Cosuppression of NF-κB and AICDA Overcomes Acquired EGFR-TKI Resistance in Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Viability Assay
2.2. Western Blot Assay
2.3. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.4. Lentivirus Production, Infection, and Knockdown of AICDA Genes
- shAICDA#1, 5′-CCGGACCACGAAAGAACTTTCAAAGCTCGAGCTTTGAAAGTTCTTTCGTGGTTTTTTTG-3′;
- shAICDA#2, 5′-CCGGCATTTCGTACTTTGGGACTTTCTCGAGAAAGTCCCAAAGTACGAAATGTTTTTG-3′;
- shAICDA#3, 5′-CCGGCATTTCGTACTTTGGGACTTTCTCGAGAAAGTCCCAAAGTACGAAATGTTTTTG-3′.
2.5. Immunohistochemical Staining and Analysis
2.6. Statistical Analysis
3. Results
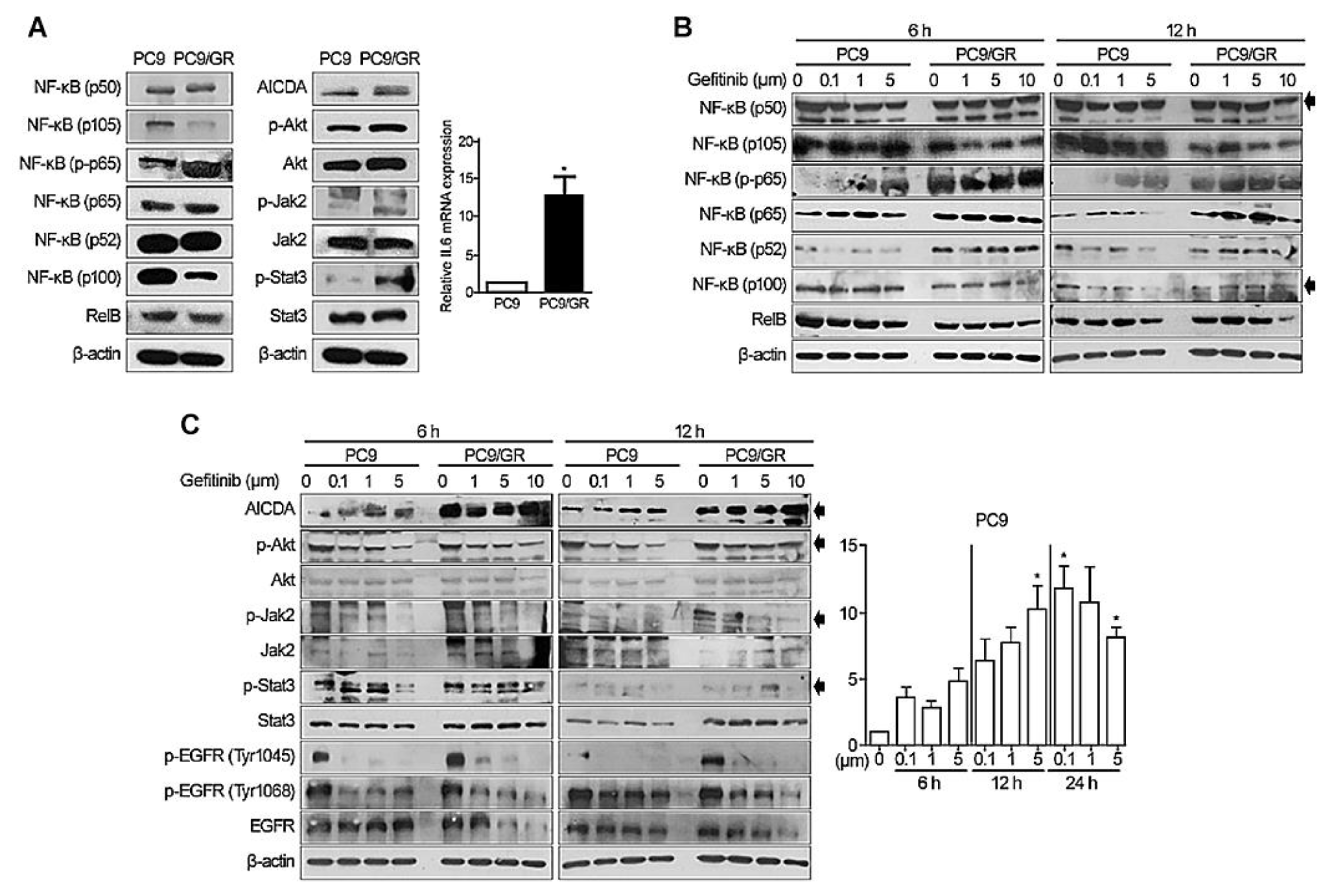
3.1. Expression of NF-κB, AICDA, Akt, JAK2/STAT3, and IL6 by EGFR-Mutated LACs
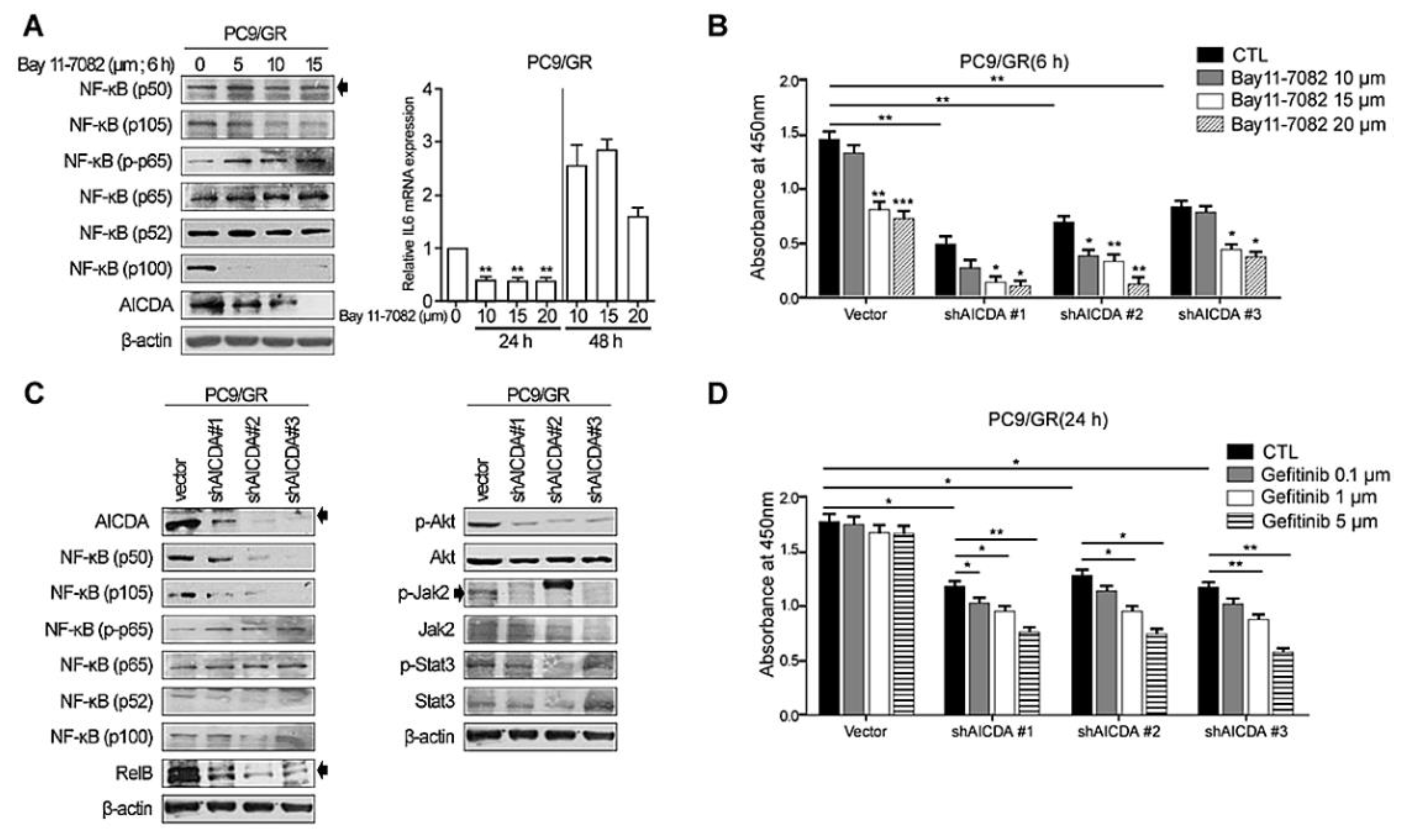
3.2. Knockdown of NF-κB and AICDA Restores the Sensitivity of TKI-Resistant Lung Cancer Cells to EGFR-TKI
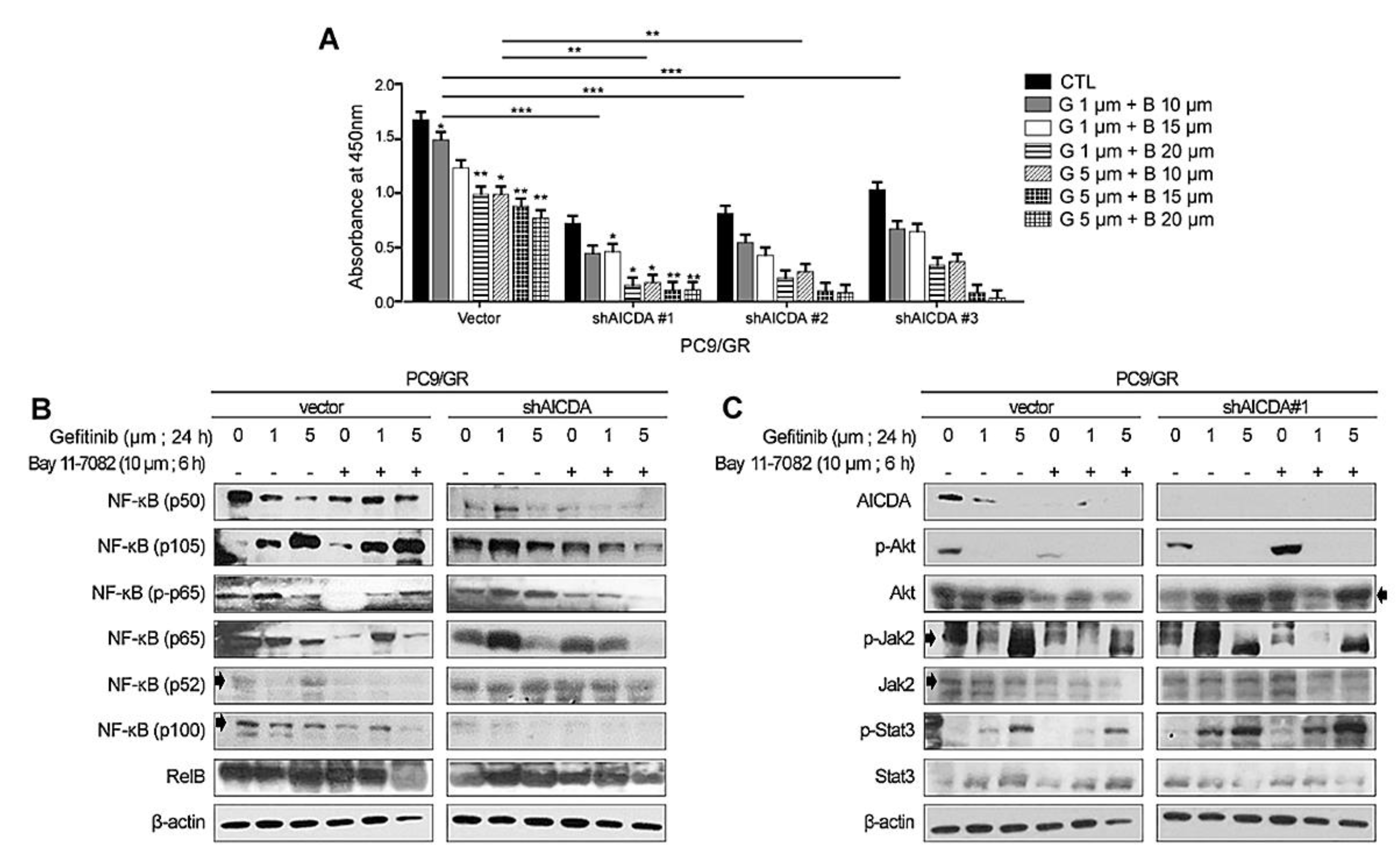
3.3. Therapeutic Effects of Combined Treatment with an EGFR-TKI Plus Cosuppression of NF-κB and AICDA
3.4. Immunohistochemical Analysis of Specimens Showing Initial and Acquired EGFR-TKI Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.; Yao, L.D. Strategies to Improve Outcomes of Patients with EGRF-Mutant Non-Small Cell Lung Cancer: Review of the Literature. J. Thorac. Oncol. 2016, 11, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Cortot, A.B.; Janne, P.A. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur. Respir. Rev. 2014, 23, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef]
