Association of Allostatic Load and All Cancer Risk in the SWAN Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Population
2.2. AL Score Construction
3. Statistical Analysis
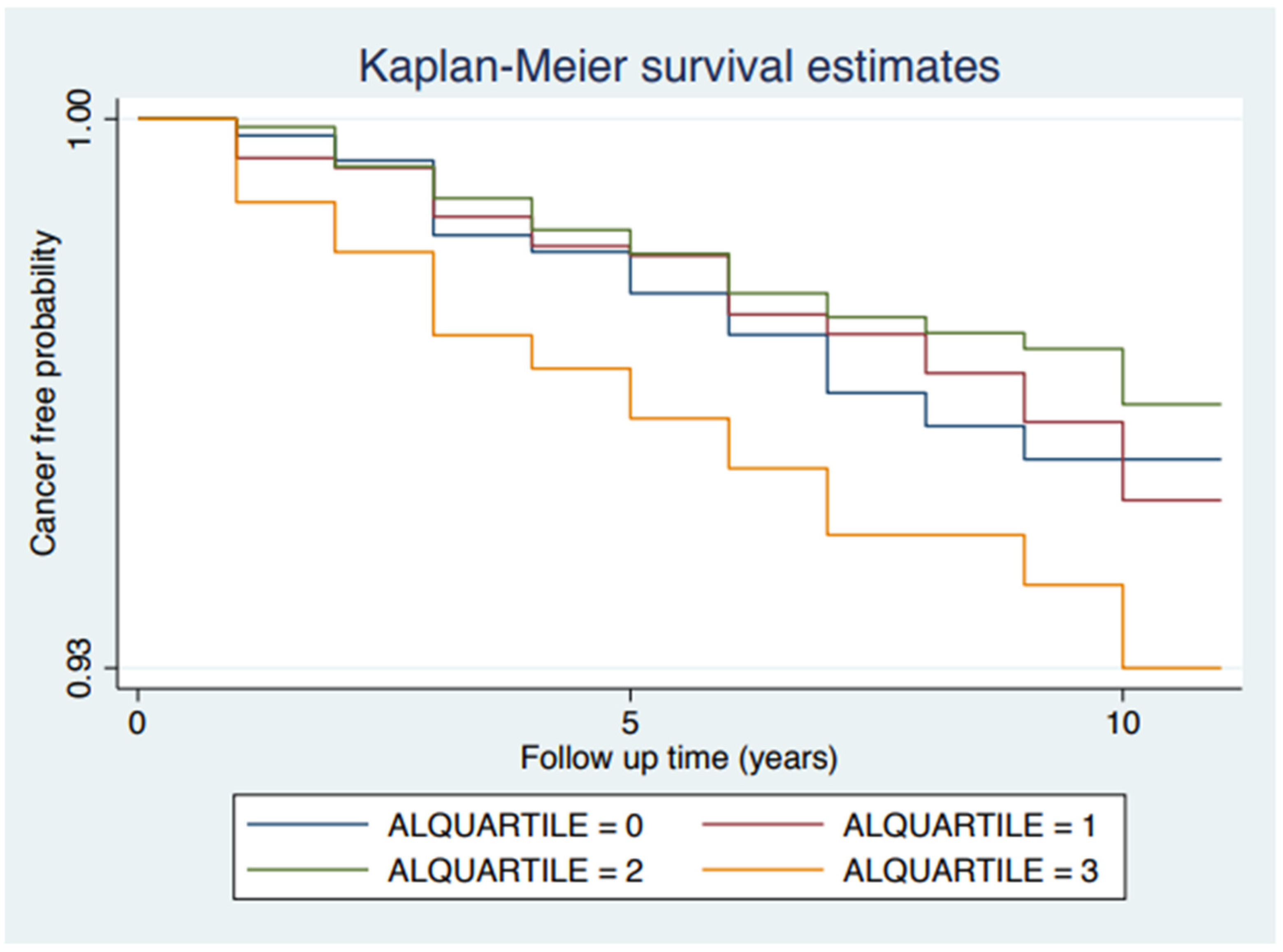
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Ranabir, S.; Reetu, K. Stress and hormones. Indian J. Endocrinol. Metab. 2011, 15, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez, E.J.; Gregorich, S.E.; Livaudais-Toman, J.; Perez-Stable, E.J. Coping With Chronic Stress by Unhealthy Behaviors: A Re-Evaluation Among Older Adults by Race/Ethnicity. J. Aging Health 2017, 29, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Freisling, H.; Viallon, V.; Lennon, H.; Bagnardi, V.; Ricci, C.; Butterworth, A.S.; Sweeting, M.; Muller, D.; Romieu, I.; Bazelle, P.; et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: A multinational cohort study. BMC Med. 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Duijts, S.F.; Zeegers, M.P.; Borne, B.V. The association between stressful life events and breast cancer risk: A meta-analysis. Int. J. Cancer 2003, 107, 1023–1029. [Google Scholar] [CrossRef]
- Svensson, T.; Inoue, M.; Sawada, N.; Charvat, H.; Iwasaki, M.; Sasazuki, S.; Shimazu, T.; Yamaji, T.; Kawamura, N.; Shibuya, K.; et al. Coping strategies and cancer incidence and mortality: The Japan Public Health Center-based prospective study. Cancer Epidemiol. 2016, 40, 126–133. [Google Scholar] [CrossRef]
- Sawada, T.; Nishiyama, T.; Kikuchi, N.; Wang, C.; Lin, Y.; Mori, M.; Tanno, K.; Tamakoshi, A.; Kikuchi, S. The influence of personality and perceived stress on the development of breast cancer: 20-year follow-up of 29,098 Japanese women. Sci. Rep. 2016, 6, 32559. [Google Scholar] [CrossRef]
- Nielsen, N.R.; Strandberg-Larsen, K.; Gronbaek, M.; Kristensen, T.S.; Schnohr, P.; Zhang, Z.F. Self-reported stress and risk of endometrial cancer: A prospective cohort study. Psychosom. Med. 2007, 69, 383–389. [Google Scholar] [CrossRef]
- Coyne, J.C.; Ranchor, A.V.; Palmer, S.C. Meta-analysis of stress-related factors in cancer. Nat. Rev. Clin. Oncol. 2010, 7, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Saito, E.; Sawada, N.; Abe, S.K.; Hidaka, A.; Shimazu, T.; Yamaji, T.; Goto, A.; Iwasaki, M.; Sasazuki, S.; et al. Perceived stress level and risk of cancer incidence in a Japanese population: The Japan Public Health Center (JPHC)-based Prospective Study. Sci. Rep. 2017, 7, 12964. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.J.; Fernandez Poole, S.; White, M.; Lyn, R.; Flores, D.A.; Haile, H.G.; Williams, D.R. The Role of Stress in Breast Cancer Incidence: Risk Factors, Interventions, and Directions for the Future. Int. J. Environ. Res. Public Health 2021, 18, 1871. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.; Epel, E.; Gruenewald, T.; Karlamangla, A.; McEwen, B.S. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann. N. Y. Acad. Sci. 2010, 1186, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.Y.; Doose, M.; Qin, B.; Lin, Y.; Plascak, J.J.; Omene, C.; He, C.; Demissie, K.; Hong, C.C.; Bandera, E.V.; et al. Prediagnostic Allostatic Load as a Predictor of Poorly Differentiated and Larger Sized Breast Cancers among Black Women in the Women’s Circle of Health Follow-Up Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 216–224. [Google Scholar] [CrossRef]
