A Frailty-Adjusted Stratification Score to Predict Surgical Risk, Post-Operative, Long-Term Functional Outcome, and Quality of Life after Surgery in Intracranial Meningiomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Type and Participants
2.2. Variables of Interest and Outcomes
2.2.1. Biometric/Functional Data Extraction
2.2.2. Radiological Data Extraction
2.2.3. Surgical Management
2.2.4. Post-Operative and Follow-Up Data Extraction
Early Post-Operative and Long-Term Parameters of Interest
Primary Post-Operative and Follow-Up Outcomes
- (1)
- The primary post-operative outcome (“early post-operative functional deterioration”) was designed to address patient dependence status and was computed as a drop in post-operative KPS of at least 20 points at discharge compared with the pre-operative assessment. The selected cut-off represented the occurrence of any general or neurological complication affecting the overall functional performance of patients undergoing surgery well [21,35].
- (2)
- The long-term follow-up primary outcome (“unfavourable long-term functional autonomy and quality of life”) was designed to address patient dependence and QoL and was defined as a decrease of ≥ 20 points in KPS at the follow-up interview compared with the pre-operative one plus an overall quality of life (QOL) under the 75th percentile of the examined population.
2.3. Statistical Analysis
3. Results
3.1. Population Description
3.2. Regression Analysis of Early/Long-Term Post-Operative Functional Outcome and Score Design
3.2.1. MBBS Part A
3.2.2. MBBS Part B
3.3. Internal Retrospective and External Comparative Validation
4. Discussion
4.1. Points of Strength
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achey, R.L.; Gittleman, H.; Schroer, J.; Khanna, V.; Kruchko, C.; Barnholtz-Sloan, J.S. Nonmalignant and malignant meningioma incidence and survival in the elderly, 2005–2015, using the Central Brain Tumor Registry of the United States. Neuro-Oncology 2019, 21, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22, IV1–IV96. [Google Scholar] [CrossRef] [PubMed]
- Wiemels, J.; Wrensch, M.; Claus, E.B. Epidemiology and etiology of meningioma. J. Neurooncol. 2010, 99, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIsaac, D.I.; Beaulé, P.E.; Bryson, G.L.; Van Walraven, C. The impact of frailty on outcomes and healthcare resource usage after total joint arthroplasty: A population-based cohort study. Bone Jt. J. 2016, 98-B, 799–805. [Google Scholar] [CrossRef]
- Robinson, T.N.; Wu, D.S.; Stiegmann, G.V.; Moss, M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am. J. Surg. 2011, 202, 511–514. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, J.G.; Evans, J.L.; Prato, B.S.; Hess, S.A.; MacGillivray, D.C.; Fitzgerald, T.L. Frailty Cost: Economic Impact of Frailty in the Elective Surgical Patient. J. Am. Coll. Surg. 2019, 228, 861–870. [Google Scholar] [CrossRef]