- Sequist, L.V.; Martins, R.G.; Spigel, D.; Grunberg, S.M.; Spira, A.; Janne, P.A.; Joshi, V.A.; McCollum, D.; Evans, T.L.; Muzikansky, A.; et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J. Clin. Oncol. 2008, 26, 2442–2449. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Sun, J.M.; Ahn, M.J.; Choi, Y.L.; Ahn, J.S.; Park, K. Clinical implications of T790M mutation in patients with acquired resistance to EGFR tyrosine kinase inhibitors. Lung Cancer 2013, 82, 294–298. [Google Scholar] [CrossRef]
- Yun, C.H.; Mengwasser, K.E.; Toms, A.V.; Woo, M.S.; Greulich, H.; Wong, K.K.; Meyerson, M.; Eck, M.J. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl. Acad. Sci. USA 2008, 105, 2070–2075. [Google Scholar] [CrossRef]
- Hata, A.N.; Niederst, M.J.; Archibald, H.L.; Gomez-Caraballo, M.; Siddiqui, F.M.; Mulvey, H.E.; Maruvka, Y.E.; Ji, F.; Bhang, H.E.; Krishnamurthy Radhakrishna, V.; et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 2016, 22, 262–269. [Google Scholar] [CrossRef]
- Mehlman, C.; Cadranel, J.; Rousseau-Bussac, G.; Lacave, R.; Pujals, A.; Girard, N.; Callens, C.; Gounant, V.; Theou-Anton, N.; Friard, S.; et al. Resistance mechanisms to osimertinib in EGFR-mutated advanced non-small-cell lung cancer: A multicentric retrospective French study. Lung Cancer 2019, 137, 149–156. [Google Scholar] [CrossRef]