- Zhao, H.; Song, R.; Ye, Y.; Chow, W.H.; Shen, J. Allostatic score and its associations with demographics, healthy behaviors, tumor characteristics, and mitochondrial DNA among breast cancer patients. Breast Cancer Res. Treat. 2021, 187, 587–596. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Wilson, L.E.; Deveaux, A.; Aslibekyan, S.; Cushman, M.; Gilchrist, S.; Safford, M.; Judd, S.; Howard, V. Association of Allostatic Load with All-Cause andCancer Mortality by Race and Body Mass Index in theREGARDS Cohort. Cancers 2020, 12, 1695. [Google Scholar] [CrossRef]
- Mathew, A.; Doorenbos, A.Z.; Li, H.; Jang, M.K.; Park, C.G.; Bronas, U.G. Allostatic Load in Cancer: A Systematic Review and Mini Meta-Analysis. Biol. Res. Nurs. 2021, 23, 341–361. [Google Scholar] [CrossRef]
- Howard, J.T.; Sparks, P.J. Does allostatic load calculation method matter? Evaluation of different methods and individual biomarkers functioning by race/ethnicity and educational level. Am. J. Hum. Biol. 2016, 28, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Sutton-Tyrrell, K. The SWAN song: Study of Women’s Health Across the Nation’s recurring themes. Obstet. Gynecol. Clin. N. Am. 2011, 38, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chyu, L.; Upchurch, D.M. A Longitudinal Analysis of Allostatic Load among a Multi-Ethnic Sample of Midlife Women: Findings from the Study of Women’s Health Across the Nation. Womens Health Issues 2018, 28, 258–266. [Google Scholar] [CrossRef]
- Oken, B.S.; Chamine, I.; Wakeland, W. A systems approach to stress, stressors and resilience in humans. Behav. Brain Res. 2015, 282, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Dowd, J.B.; Simanek, A.M.; Aiello, A.E. Socio-economic status, cortisol and allostatic load: A review of the literature. Int. J. Epidemiol. 2009, 38, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Flint, M.S.; Bovbjerg, D.H. DNA damage as a result of psychological stress: Implications for breast cancer. Breast Cancer Res. 2012, 14, 320. [Google Scholar] [CrossRef]
- Lund, M.; Melbye, M.; Diaz, L.J.; Duno, M.; Wohlfahrt, J.; Vissing, J. Mitochondrial dysfunction and risk of cancer. Br. J. Cancer 2015, 112, 1134–1140. [Google Scholar] [CrossRef]
- Borena, W.; Stocks, T.; Jonsson, H.; Strohmaier, S.; Nagel, G.; Bjorge, T.; Manjer, J.; Hallmans, G.; Selmer, R.; Almquist, M.; et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control 2011, 22, 291–299. [Google Scholar] [CrossRef]