- Dasenbrock, H.H.; Liu, K.X.; Devine, C.A.; Chavakula, V.; Smith, T.R.; Gormley, W.B.; Dunn, I.F. Length of hospital stay after craniotomy for tumor: A National Surgical Quality Improvement Program analysis. Neurosurg. Focus 2015, 39, E12. [Google Scholar] [CrossRef] [Green Version]
- Cloney, M.; D’Amico, R.; Lebovic, J.; Nazarian, M.; Zacharia, B.E.; Sisti, M.B.; Bruce, J.N.; McKhann, G.M.; Iwamoto, F.M.; Sonabend, A.M. Frailty in Geriatric Glioblastoma Patients: A Predictor of Operative Morbidity and Outcome. World Neurosurg. 2016, 89, 362–367. [Google Scholar] [CrossRef]
- Imaoka, Y.; Kawano, T.; Hashiguchi, A.; Fujimoto, K.; Yamamoto, K.; Nishi, T.; Otsuka, T.; Yano, S.; Mukasa, A. Modified frailty index predicts postoperative outcomes of spontaneous intracerebral hemorrhage. Clin. Neurol. Neurosurg. 2018, 175, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Harland, T.A.; Wang, M.; Gunaydin, D.; Fringuello, A.; Freeman, J.; Hosokawa, P.W.; Ormond, D.R. Frailty as a Predictor of Neurosurgical Outcomes in Brain Tumor Patients. World Neurosurg. 2020, 133, e813–e818. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.; Mukherjee, D.; Chang, D.C.; Purtell, M.; Lim, M.; Brem, H.; Quiñones-Hinojosa, A. Predictors of inpatient death and complications among postoperative elderly patients with metastatic brain tumors. Ann. Surg. Oncol. 2011, 18, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gani, F.; Canner, J.K.; Pawlik, T.M. Use of the modified frailty index in the American college of surgeons national surgical improvement program database: Highlighting the problem of missing data. JAMA Surg. 2017, 152, 205–207. [Google Scholar] [CrossRef]
- Jakola, A.S.; Gulati, M.; Gulati, S.; Solheim, O. The influence of surgery on quality of life in patients with intracranial meningiomas: A prospective study. J. Neurooncol. 2012, 110, 137–144. [Google Scholar] [CrossRef]
- Meskal, I.; Gehring, K.; Rutten, G.J.M.; Sitskoorn, M.M. Cognitive functioning in meningioma patients: A systematic review. J. Neurooncol. 2016, 128, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Lu, X.; Qiu, Y.; Jiang, J.; Lin, Y. A multivariate analysis of prognostic factors for health-related quality of life in patients with surgically managed meningioma. J. Clin. Neurosci. 2010, 17, 446–449. [Google Scholar] [CrossRef]
- Nassiri, F.; Price, B.; Shehab, A.; Au, K.; Cusimano, M.D.; Jenkinson, M.D.; Jungk, C.; Mansouri, A.; Santarius, T.; Suppiah, S.; et al. Life after surgical resection of a meningioma: A prospective cross-sectional study evaluating health-related quality of life. Neuro-oncology 2019, 21, I32–I43. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.M.; Zhang, L.M.; Hornor, M.A.; Robinson, T.; Rosenthal, R.A.; Ko, C.Y.; Russell, M.M. Evaluation of Postoperative Functional Health Status Decline among Older Adults. JAMA Surg. 2020, 155, 950–958. [Google Scholar] [CrossRef]