- Blakely, C.M.; Pazarentzos, E.; Olivas, V.; Asthana, S.; Yan, J.J.; Tan, I.; Hrustanovic, G.; Chan, E.; Lin, L.; Neel, D.S.; et al. NF-kappaB-activating complex engaged in response to EGFR oncogene inhibition drives tumor cell survival and residual disease in lung cancer. Cell Rep. 2015, 11, 98–110. [Google Scholar] [CrossRef]
- Fukuoka, M.; Yoshioka, K.; Hohjoh, H. NF-kappaB activation is an early event of changes in gene regulation for acquiring drug resistance in human adenocarcinoma PC-9 cells. PLoS ONE 2018, 13, e0201796. [Google Scholar] [CrossRef] [PubMed]
- El Kadi, N.; Wang, L.; Davis, A.; Korkaya, H.; Cooke, A.; Vadnala, V.; Brown, N.A.; Betz, B.L.; Cascalho, M.; Kalemkerian, G.P.; et al. The EGFR T790M Mutation Is Acquired through AICDA-Mediated Deamination of 5-Methylcytosine following TKI Treatment in Lung Cancer. Cancer Res. 2018, 78, 6728–6735. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, W.; Chang, H.; Han, Z.; Yu, X.; Zhang, T. Reciprocal regulation of miR-206 and IL-6/STAT3 pathway mediates IL6-induced gefitinib resistance in EGFR-mutant lung cancer cells. J. Cell Mol. Med. 2019, 23, 7331–7341. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.S.; Li, M.; Xu, C.X.; Wang, D. APE1 stimulates EGFR-TKI resistance by activating Akt signaling through a redox-dependent mechanism in lung adenocarcinoma. Cell Death Dis. 2018, 9, 1111. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, H.S.; Lee, D.; Yoo, G.; Kim, T.; Jeon, H.; Yeo, M.K.; Lee, C.S.; Moon, J.Y.; Jung, S.S.; et al. Hippo pathway effector YAP inhibition restores the sensitivity of EGFR-TKI in lung adenocarcinoma having primary or acquired EGFR-TKI resistance. Biochem. Biophys Res. Commun 2016, 474, 154–160. [Google Scholar] [CrossRef]
- Kang, D.H.; Jung, S.S.; Yeo, M.K.; Lee, D.H.; Yoo, G.; Cho, S.Y.; Oh, I.J.; Kim, J.O.; Park, H.S.; Chung, C.; et al. Suppression of Mig-6 overcomes the acquired EGFR-TKI resistance of lung adenocarcinoma. BMC Cancer 2020, 20, 571. [Google Scholar] [CrossRef]
- Deng, Q.F.; Su, B.O.; Zhao, Y.M.; Tang, L.; Zhang, J.; Zhou, C.C. Integrin beta1-mediated acquired gefitinib resistance in non-small cell lung cancer cells occurs via the phosphoinositide 3-kinase-dependent pathway. Oncol. Lett. 2016, 11, 535–542. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, J.; Gan, R.; Wu, Z.; Luo, H.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Synergistic inhibition of lung cancer cells by EGCG and NF-kappaB inhibitor BAY11-7082. J. Cancer 2019, 10, 6543–6556. [Google Scholar] [CrossRef]
- Kaewpiboon, C.; Srisuttee, R.; Malilas, W.; Moon, J.; Kaowinn, S.; Cho, I.R.; Johnston, R.N.; Assavalapsakul, W.; Chung, Y.H. Extract of Bryophyllum laetivirens reverses etoposide resistance in human lung A549 cancer cells by downregulation of NF-kappaB. Oncol. Rep. 2014, 31, 161–168. [Google Scholar] [CrossRef][Green Version]