- Trabert, B.; Hathaway, C.A.; Rice, M.S.; Rimm, E.B.; Sluss, P.M.; Terry, K.L.; Zeleznik, O.A.; Tworoger, S.S. Ovarian Cancer Risk in Relation to Blood Cholesterol and Triglycerides. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2044–2051. [Google Scholar] [CrossRef]
- Allin, K.H.; Nordestgaard, B.G. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit. Rev. Clin. Lab. Sci. 2011, 48, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hernandez, D.; McNeill, L.H.; Chow, W.H.; Zhao, H. Associations of serum CRP levels with demographics, health behaviors, and risk of cancer among the Mexican American Mano A Mano Cohort. Cancer Epidemiol. 2019, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peek, M.K.; Cutchin, M.P.; Salinas, J.J.; Sheffield, K.M.; Eschbach, K.; Stowe, R.P.; Goodwin, J.S. Allostatic load among non-Hispanic Whites, non-Hispanic Blacks, and people of Mexican origin: Effects of ethnicity, nativity, and acculturation. Am. J. Public Health 2010, 100, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M.; Kim, J.K.; Seeman, T.E. Poverty and biological risk: The earlier “aging” of the poor. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 286–292. [Google Scholar] [CrossRef]
- Robertson, T.; Benzeval, M.; Whitley, E.; Popham, F. The role of material, psychosocial and behavioral factors in mediating the association between socioeconomic position and allostatic load (measured by cardiovascular, metabolic and inflammatory markers). Brain Behav. Immun. 2015, 45, 41–49. [Google Scholar] [CrossRef]
- Moffatt, R.J. Effects of cessation of smoking on serum lipids and high density lipoprotein-cholesterol. Atherosclerosis 1988, 74, 85–89. [Google Scholar] [CrossRef]
- Tonstad, S.; Cowan, J.L. C-reactive protein as a predictor of disease in smokers and former smokers: A review. Int. J. Clin. Pract. 2009, 63, 1634–1641. [Google Scholar] [CrossRef]
- Will, J.C.; Galuska, D.A.; Ford, E.S.; Mokdad, A.; Calle, E.E. Cigarette smoking and diabetes mellitus: Evidence of a positive association from a large prospective cohort study. Int. J. Epidemiol. 2001, 30, 540–546. [Google Scholar] [CrossRef]
- Gay, J.L.; Salinas, J.J.; Buchner, D.M.; Mirza, S.; Kohl, H.W., 3rd; Fisher-Hoch, S.P.; McCormick, J.B. Meeting physical activity guidelines is associated with lower allostatic load and inflammation in Mexican Americans. J. Immigr. Minor. Health 2015, 17, 574–581. [Google Scholar] [CrossRef]
- Hampson, S.E.; Goldberg, L.R.; Vogt, T.M.; Hillier, T.A.; Dubanoski, J.P. Using physiological dysregulation to assess global health status: Associations with self-rated health and health behaviors. J. Health Psychol. 2009, 14, 232–241. [Google Scholar] [CrossRef][Green Version]
