossRef]
- Huynh, E.; Coroller, T.; Narayan, V.; Agrawal, V.; Hou, Y.; Romano, J.; Franco, I.; Mak, R.H.; Aerts, H.J. CT-based radiomic analysis of stereotactic body radiation therapy patients with lung cancer. Radiother. Oncol. 2016, 120, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, Y.; He, L.; Chen, X.; Ma, Z.; Dong, D.; Tian, J.; Liang, C.; Liu, Z. The development and validation of a CT-based radiomics signature for the preoperative discrimination of stage I-II and stage III-IV colorectal cancer. Oncotarget 2016, 7, 31401–31412. [Google Scholar] [CrossRef]
- Hawkins, S.; Wang, H.; Liu, Y.; Garcia, A.; Stringfield, O.; Krewer, H.; Li, Q.; Cherezov, D.; Gatenby, R.A.; Balagurunathan, Y.; et al. Predicting Malignant Nodules from Screening CT Scans. J. Thorac. Oncol. 2016, 11, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.; Sze, W.; Au, J.S.; Leung, S.; Leung, T.; Chua, D.T.; Zee, B.C.-Y.; Law, S.C.; Teo, P.M.; Tung, S.Y.; et al. Treatment results for nasopharyngeal carcinoma in the modern era: The Hong Kong experience. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1107–1116. [Google Scholar] [CrossRef]
- Lv, W.; Yuan, Q.; Wang, Q.; Ma, J.; Feng, Q.; Chen, W.; Rahmim, A.; Lu, L. Radiomics Analysis of PET and CT Components of PET/CT Imaging Integrated with Clinical Parameters: Application to Prognosis for Nasopharyngeal Carcinoma. Mol. Imaging Biol. 2019, 21, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Dong, D.; Fang, M.-J.; Li, L.; Tang, L.-L.; Chen, L.; Li, W.-F.; Mao, Y.-P.; Fan, W.; Liu, L.-Z.; et al. Prognostic Value of Deep Learning PET/CT-Based Radiomics: Potential Role for Future Individual Induction Chemotherapy in Advanced Nasopharyngeal Carcinoma. Clin. Cancer Res. 2019, 25, 4271–4279. [Google Scholar] [CrossRef]
- Gu, B.; Meng, M.; Bi, L.; Kim, J.; Feng, D.D.; Song, S. Prediction of 5-year Progression-Free Survival in Advanced Nasopharyngeal Carcinoma with Pretreatment PET/CT using Multi-Modality Deep Learning-based Radiomics. arXiv 2021, arXiv:210305220. [Google Scholar]
- Wong, K.; Huang, S.H.; O’Sullivan, B.; Lockwood, G.; Dale, D.; Michaelson, T.; Waldron, J.; Bayley, A.; Cummings, B.; Dawson, L.A.; et al. Point-of-care outcome assessment in the cancer clinic: Audit of data quality. Radiother. Oncol. 2010, 95, 339–343. [Google Scholar] [CrossRef]