- Bette, S.; Gradtke, C.V.; Cammardella, J.H.; Albertshauser, J.; Wiestler, B.; Barz, M.; Meyer, B.; Zimmer, C.; Ryang, Y.M.; Ringel, F.; et al. Perioperative neurocognitive functions in patients with neuroepithelial intracranial tumors. J. Neurooncol. 2020, 147, 77–89. [Google Scholar] [CrossRef]
- Stienen, M.N.; Zhang, D.Y.; Broggi, M.; Seggewiss, D.; Villa, S.; Schiavolin, S.; Bozinov, O.; Krayenbühl, N.; Sarnthein, J.; Ferroli, P.; et al. The influence of preoperative dependency on mortality, functional recovery and complications after microsurgical resection of intracranial tumors. J. Neurooncol. 2018, 139, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmer, M.; Seibl-Leven, M.; Wittenstein, K.; Grau, S.; Stavrinou, P.; Röhn, G.; Krischek, B.; Goldbrunner, R. Long-Term Outcome and Health-Related Quality of Life of Elderly Patients after Meningioma Surgery. World Neurosurg. 2019, 125, e697–e710. [Google Scholar] [CrossRef] [PubMed]
- DeMonte, F.; McDermott, M.W.; Al-Mefty, O. Al-Mefty’s Meningiomas; Thieme Medical Publishers: New York, NY, USA, 2011; p. 432. [Google Scholar]
- Wen, P.Y.; Huse, J.T. 2016 World Health Organization Classification of Central Nervous System Tumors. Contin. Lifelong Learn. Neurol. 2017, 23, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.A.; Brown, P.D. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J. Clin. Oncol. 2006, 24, 1305–1309. [Google Scholar] [CrossRef]
- Schag, C.C.; Heinrich, R.L.; Ganz, P.A. Karnofsky performance status revisited: Reliability, validity, and guidelines. J. Clin. Oncol. 1984, 2, 187–193. [Google Scholar] [CrossRef]
- Searle, S.D.; Rockwood, K. Frailty and the risk of cognitive impairment. Alzheimer’s Res. Ther. 2015, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Loewenstern, J.; Aggarwal, A.; Pain, M.; Barthélemy, E.; Costa, A.; Bederson, J.; Shrivastava, R.K. Peritumoral Edema Relative to Meningioma Size Predicts Functional Outcomes after Resection in Older Patients. Oper. Neurosurg. 2019, 16, 281–291. [Google Scholar] [CrossRef]
- Sindou, M.P.; Alvernia, J.E. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J. Neurosurg. 2006, 105, 514–525. [Google Scholar] [CrossRef]
- Nakasu, S.; Nakasu, Y.; Nakajima, M.; Matsuda, M.; Handa, J. Preoperative identification of meningiomas that are highly likely to recur. J. Neurosurg. 1999, 90, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Weitzner, M.A.; Meyers, C.A.; Gelke, C.K.; Byrne, K.S.; Levin, V.A.; Cella, D.F. The functional assessment of cancer therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 1995, 75, 1151–1161. [Google Scholar] [CrossRef]