- Hu, P.; Wagle, N.; Goldman, N.; Weinstein, M.; Seeman, T.E. The associations between socioeconomic status, allostatic load and measures of health in older Taiwanese persons: Taiwan social environment and biomarkers of aging study. J. Biosoc. Sci. 2007, 39, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y.; Crews, D.E.; Iwamoto, A.; Sone, Y.; Aoyagi, K.; Maeda, T.; Leahy, R. Allostatic load differs by sex and diet, but not age in older Japanese from the Goto Islands. Ann. Hum. Biol. 2016, 43, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, D.M.; Rainisch, B.W.; Chyu, L. Greater Leisure Time Physical Activity Is Associated with Lower Allostatic Load in White, Black, and Mexican American Midlife Women: Findings from the National Health and Nutrition Examination Survey, 1999 through 2004. Womens Health Issues 2015, 25, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.H.; Casement, M.D.; Troxel, W.M.; Matthews, K.A.; Bromberger, J.T.; Kravitz, H.M.; Krafty, R.T.; Buysse, D.J. Chronic Stress is Prospectively Associated with Sleep in Midlife Women: The SWAN Sleep Study. Sleep 2015, 38, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; Costanzo, S.; Bagnardi, V.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Alcohol dosing and total mortality in men and women: An updated meta-analysis of 34 prospective studies. Arch. Intern. Med. 2006, 166, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.C.; Jimenez, J.A.; Shivpuri, S.; Espinosa de los Monteros, K.; Mills, P.J. Domains of chronic stress, lifestyle factors, and allostatic load in middle-aged Mexican-American women. Ann. Behav. Med. 2011, 41, 21–31. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bybee, K.A.; Lavie, C.J. Alcohol and cardiovascular health: The razor-sharp double-edged sword. J. Am. Coll. Cardiol. 2007, 50, 1009–1014. [Google Scholar] [CrossRef]
- Forrester, S.N.; Leoutsakos, J.M.; Gallo, J.J.; Thorpe, R.J., Jr.; Seeman, T.E. Association between allostatic load and health behaviours: A latent class approach. J. Epidemiol. Community Health 2019, 73, 340–345. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Avogaro, A.; Barba, G.; Bellentani, S.; Bucci, M.; Cambieri, R.; Catapano, A.L.; Costanzo, S.; Cricelli, C.; et al. Moderate alcohol use and health: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 487–504. [Google Scholar] [CrossRef]
- McCrory, C.; Fiorito, G.; Ni Cheallaigh, C.; Polidoro, S.; Karisola, P.; Alenius, H.; Layte, R.; Seeman, T.; Vineis, P.; Kenny, R.A. How does socio-economic position (SEP) get biologically embedded? A comparison of allostatic load and the epigenetic clock(s). Psychoneuroendocrinology 2019, 104, 64–73. [Google Scholar] [CrossRef]
- Seeman, T.E.; Singer, B.H.; Rowe, J.W.; Horwitz, R.I.; McEwen, B.S. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Arch. Intern. Med. 1997, 157, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Parente, V.; Hale, L.; Palermo, T. Association between breast cancer and allostatic load by race: National Health and Nutrition Examination Survey 1999–2008. Psychooncology 2013, 22, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.J.; Mentz, G.; Lachance, L.; Johnson, J.; Gaines, C.; Israel, B.A. Associations between socioeconomic status and allostatic load: Effects of neighborhood poverty and tests of mediating