- Parvez, A.; Tau, N.; Hussey, D.; Maganti, M.; Metser, U. (18)F-FDG PET/CT metabolic tumor parameters and radiomics features in aggressive non-Hodgkin’s lymphoma as predictors of treatment outcome and survival. Ann. Nucl. Med. 2018, 32, 410–416. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Orlhac, F.; Soussan, M.; Maisonobe, J.A.; Garcia, C.A.; Vanderlinden, B.; Buvat, I. Tumor texture analysis in 18F-FDG PET: Relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J. Nucl. Med. 2014, 55, 414–422. [Google Scholar] [CrossRef]
- Clarke, L.P.; Schilling, L.B. Imaging as a Biomarker: Standards for Change Measurements in Therapy workshop summary. Acad. Radiol. 2008, 15, 501–530. [Google Scholar] [CrossRef] [PubMed]
- Shankar, L.K.; Hoffman, J.M.; Bacharach, S.; Graham, M.M.; Karp, J.; Lammertsma, A.A.; Larson, S.; Mankoff, D.A.; Siegel, B.A.; Van den Abbeele, A.; et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J. Nucl. Med. 2006, 47, 1059–1066. [Google Scholar] [PubMed]
- Clarke, L.P.; Nordstrom, R.J.; Zhang, H.; Tandon, P.; Zhang, Y.; Redmond, G.; Farahani, K.; Kelloff, G.; Henderson, L.; Shankar, L.; et al. The Quantitative Imaging Network: NCI’s Historical Perspective and Planned Goals. Transl. Oncol. 2014, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Buckler, A.J.; Dunnick, N.R.; Sullivan, D.C.; Group. A collaborative enterprise for multi-stakeholder participation in the advancement of quantitative imaging. Radiology 2011, 258, 906–914. [Google Scholar] [CrossRef]
- Brooks, F.J.; Grigsby, P.W. The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J. Nucl. Med. 2014, 55, 37–42. [Google Scholar] [CrossRef]
- Hatt, M.; Majdoub, M.; Vallières, M.; Tixier, F.; Le Rest, C.C.; Groheux, D.; Hindié, E.; Martineau, A.; Pradier, O.; Hustinx, R.; et al. 18F-FDG PET uptake characterization through texture analysis: Investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J. Nucl. Med. 2015, 56, 38–44. [Google Scholar] [CrossRef]
- Rios Velazquez, E.; Aerts, H.J.W.L.; Gu, Y.; Goldgof, D.B.; De Ruysscher, D.; Dekker, A.; Korn, R.; Gillies, R.J.; Lambin, P. A semiautomatic CT-based ensemble segmentation of lung tumors: Comparison with oncologists’delineations and with the surgical specimen. Radiother. Oncol. 2012, 105, 167–173. [Google Scholar] [CrossRef]
- van Dam, I.E.; van Sörnsen de Koste, J.R.; Hanna, G.G.; Muirhead, R.; Slotman, B.J.; Senan, S. Improving target delineation on 4-dimensional CT scans in stage I NSCLC using a deformable registration tool. Radiother. Oncol. 2010, 96, 67–72. [Google Scholar] [CrossRef]