- Ferroli, P.; Broggi, M.; Schiavolin, S.; Acerbi, F.; Bettamio, V.; Caldiroli, D.; Cusin, A.; La Corte, E.; Leonardi, M.; Raggi, A.; et al. Predicting functional impairment in brain tumor surgery: The Big Five and the Milan Complexity Scale. Neurosurg. Focus 2015, 39, E14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Velanovich, V.; Antoine, H.; Swartz, A.; Peters, D.; Rubinfeld, I. Accumulating deficits model of frailty and postoperative mortality and morbidity: Its application to a national database. J. Surg. Res. 2013, 183, 104–110. [Google Scholar] [CrossRef]
- Pisica, D.; Volovici, V. Letter: Assessing Frailty in Neurosurgical Patients: Less is not Always More. Is There Any Construct Validity Left in the Modified Frailty Index? Neurosurgery 2021, 88, E292–E293. [Google Scholar] [CrossRef]
- Movsas, B. Quality of life in oncology trials: A clinical guide. Semin. Radiat. Oncol. 2003, 13, 235–247. [Google Scholar] [CrossRef]
- Caroli, M.; Locatelli, M.; Prada, F.; Beretta, F.; Martinelli-Boneschi, F.; Campanella, R.; Arienta, C. Surgery for intracranial meningiomas in the elderly: A clinical- radiological grading system as a predictor of outcome. J. Neurosurg. 2005, 102, 290–294. [Google Scholar] [CrossRef]
- Delgado-Fernández, J.; García-Pallero, M.A.; Gil-Simoes, R.; Blasco, G.; Frade-Porto, N.; Pulido, P.; Sola, R.G. Validation of Grading Scores and Outcome Prognostic Factors in Intracranial Meningiomas in Elderly Patients. World Neurosurg. 2018, 114, e1057–e1065. [Google Scholar] [CrossRef]
- Grossman, R.; Mukherjee, D.; Chang, D.C.; Bennett, R.; Brem, H.; Olivi, A.; Quiñones-Hinojosa, A. Preoperative Charlson comorbidity score predicts postoperative outcomes among older intracranial meningioma patients. World Neurosurg. 2011, 75, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Soustiel, J.F.; Zaaroor, M. Meningiomas in the elderly, the surgical benefit and a new scoring system. Acta Neurochir. 2010, 152, 87–97. [Google Scholar] [CrossRef] [PubMed]

| 34-Items Frailty-Index for Pre-Operative Assessment in Craniotomy Surgery | |||||||
| Items | Code | YES | NO | Items | Code | YES | NO |
| 1—Smoking status | Smoking | 18—Thyroid disease | Thyroid | ||||
| 2—Balance disorders | Balance | 19—Cancer | Cancer | ||||
| 3—Osteoporosis | Osteop | 20—Chirrhosis | Liver | ||||
| 4—Arthritis/Deformant arthrosis | Bone | 21—Urinary or bowel incontinence | Incontinence | ||||
| 5—Hypertension (>140/90 mmHg) | HTN | 22—Stayed in bed > half of the day due to health (last month) | Bed | ||||
| 6—Ischemic heart disease, CAD, PAD | Ischemia | 23—Parkinsonism | Park | ||||
| 7—Chronic heart failure (CHF) | Heart | 24—Focal neurological signs | Neuro | ||||
| 8—Arrhythmia | I | 25—Hearing impairment | Hearing | ||||
| 9—COPD or other respiratory disorders | Lung | 26—Mobility disability (200 m walking test) | Mobility | ||||
| 10—History of previous blood clot (DVT, PE, TIA or Stroke) | Clot | 27—Depression (feeling downhearted/depressed most of the time) | Depression | ||||