pathways. Am. J. Public Health 2012, 102, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Geronimus, A.T.; Hicken, M.; Keene, D.; Bound, J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 2006, 96, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Bingham, B.A.; Aldana, P.C.; Chung, S.T.; Sumner, A.E. Variation in the Calculation of Allostatic Load Score: 21 Examples from NHANES. J. Racial Ethn. Health Disparities 2017, 4, 455–461. [Google Scholar] [CrossRef]
- Karlamangla, A.S.; Singer, B.H.; Seeman, T.E. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosom. Med. 2006, 68, 500–507. [Google Scholar] [CrossRef]
- Sabbah, W.; Tsakos, G.; Sheiham, A.; Watt, R.G. The effects of income and education on ethnic differences in oral health: A study in US adults. J. Epidemiol. Community Health 2009, 63, 516–520. [Google Scholar] [CrossRef]
| Biomarkers | Mean (SD) | Cutoff Value | N (%) at Risk |
|---|---|---|---|
| SBP (mm Hg) | 118.23 (17.24) | ≥140 | 377 (11.45) |
| DBP (mm Hg) | 75.57 (10.80) | ≥90 | 394 (11.98) |
| HDL (mg/dL) | 55.90 (14.56) | <50 | 1173 (35.77) |
| LDL (mg/dL) | 116.086 (31.01) | >130 | 926 (30.06) |
| Total cholesterol (mg/dL) | 194.57 (34.89) | ≥240 | 343 (10.46) |
| Triglycerides (mg/dL) | 113.45 (84.63) | ≥150 | 592 (19.00) |
| Waist to hip ratio | 0.80 (0.07) | ≥0.85 | 798 (24.53) |
| Glucose level (mg/dL) | 98.08 (31.09) | ≥110 | 341 (10.93) |
| CRP (mg/L) | 3.90 (6.26) | >3 | 1116 (34.39) |
| DHAS (ug/dL) | 129.77 (78.95) | >240 | 301 (9.12) |
| History of medication to control metabolic diseases and hypertension | Yes | Yes | 129 (3.92) |
| AL Score | Number | Percentage |
|---|---|---|
| 0 | 888 | 29.45 |
| 1 | 754 | 25.01 |
| 2 | 555 | 18.41 |
| 3 | 375 | 12.44 |
| 4 | 227 | 7.53 |
| 5 | 118 | 3.91 |
| 6 | 66 | 2.19 |
| 7 | 22 | 0.73 |
| 8 | 9 | 0.3 |
| 9 | 1 | 0.03 |
| AL category | Number | Percentage |
| 1 (AL score = 0) | 888 | 29.45 |
| 2 (AL score = 1) | 754 | 25.01 |
| 3 (AL score = 2–3) | 930 | 30.85 |
| 4 (Al score = 4–9) | 443 | 14.65 |
| Category 1 | Category 2 | Category 3 | Category 4 | p-Value | |
|---|---|---|---|---|---|
| n = 888 | n = 754 | n = 930 | n = 443 | ||
| Age, Mean (SD) | 45.75 (2.67) | 45.68 (2.61) | 45.99 (2.70) | 46.12 (2.82) | 0.004 |
| Race/ethnicity, N (%) | |||||
| African American | 152 (17.12) | 206 (27.32) | 323 (34.73) | 173 (39.05) | |
| Chinese American | 90 (10.14) | 57 (7.56) | 56 (6.02) | 20 (4.51) | |
| Japanese American | 120 (13.51) | 76 (10.08) | 55 (5.91) | 11 (2.48) | |
| White | 492 (55.41) | 358 (47.48) | 392 (42.15) | 185 (41.76) | |
| Hispanic | 34 (3.83) | 57 (7.56) | 104 (11.18) | 54 (12.19) | <0.001 |
| Ever smoked regularly, N (%) | |||||
| No | 547 (62.30) | 448 (59.57) | 510 (55.14) | 214 (49.20) | |
| Yes | 331 (37.70) | 304 (40.43) | 415 (44.86) | 221 (50.80) | <0.001 |
| Ever drank alcohol regularly, N (%) | |||||
| No | 347 (39.08) | 334 (44.30) | 484 (52.04) | 254 (57.34) | |
| Yes | 541 (60.92) | 420 (55.70) | 446 (47.96) | 189 (42.66) | <0.001 |