- Pfister, D.G.; Ang, K.; Brockstein, B.; Colevas, A.D.; Ellenhorn, J.; Goepfert, H.; Hicks, W.L.; Hong, W.K.; Kies, M.S.; Lydiatt, W.; et al. NCCN Practice Guidelines for Head and Neck Cancers. Oncology 2000, 14, 163–194. [Google Scholar]
- Intarak, S.; Chongpison, Y.; Vimolnoch, M.; Oonsiri, S.; Kitpanit, S.; Prayongrat, A.; Kannarunimit, D.; Chakkabat, C.; Sriswasdi, S.; Lertbutsayanukul, C.; et al. Tumor Prognostic Prediction of Nasopharyngeal Carcinoma Using CT-Based Radiomics in Non-Chinese Patients. Front. Oncol. 2022, 12, 775248. [Google Scholar] [CrossRef]
- Yan, C.; Shen, D.-S.; Chen, X.-B.; Su, D.-K.; Liang, Z.-G.; Chen, K.-H.; Li, L.; Liang, X.; Liao, H.; Zhu, X.-D. CT-Based Radiomics Nomogram for Prediction of Progression-Free Survival in Locoregionally Advanced Nasopharyngeal Carcinoma. Cancer Manag. Res. 2021, 13, 6911. [Google Scholar] [CrossRef] [PubMed]
- Keek, S.; Wesseling, F.; Woodruff, H.; van Timmeren, J.; Nauta, I.; Hoffmann, T.; Cavalieri, S.; Calareso, G.; Primakov, S.; Leijenaar, R.; et al. A Prospectively Validated Prognostic Model for Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck Based on Radiomics of Computed Tomography Images. Cancers 2021, 13, 3271. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Leijenaar, R.T.H.; Grossmann, P.; Velazquez, E.R.; Bussink, J.; Rietveld, D.; Rietbergen, M.M.; Haibe-Kains, B.; Lambin, P.; Aerts, H.J. Radiomic feature clusters and prognostic signatures specific for Lung and Head & Neck cancer. Sci. Rep. 2015, 5, 11044. [Google Scholar] [CrossRef]
- Bogowicz, M.; Riesterer, O.; Ikenberg, K.; Stieb, S.; Moch, H.; Studer, G.; Guckenberger, M.; Tanadini-Lang, S. Computed Tomography Radiomics Predicts HPV Status and Local Tumor Control After Definitive Radiochemotherapy in Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Bogowicz, M.; Riesterer, O.; Stark, L.S.; Studer, G.; Unkelbach, J.; Guckenberger, M.; Tanadini-Lang, S. Comparison of PET and CT radiomics for prediction of local tumor control in head and neck squamous cell carcinoma. Acta Oncol. 2017, 56, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Bogowicz, M.; Leijenaar, R.T.; Tanadini-Lang, S.; Riesterer, O.; Pruschy, M.; Studer, G.; Unkelbach, J.; Guckenberger, M.; Konukoglu, E.; Lambin, P. Post-radiochemotherapy PET radiomics in head and neck cancer—The influence of radiomics implementation on the reproducibility of local control tumor models. Radiother. Oncol. 2017, 125, 385–391. [Google Scholar] [CrossRef]