| 11—Bleeding disorders (thrombocytopenias, NOAC, VKA, other haematological conditions) | Bleed | 28—Anxiety | Anxiety | ||||
| 12—Dislipidaemia | Lipids | 29—Sleep disorders (difficulty sleeping > 6 h or takes sleep pilhs) | Sleep | ||||
| 13—Obesity (BMI > 30) or underweight (BMI < 18.5) | Obesity | 30—Haemoglobin (<13.5 g/dL in males, <12.0 g/dL in females) | HB | ||||
| 14—Gastric disorder | Gastric | 31—HCT < 26% | HCT | ||||
| 15—Intestinal disorder | Bowel | 32—Creatinine (<0.6 mg/dL) | Creatinine | ||||
| 16—Diabetes | DM | 33—Albumin (<3.5 g/dL) | Albumin | ||||
| 17—Chronic kidney disease | Renal | 34—White blood cells (<4 × 103/mm3) | WBC | ||||
| Total | /34 | ||||||
| Variables | Median (IQR) | Count (N) | N% | Variables | Median (IQR) | Count (N) | N% | |
| Age | Clavien-Dindo Classification Grade | No complication | 82 | 49.70% | ||||
| Overall population Age | 63 (52–72) | Grade 1 | 13 | 7.88% | ||||
| 18–64 | 87 | 52.41% | Grade 2 | 61 | 36.97% | |||
| 65–79 | 60 | 36.14% | Grade 3 | 5 | 3.03% | |||
| >80 | 18 | 10.84% | Grade 4 | 1 | 0.61% | |||
| Gender | Grade 5 | 3 | 1.82% | |||||
| Female | 116 | 70.30% | Length of stay (LOS) | 11 (8–16) | ||||
| Male | 49 | 29.70% | ICU discharge > 24 h | 14 | 8.48% | |||
| Anatomical location | Post-operative KPS | 90 (80–90) | ||||||
| Convexity | 61/165 | 37.58% | Unfavorable out–ome—Post-operative | 22 | 13.33% | |||
| Parasagittal | 6/165 | 3.64% | Operation time (min) | 216 (155–310) | ||||
| Falx | 19/165 | 11.52% | ICH | 36 | 21.82% | |||
| Tentorium | 3/165 | 1.82% | Seizure | 7 | 4.24% | |||
| Cerebellar convexity | 2/165 | 1.21% | Infections | 8 | 4.85% | |||
| CPA | 9/165 | 5.45% | Pulmonary embolism | 43 | 26.06% | |||
| Sphenoid wing | 9/165 | 5.45% | Post-operative tumor volume (mL) | 0.41 (2,12) | ||||
| Tuberculum/Dorsum sellae/Planum/Clinoid | 10/165 | 6.06% | Gross total resection (GTR) | 128 | 81.01% | |||
| Middle Fossa | 16/165 | 9.70% | KPS at follow-up | 90 (80–90) | ||||
| Olfactory Groove | 20/165 | 12.12% | Unfavourable outcome at FU | 28 | 16.97% | |||
| Clival/Petroclival | 8/165 | 4.85% | Mortality: | |||||
| Foramen Magnum | 1/165 | 0.61% | 30-day mortality | 3 | 1.82% | |||
| Intraventricular | 1/165 | 0.61% | 6-month mortality | 5 | 3.03% | |||
| WHO grade | 1-year mortality | 5 | 3.03% | |||||
| I | 126 | 79.25% | 3-year mortality | 8 | 4.85% | |||
| II | 31 | 19.50% | 5-year mortality | 9 | 5.45% | |||
| III | 2 | 1.26% | Functional and patient-reported assessment (FACT-Br): | |||||
| KI67 > 4% | 43 | 26.06% | PWB | 26 (22–28) | ||||
| Side (Hemisphere) | SWB | 20,57 (18–20,57) | ||||||
| Left | 88 | 53.33% | EWB | 23 (19–24) | ||||
| Right | 71 | 43.03% | FWB | 22 (18–27) | ||||
| Midline/Bilateral | 6 | 3.64% | BrCS | 81.65 (75.27–85.79) | ||||