| Leisure physical activity, N (%) | |||||
| No | 162 (18.39) | 174 (23.23) | 309 (33.77) | 164 (37.10) | |
| Yes | 719 (81.61) | 575 (76.77) | 606 (66.23) | 278 (62.90) | <0.001 |
| Self-rated sleep quality, N (%) | |||||
| Sound and restful | 373 (42.19) | 304 (40.37) | 331 (35.90) | 146 (32.96) | |
| Average | 364 (41.18) | 312 (41.43) | 369 (40.02) | 183 (41.31) | |
| Restless | 147 (16.63) | 137 (18.19) | 222 (24.08) | 114 (25.73) | <0.001 |
| Family total income, N (%) | |||||
| <20 k per year | 71 (8.21) | 83 (11.31) | 171 (19.02) | 110 (25.40) | |
| 20–50 k per year | 259 (29.94) | 245 (33.38) | 325 (36.15) | 157 (36.26) | |
| 50–100 k per year | 350 (40.46) | 288 (39.24) | 304 (33.82) | 135 (31.18) | |
| ≥100 k per year | 185 (21.39) | 118 (16.08) | 99 (11.01) | 31 (7.16) | <0.001 |
| Health insurance, N (%) | |||||
| No | 51 (5.76) | 51 (6.76) | 103 (11.08) | 40 (9.05) | |
| Yes | 835 (94.24) | 703 (93.24) | 827 (88.92) | 402 (90.95) | <0.001 |
| Cancer status, N (%) | |||||
| No | 847 (95.38) | 715 (94.83) | 894 (96.13) | 410 (92.55) | |
| Yes | 41 (4.62) | 39 (5.17) | 36 (3.87) | 33 (7.45) | 0.037 |
| AL Category | Unadjusted (HR, 95% CI) | Model 1 * (HR, 95% CI) | Model 2 # (HR, 95% CI) | Model 3 @ (HR, 95% CI) |
|---|---|---|---|---|
| 1 | reference | reference | reference | reference |
| 2 | 1.12 (0.72, 1.73) | 1.19 (0.77, 1.85) | 1.25 (0.80, 1.96) | 1.27 (0.82, 1.99) |
| 3 | 0.83 (0.53, 1.30) | 0.93 (059, 1.47) | 0.99 (0.62, 1.59) | 0.99 (0.61, 1.58) |
| 4 | 1.64 (1.04, 2.59) | 1.88 (1.17, 3.02) | 2.09 (1.29, 3.41) | 2.08 (1.26, 3.42) |
| P for trend | 0.224 | 0.072 | 0.031 | 0.040 |
| HR * (95% CI) | |
|---|---|
| Higher SBP | 1.07 (0.63, 1.82) |
| Higher DBP | 0.89 (0.52, 1.53) |
| Higher HDL | 1.33 (0.96, 1.85) |
| Higher total cholesterol | 1.38 (0.87, 2.20) |
| Higher triglycerides | 1.68 (1.16, 2.43) |
| Higher waist to hip ratio | 1.38 (0.95, 1.99) |
| Higher glucose level | 1.24 (0.74, 2.06) |
| Higher CRP | 1.42 (1.01, 2.01) |
| Higher DHAS | 0.75 (0.40, 1.39) |
| History of medication to control metabolic diseases and hypertension | 1.58 (0.79, 3.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Fuemmeler, B.F.; Guan, Y.; Zhao, H. Association of Allostatic Load and All Cancer Risk in the SWAN Cohort. Cancers 2022, 14, 3044. https://doi.org/10.3390/cancers14133044
Shen J, Fuemmeler BF, Guan Y, Zhao H. Association of Allostatic Load and All Cancer Risk in the SWAN Cohort. Cancers. 2022; 14(13):3044. https://doi.org/10.3390/cancers14133044
Chicago/Turabian StyleShen, Jie, Bernard F. Fuemmeler, Yufan Guan, and Hua Zhao. 2022. "Association of Allostatic Load and All Cancer Risk in the SWAN Cohort" Cancers 14, no. 13: 3044. https://doi.org/10.3390/cancers14133044
APA StyleShen, J., Fuemmeler, B. F., Guan, Y., & Zhao, H. (2022). Association of Allostatic Load and All Cancer Risk in the SWAN Cohort. Cancers, 14(13), 3044. https://doi.org/10.3390/cancers14133044