- Vallières, M.; Kay-Rivest, E.; Perrin, L.J.; Liem, X.; Furstoss, C.; Aerts, H.J.W.L.; Khaouam, N.; Nguyen-Tan, P.F.; Wang, C.-S.; Sultanem, K.; et al. Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci. Rep. 2017, 7, 10117. [Google Scholar] [CrossRef]
- Folkert, M.R.; Setton, J.; Apte, A.P.; Grkovski, M.; Young, R.J.; Schöder, H.; Thorstad, W.L.; Lee, N.Y.; Deasy, J.O.; Oh, J.H. Predictive modeling of outcomes following definitive chemoradiotherapy for oropharyngeal cancer based on FDG-PET image characteristics. Phys. Med. Biol. 2017, 62, 5327–5343. [Google Scholar] [CrossRef]
- Cheng, N.-M.; Fang, Y.-H.D.; Chang, J.T.-C.; Huang, C.-G.; Tsan, D.-L.; Ng, S.-H.; Wang, H.-M.; Lin, C.-Y.; Liao, C.-T.; Yen, T.-C. Textural features of pretreatment 18F-FDG PET/CT images: Prognostic significance in patients with advanced T-stage oropharyngeal squamous cell carcinoma. J. Nucl. Med. 2013, 54, 1703–1709. [Google Scholar] [CrossRef]
- Apostolova, I.; Steffen, I.G.; Wedel, F.; Lougovski, A.; Marnitz, S.; Derlin, T.; Amthauer, H.; Buchert, R.; Hofheinz, F.; Brenner, W. Asphericity of pretherapeutic tumour FDG uptake provides independent prognostic value in head-and-neck cancer. Eur. Radiol. 2014, 24, 2077–2087. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer, J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Yi, J.-L.; Gao, L.; Huang, X.-D.; Li, S.-Y.; Luo, J.-W.; Cai, W.-M.; Xiao, J.-P.; Xu, G.-Z. Nasopharyngeal carcinoma treated by radical radiotherapy alone: Ten-year experience of a single institution. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, J.; Dong, D.; Gu, D.; Dong, Y.; Zhang, L.; Lian, Z.; Liu, J.; Luo, X.; Pei, S.; et al. Radiomics Features of Multiparametric MRI as Novel Prognostic Factors in Advanced Nasopharyngeal Carcinoma. Clin. Cancer Res. 2017, 23, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.-S.; Guo, B.-L.; Zhang, B.; Dong, Y.-H.; Zhang, L.; Mo, X.-K.; Huang, W.-H.; Zhang, S.-X.; Hu, Q.-G. Exploration and validation of radiomics signature as an independent prognostic biomarker in stage III-IVb nasopharyngeal carcinoma. Oncotarget 2017, 8, 74869–74879. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, B.; Ouyang, F.; Gu, D.; Dongsheng, G.; Zhang, L.; Mo, X.; Huang, W.; Zhang, S. Advanced nasopharyngeal carcinoma: Pre-treatment prediction of progression based on multi-parametric MRI radiomics. Oncotarget 2017, 8, 72457–72465. [Google Scholar] [CrossRef]