| Surgical parameters | Overall quality of life (QoL) | 169.57 (157.93–183.07) | ||||||
| Skull base location | 61 | 36.97% | Biological/Functional assessment | |||||
| Infratentorial location | 20 | 12.12% | ASA | 1 or 2 | 131 | 79.39% | ||
| Max diameter | 1.86 (0.57–2.94) | 3 or 4 | 34 | 20.61% | ||||
| Diameter > 25 mm | 54 | 32.73% | Pre-operative KPS | 90 (80–90) | ||||
| Preoperative tumour volume (mL) | 27.93 (8.54–44.10) | |||||||
| Frailty index (FI) | 0.16 (0.06–0.18) | |||||||
| FI profiles | Fit | 69 | 41.82% | |||||
| Semi-Fit | 77 | 46.67% | ||||||
| Frail | 19 | 11.52% | ||||||
| KPS Postop—20 Drop | KPS FU—PRE ≤ 20 + QOL < 183 | ||||||||
| Univariable | Multivariable | Univariable | Multivariable | ||||||
| Parameters | OR (95% C.I.) | p Value | OR (95% C.I.) | p Value | OR (95% C.I.) | p Value | OR (95% C.I.) | p Value | |
| Demographics | Age > 65 | 5.890 (0.673–51.568) | 0.109 | 2.310 (0.994–5.369) | 0.002 ** | 0.420 (0.127–1.392) | 0.156 | ||
| Age > 70 | 4.235 (0.751–23.880) | 0.102 | 1.960 (0.858–4.481) | 0.111 | |||||
| Age > 80 | 9.600 (1.778–21.835) | 0.009 ** | 0.720 (0.038–13.569) | 0.827 | 2.841 (0.965–5.363) | 0.002 ** | 0.956 (0.212–4.312) | 0.953 | |
| Previous surgery | 1.390 (0.154–12.515) | 0.769 | 2.218 (0.776–6.339) | 0.137 | |||||
| Clinical and Functional | ASA Score | 22.414 (2.523–39.139) | 0.005 ** | 5.553 (1.760–7.642) | 0.023 * | 2.616 (1.074–5.368) | 0.034 * | 2.620 (0.815–8.429) | 0.106 |
| KPS pre-op | 0.682 (0.539–0.862) | 0.001 ** | // | 0.923 (0.863–0.988) | 0.021 * | // | |||
| KPS pre-op < 80 | 15.800 (11.523–19.498) | <0.001 *** | 1.46 (0.017–7.494) | 0.98 | 16.320 (1.631–26.269) | 0.017 * | 4.824 (0.274–85.050) | 0.282 | |
| Frailty Index (FI) | 4.100 (1.595–10.538) | 0.003 ** | // | 3.107 (1.744–5.534) | <0.001 *** | // | |||
| FI > 0.10 (Semi-Fit) | 0.560 (0.100–3.145) | 0.510 | 1.983 (0.865–4.549) | 0.016 * | 12.479 (2.764–16.349) | 0.001 ** | |||
| FI > 0.20 (Frail) | 8.937 (1.663–28.032) | 0.011 * | 14.752 (1.463–148.777) | 0.022 * | 4.582 (1.643–8.778) | 0.004 ** | 35.457 (25.210–41.318) | <0.001 *** | |
| Surgical | Skull base location | 9.196 (1.048–20.669) | 0.045 * | 4.232 (0.280–63.975) | 0.050 * | 1.607 (0.707–3.654) | 0.002 ** | 0.821 (0.228–2.961) | 0.763 |
| Infratentorial location | 3.917 (0.669–22.922) | 0.013 * | 6.079 (1.573–9.282) | 0.028 * | 4.167 (1.515–9.457) | 0.006 ** | 7.514 (1.514–37.280) | 0.014 * | |
| Diameter > 2.5 cm | 11.224 (1.277–18.625) | 0.029 * | 16.078 (0.939–27.310) | 0.050 * | 2.899 (1.264–6.651) | 0.012 * | 4.983 (1.720–14.440) | 0.003 ** | |
| Diameter > 3 cm | 3.543 (0.685–18.331) | 0.013 * | 4.363 × 106 (0.000—//) | 0.989 | 1.764 (0.722–4.308) | 0.213 | |||
| Diameter > 4 cm | 6.950 (1.312–16.828) | 0.023 * | 2.754 × 109 (0.000—//) | 0.998 | 1.925 (0.683–5.424) | 0.215 | |||
| Radiological | Calcification | 1.051 (0.186–5.935) | 0.955 | 1.206 (0.512–2.841) | 0.668 | ||||
| Severe peritumoral edema | 1.714 (0.335–8.784) | 0.518 | 3.238 (1.394–7.522) | 0.006 ** | 4.162 (1.299–13.331) | 0.016 * | |||
| Necrosis | 2.177e^–8 (0.000—//) | 0.995 | 1.059 (0.419–2.677) | 0.904 | |||||
| Hyperostosis | 1.527 (0.269–8.678) | 0.633 | 1.000 (0.389 2.571) | 0.997 | |||||
| Heterogeneous Gd enhancement | 2.167 (0.350–3.406) | 0.004 ** | 2.251 (0.348–14.570) | 0.394 | 1.232 (0.508–2.990) | 0.04 * | 0.850 (0.281–2.567) | 0.773 | |
| Sinus invasion | 1.935 (0.339–11.057) | 0.004 ** | 2.064 (0.313–13.603) | 0.451 | 2.560 (1.046–6.265) | 0.039 * | 4.458 (1.392–14.279) | 0.012 * | |
| Tumor shape (Multilobated > 2) | 1.679 (0.298–9.449) | 0.557 | 1.500 (0.638–3.526) | 0.353 | |||||
| DWI hyperintensity | 1.829 (0.248–13.470) | 0.553 | 2.303 (0.887–5.981) | 0.037 * | 3.208 (1.040–9.891) | 0.042 * | |||
| Absence of a Tumor-Brain cleft | 4.567 (0.771–7.056) | 0.044 ** | 5.910 (0.880–39.675) | 0.047 * | 2.138 (0.687–6.650) | 0.001 ** | 4.350 (1.006–18.818) | 0.049 * | |
| Nagelkerke R²: 0.560 AIC: 40.695 | Nagelkerke R²: 0.347 AIC: 129.927 | ||||||||
| Milan Biometric Surgical Score for Intracranial Meningiomas (MBSS-Men Score; Part A) | ||||||||
| ITEM | MEASURE | SCORE VALUE | ||||||
| ASA Score | 1–2 | 0 | ||||||
| >2 | 3 | |||||||
| Frailty Index | <0.10 | 0 | Multivariate regression analysis | Odd Ratio (OD) | Standard Error (S.E.) | p Value | 95% C.I. | |
| 0.10–0.20 | 2 | |||||||
| >0.20 | 3 | Post-operative prognostic Score | MBSS (Part A) | 2.611 | 0.293 | 0.001 | 1.469–4.640 | |
| Skull base location | Yes | 1 | Constant | 0 | 3.056 | 0 | ||
| No | 0 | |||||||
| Infratentorial location | Yes | 3 | AUR-ROC analysis | Area (AUC) | Std. Error (S.E.) | p Value | 95% C.I. | |
| No | 0 | |||||||
| Diameter > 2.5 cm | >25 mm | 3 | Overall population | 0.956 | 0.034 | 0 | 0.890–1.022 | |
| <25 mm | 0 | Age 18–65 years | 0.878 | 0.042 | 0 | 0.794–0.961 | ||
| Tumor-Brain cleft on T2WI | Absent | 2 | Age > 65 years | 0.981 | 0.017 | 0 | 0.948–1.013 | |
| Present | 0 | Age > 70 years | 0.973 | 0.024 | 0 | 0.926–1.020 | ||
| RANGE | 0–15 | Age > 80 years | 0.911 | 0.074 | 0 | 0.765–1.057 | ||
| Milan Biometric Surgical Score for intracranial meningiomas (MBSS-Men Score; Part B) | ||||||||
| Frailty Index | <0.10 | 0 | ||||||
| 0.10–0.20 | 1 | |||||||
| >0.20 | 3 | |||||||
| Infratentorial location | Yes | 2 | ||||||
| No | 0 | Multivariate regression analysis | Odd Ratio (OD) | Standard Error (S.E.) | p Value | 95% C.I. | ||
| Diameter > 2.5 cm | >25 mm | 1 | ||||||
| <25 mm | 0 | Follow-up prognostic Score | MBSS (Part B) | 2.961 | 0.203 | 0 | 1.988–4.411 | |
| Severe peritumoral edema | Yes | 1 | Constant | 0.004 | 0.876 | 0 | ||
| No | 0 | |||||||
| Sinus Invasion | Yes | 1 | AUR-ROC analysis | Area (AUC) | Std. Error (S.E.) | p Value | 95% C.I. | |