- Liu, J.; Mao, Y.; Li, Z.; Zhang, D.; Zhang, Z.; Hao, S.; Li, B. Use of texture analysis based on contrast-enhanced MRI to predict treatment response to chemoradiotherapy in nasopharyngeal carcinoma. J. Magn. Reason. Imaging 2016, 44, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, q.; Zhang, Y.; Pan, H.; Yao, Z.; Hu, S.; Shi, W.; Zhu, B.; Zhang, Y.; Hu, C. Pretreatment (18)F-FDG uptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma—A retrospective study. Radiat. Oncol. 2015, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Cho, Y.S.; Son, Y.-I.; Ahn, Y.C.; Ahn, M.-J.; Choi, J.Y.; Kim, B.-T.; Lee, K.-H. Value of (18)F-FDG heterogeneity for discerning metastatic from benign lymph nodes in nasopharyngeal carcinoma patients with suspected recurrence. Br. J. Radiol. 2016, 89, 20160109. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chan, T.; Kwong, D.L.; Chan, W.K.; Khong, P.L. Nasopharyngeal carcinoma: Investigation of intratumoral heterogeneity with FDG PET/CT. Am. J. Roentgenol. 2012, 199, 169–174. [Google Scholar] [CrossRef]
- Zhong, L.; Dong, D.; Fang, X.; Zhang, F.; Zhang, N.; Zhang, L.; Fang, M.; Jiang, W.; Liang, S.; Li, C.; et al. A deep learning-based radiomic nomogram for prognosis and treatment decision in advanced nasopharyngeal carcinoma: A multicentre study. EBioMedicine 2021, 70, 103522. [Google Scholar] [CrossRef]
- Feng, Q.; Liang, J.; Wang, L.; Niu, J.; Ge, X.; Pang, P.; Ding, Z. Radiomics Analysis and Correlation With Metabolic Parameters in Nasopharyngeal Carcinoma Based on PET/MR Imaging. Front. Oncol. 2020, 10, 1619. [Google Scholar] [CrossRef]
- Shafiq-Ul-Hassan, M.; Zhang, G.G.; Latifi, K.; Ullah, G.; Hunt, D.C.; Balagurunathan, Y.; Abdalah, M.A.; Schabath, M.; Goldgof, D.G.; Mackin, D.; et al. Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med. Phys. 2017, 44, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Nyflot, M.J.; Yang, F.; Byrd, D.; Bowen, S.R.; Sandison, G.A.; Kinahan, P.E. Quantitative radiomics: Impact of stochastic effects on textural feature analysis implies the need for standards. J. Med. Imaging 2015, 2, 041002. [Google Scholar] [CrossRef]
| Characteristic | n = 60 (%) |
|---|---|
| Age, median (range), years | 51.2 (18.3–74.8) |
| Male Female | 37 (62%) 23 (38%) |
| Smoking Status | |
| Current | 4 (8%) |
| Former | 13 (22%) |
| Non-smoker | 41 (71%) |
| Unknown | 2 (4%) |
| Pathology | |
| WHO I | 1 (2%) |
| WHO IIA | 10 (16%) |
| WHO IIB | 49 (82%) |
| Stage | |
| I | 2 (4%) |
| II | 11 (21%) |
| III | 19 (37%) |
| IVa | 12 (23%) |
| IVb | 7 (13%) |
| IVc | 1 (2%) |
| T | |
| T1 | 24 (40%) |
| T2 | 12 (20%) |
| T3 | 7 (12%) |
| T4 | 17 (28%) |
| N | |
| N0 | 4 (7%) |
| N1 | 22 (20%) |
| N2 | 26 (43%) |
| N3 | 1 (2%) |
| N3A | 5 (8%) |
| N3B | 2 (3%) |
| M | |
| M0 | 57 (95%) |
| M1 | 3 (5%) |
| Status | |
| Alive | 42 (70%) |
| Dead | 18 (30%) |
| Treatment | |
| Radiotherapy alone | 6 (10%) |
| Chemoradiotherapy | 54 (90%) |
| Local Failure | 4 (7%) |
| Regional Failure | 5 (8%) |
| Distant Failure | 8 (13%) |
| Overall Survival | Time-Dependent Model | Baseline Model | |||
|---|---|---|---|---|---|
| Modality | Radiomic Features | HR (95%CI) | p-Value | HR (95%CI) | p-Value |
| CT + PET 40% | CT_NGLDM_Busyness | 2.54 (1.29, 5.00) | 0.0069 | 0.98 (0.73, 1.33) | 0.90 |
| PET_CONVENTIONAL_SUVbwmax | 2.66 (1.56, 4.55) | 0.0004 | 1.08 (0.74, 1.57) | 0.69 | |
| PET_GLZLM_GLNU | 2.26 (1.46, 3.49) | 0.0002 | 1.14 (0.95, 1.37) | 0.16 | |
| CT + PET 70% | CT_SHAPE_ Volume.vx. | 1.94 (1.34, 2.80) | 0.0004 | 1.08 (0.85, 1.38) | 0.51 |
| PET_DISCRETIZED_SUVbwmax | 2.74 (1.58, 4.74) | 0.0003 | 1.07 (0.70, 1.64) | 0.74 | |
| Progression-Free Survival | Time-Dependent model | Baseline Model | |||
| Modality | Radiomic Features | HR (95%CI) | p-Value | HR (95%CI) | p-Value |
| CT + PET 40% | PET_DISCRETIZED_ SUVbwpeakSphere0.5mL | 2.06 (1.28, 3.31) | 0.0029 | 1.08 (0.74, 1.58) | 0.68 |
| PET_GLZLM_GLNU | 1.67 (1.23, 2.26) | 0.0011 | 1.09 (0.90, 1.31) | 0.38 | |
| CT + PET 70% | PET_CONVENTIONAL_SUVbwQ1 | 1.84 (1.23, 2.76) | 0.0031 | 1.05 (0.76, 1.43) | 0.78 |
| PET_CONVENTIONAL_TLG.mL | 5.67 (1.75, 18.39) | 0.0039 | 1.14 (0.68, 1.91) | 0.62 | |
| Modality | Time Point | Significant Features | HR (95% CI) | p-Value | C-Index |
|---|---|---|---|---|---|
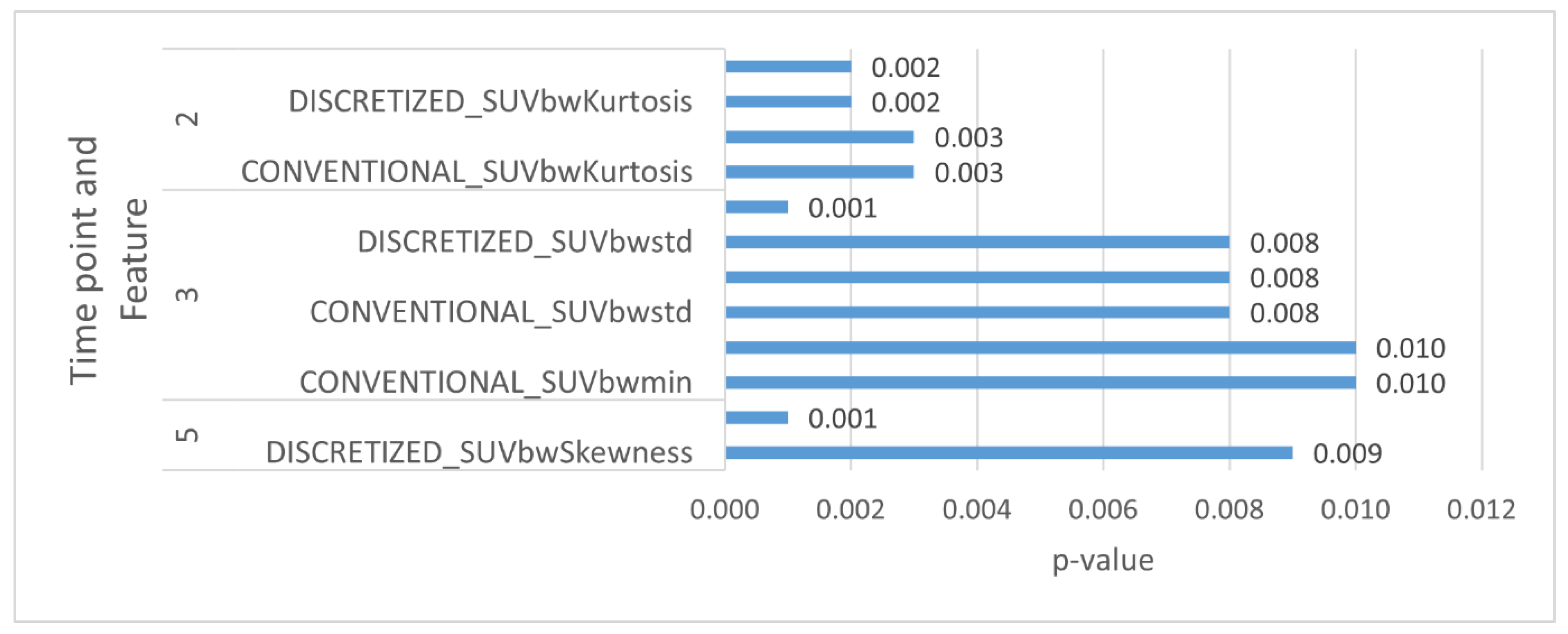
| CT | 5 | GLCM_Correlation | 0.33 (0.17, 0.62) | 0.001 | 0.792 |
| PET 40% | 2 | CONVENTIONAL_SUVbwKurtosis | 1.85 (1.23, 2.78) | 0.003 | 0.616 |
| 2 | CONVENTIONAL_SUVbwExcessKurtosis | 1.85 (1.23, 2.78) | 0.003 | 0.616 | |
| 2 | DISCRETIZED_SUVbwKurtosis | 1.94 (1.27, 2.97) | 0.002 | 0.653 | |
| 2 | DISCRETIZED_SUVbwExcessKurtosis | 1.94 (1.27, 2.97) | 0.002 | 0.653 | |
| 3 | CONVENTIONAL_SUVbwstd | 2.01 (1.2, 3.38) | 0.008 | 0.551 | |
| 3 | DISCRETIZED_SUVbwstd | 2.01 (1.2, 3.38) | 0.008 | 0.538 | |
| 3 | GLZLM_ZLNU | 2.3 (1.46, 3.64) | 0.001 | 0.707 | |