| No | 0 | |||||||
| DWI hyperintensity | Present | 1 | Overall population | 0.877 | 0.033 | 0 | 0.811–0.942 | |
| Absent | 0 | Age 18–65 years | 0.901 | 0.04 | 0 | 0.823–0.978 | ||
| Tumor-Brain cleft in T2WI | Absent | 1 | Age > 65 years | 0.854 | 0.054 | 0 | 0.749–0.959 | |
| Present | 0 | Age > 70 years | 0.85 | 0.055 | 0 | 0.741–0.959 | ||
| RANGE | 0–10 | Age > 80 years | 0.861 | 0.088 | 0 | 0.689–1.033 | ||
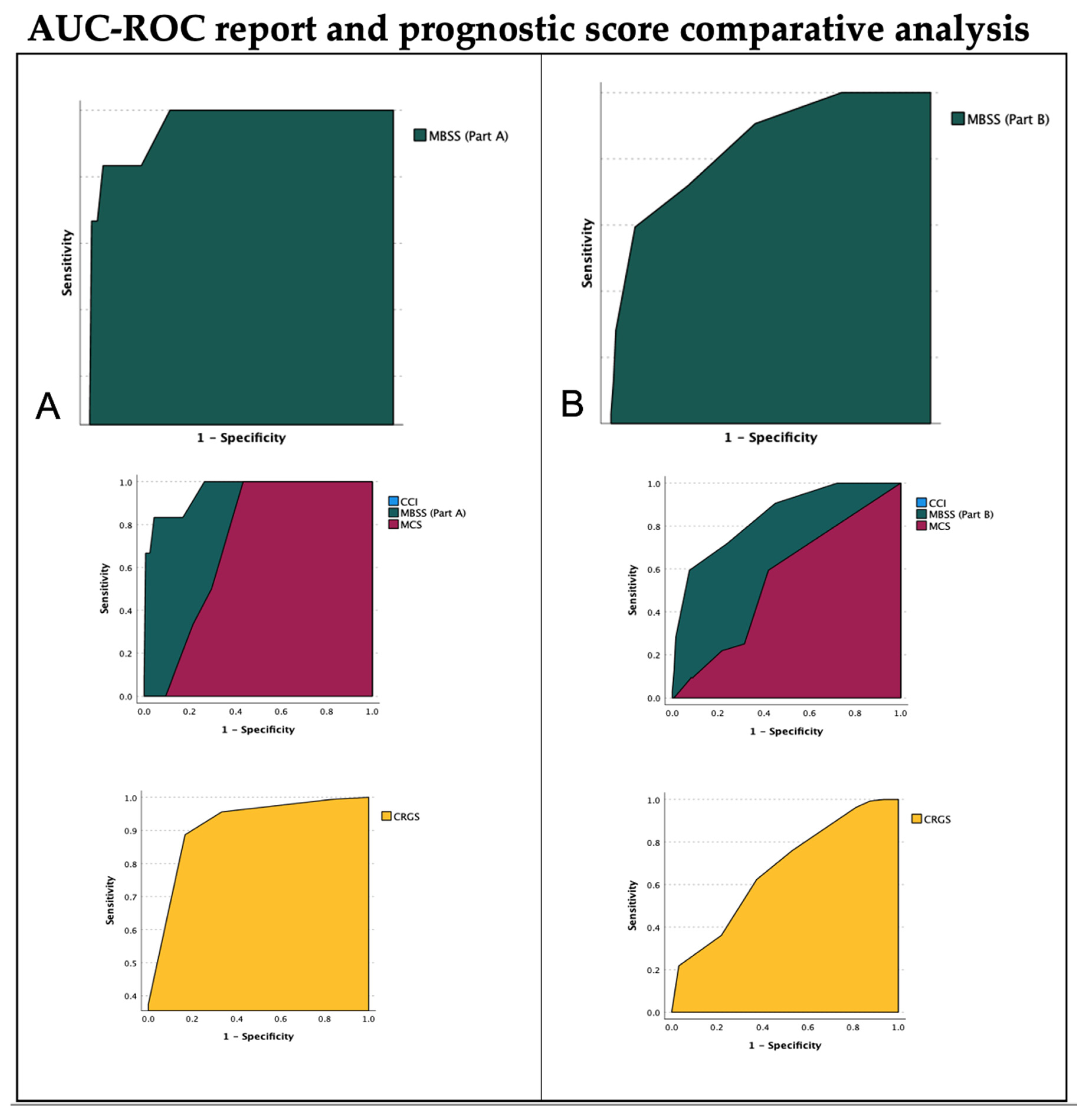
| Post-Operative Outcome Scores Comparison | ||||
| Area (AUC) | Std. Error (S.E.) | p Value | 95% C.I. | |
| MBSS (Part A) | 0.956 | 0.034 | 0.0001 | 0.890–1.022 |
| MCS | 0.724 | 0.051 | 0.0001 | 0.623–0.825 |
| CRGS * | 0.943 | 0.030 | 0.0001 | 0.885–1.000 |
| CCI | 0.551 | 0.096 | 0.594 | 0.363–0.740 |
| FI | 0.752 | 0.129 | 0.005 | 0.500–0.972 |
| Follow-up Outcome Score comparison | ||||
| Area (AUC) | Std. Error (S.E.) | p Value | 95% C.I. | |
| MBSS (Part B) | 0.877 | 0.033 | 0.0001 | 0.811–0.942 |
| MCS | 0.553 | 0.054 | 0.328 | 0.447–0.659 |
| CRGS * | 0.671 | 0.053 | 0.001 | 0.566–0.775 |
| CCI | 0.598 | 0.049 | 0.046 | 0.502–0.695 |
| FI | 0.729 | 0.054 | 0.0001 | 0.623–0.834 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariciotti, L.; Fiore, G.; Carapella, S.; Remore, L.G.; Schisano, L.; Borsa, S.; Pluderi, M.; Canevelli, M.; Marfia, G.; Caroli, M.; et al. A Frailty-Adjusted Stratification Score to Predict Surgical Risk, Post-Operative, Long-Term Functional Outcome, and Quality of Life after Surgery in Intracranial Meningiomas. Cancers 2022, 14, 3065. https://doi.org/10.3390/cancers14133065
Tariciotti L, Fiore G, Carapella S, Remore LG, Schisano L, Borsa S, Pluderi M, Canevelli M, Marfia G, Caroli M, et al. A Frailty-Adjusted Stratification Score to Predict Surgical Risk, Post-Operative, Long-Term Functional Outcome, and Quality of Life after Surgery in Intracranial Meningiomas. Cancers. 2022; 14(13):3065. https://doi.org/10.3390/cancers14133065
Chicago/Turabian StyleTariciotti, Leonardo, Giorgio Fiore, Sara Carapella, Luigi Gianmaria Remore, Luigi Schisano, Stefano Borsa, Mauro Pluderi, Marco Canevelli, Giovanni Marfia, Manuela Caroli, and et al. 2022. "A Frailty-Adjusted Stratification Score to Predict Surgical Risk, Post-Operative, Long-Term Functional Outcome, and Quality of Life after Surgery in Intracranial Meningiomas" Cancers 14, no. 13: 3065. https://doi.org/10.3390/cancers14133065
APA StyleTariciotti, L., Fiore, G., Carapella, S., Remore, L. G., Schisano, L., Borsa, S., Pluderi, M., Canevelli, M., Marfia, G., Caroli, M., Locatelli, M., & Bertani, G. (2022). A Frailty-Adjusted Stratification Score to Predict Surgical Risk, Post-Operative, Long-Term Functional Outcome, and Quality of Life after Surgery in Intracranial Meningiomas. Cancers, 14(13), 3065. https://doi.org/10.3390/cancers14133065