| 5 | DISCRETIZED_SUVbwSkewness | 0.32 (0.14, 0.75) | 0.009 | 0.736 | |
| PET 70% | 3 | CONVENTIONAL_SUVbwmin | 2.01 (1.19, 3.41) | 0.010 | 0.546 |
| 3 | CONVENTIONAL_SUVbwQ3 | 1.98 (1.17, 3.34) | 0.010 | 0.519 | |
| 3 | DISCRETIZED_SUVbwstd | 1.98 (1.2, 3.27) | 0.008 | 0.536 |
| Modality | Time Point | Significant Features | HR (95% CI) | p-Value | C-Index |
|---|---|---|---|---|---|
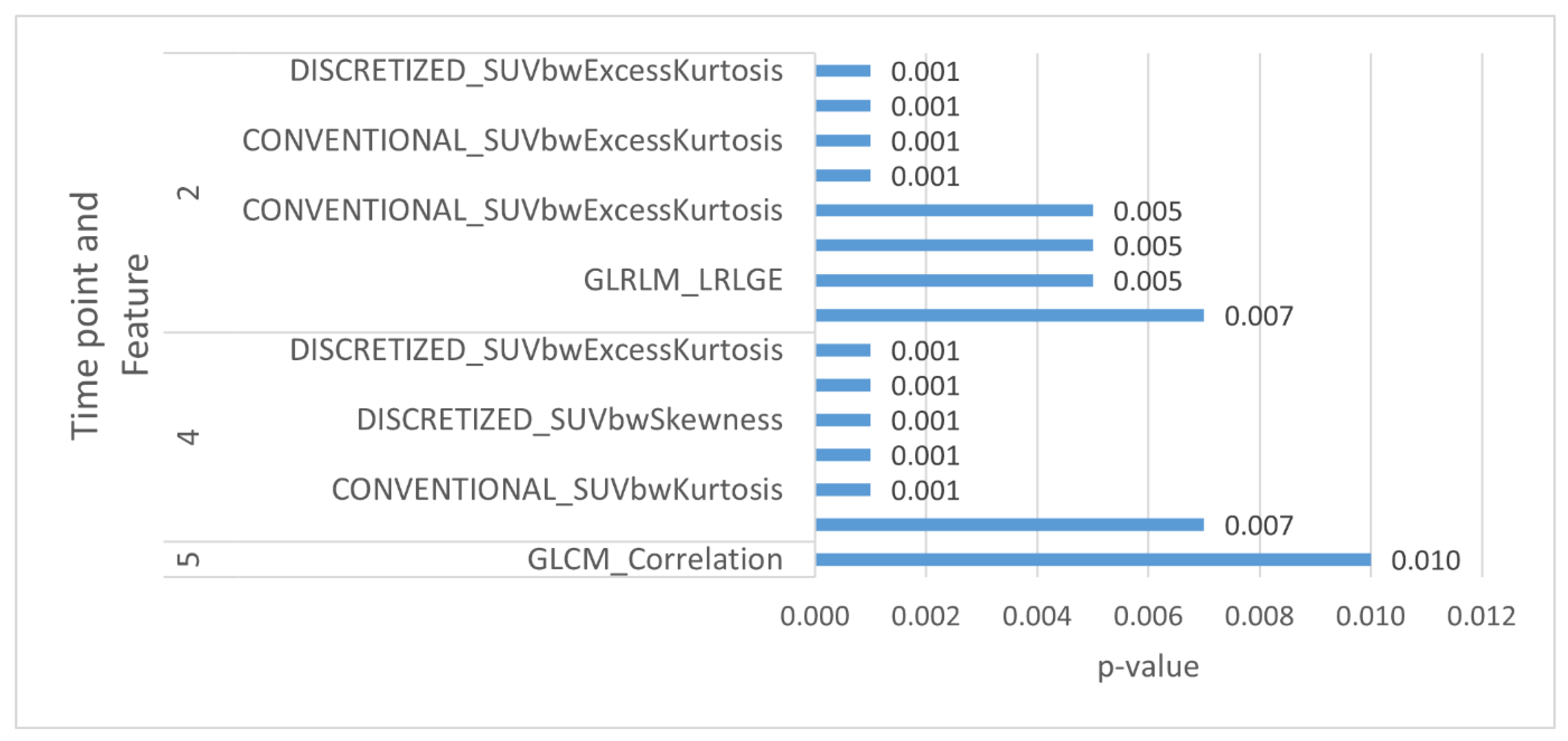
| CT | 5 | GLCM_Correlation | 0.46 (0.26, 0.83) | 0.010 | 0.690 |
| PET 40% | 2 | CONVENTIONAL_SUVbwKurtosis | 2.38 (1.54, 3.67) | 0.001 | 0.671 |
| 2 | CONVENTIONAL_SUVbwExcessKurtosis | 2.38 (1.54, 3.67) | 0.001 | 0.671 | |
| 2 | DISCRETIZED_SUVbwKurtosis | 2.44 (1.57, 3.77) | 0.001 | 0.689 | |
| 2 | DISCRETIZED_SUVbwExcessKurtosis | 2.44 (1.57, 3.77) | 0.001 | 0.689 | |
| 2 | GLCM_Energy AngularSecondMoment. | 1.66 (1.15, 2.41) | 0.007 | 0.648 | |
| 2 | GLRLM_LRLGE | 1.67 (1.17, 2.39) | 0.005 | 0.629 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmytriw, A.A.; Ortega, C.; Anconina, R.; Metser, U.; Liu, Z.A.; Liu, Z.; Li, X.; Sananmuang, T.; Yu, E.; Joshi, S.; et al. Nasopharyngeal Carcinoma Radiomic Evaluation with Serial PET/CT: Exploring Features Predictive of Survival in Patients with Long-Term Follow-Up. Cancers 2022, 14, 3105. https://doi.org/10.3390/cancers14133105
Dmytriw AA, Ortega C, Anconina R, Metser U, Liu ZA, Liu Z, Li X, Sananmuang T, Yu E, Joshi S, et al. Nasopharyngeal Carcinoma Radiomic Evaluation with Serial PET/CT: Exploring Features Predictive of Survival in Patients with Long-Term Follow-Up. Cancers. 2022; 14(13):3105. https://doi.org/10.3390/cancers14133105
Chicago/Turabian StyleDmytriw, Adam A., Claudia Ortega, Reut Anconina, Ur Metser, Zhihui A. Liu, Zijin Liu, Xuan Li, Thiparom Sananmuang, Eugene Yu, Sayali Joshi, and et al. 2022. "Nasopharyngeal Carcinoma Radiomic Evaluation with Serial PET/CT: Exploring Features Predictive of Survival in Patients with Long-Term Follow-Up" Cancers 14, no. 13: 3105. https://doi.org/10.3390/cancers14133105
APA StyleDmytriw, A. A., Ortega, C., Anconina, R., Metser, U., Liu, Z. A., Liu, Z., Li, X., Sananmuang, T., Yu, E., Joshi, S., Waldron, J., Huang, S. H., Bratman, S., Hope, A., & Veit-Haibach, P. (2022). Nasopharyngeal Carcinoma Radiomic Evaluation with Serial PET/CT: Exploring Features Predictive of Survival in Patients with Long-Term Follow-Up. Cancers, 14(13), 3105. https://doi.org/10.3390/cancers14133105