- Jensen, K.; Krusenstjerna-Hafstrom, R.; Lohse, J.; Petersen, K.H.; Derand, H. A novel quantitative immunohistochemistry method for precise protein measurements directly in formalin-fixed, paraffin-embedded specimens: Analytical performance measuring HER2. Mod Pathol 2017, 30, 180–193. [Google Scholar] [CrossRef]
- He, J.; Huang, Z.; Han, L.; Gong, Y.; Xie, C. Mechanisms and management of 3rdgeneration EGFRTKI resistance in advanced nonsmall cell lung cancer (Review). Int. J. Oncol. 2021, 59, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rasmi, R.R.; Sakthivel, K.M.; Guruvayoorappan, C. NF-kappaB inhibitors in treatment and prevention of lung cancer. Biomed Pharm. 2020, 130, 110569. [Google Scholar] [CrossRef] [PubMed]
- Frances, A.; Cordelier, P. The Emerging Role of Cytidine Deaminase in Human Diseases: A New Opportunity for Therapy? Mol Ther. 2020, 28, 357–366. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, M.-K.; Kim, Y.; Lee, D.H.; Chung, C.; Bae, G.E. Cosuppression of NF-κB and AICDA Overcomes Acquired EGFR-TKI Resistance in Non-Small Cell Lung Cancer. Cancers 2022, 14, 2940. https://doi.org/10.3390/cancers14122940
Yeo M-K, Kim Y, Lee DH, Chung C, Bae GE. Cosuppression of NF-κB and AICDA Overcomes Acquired EGFR-TKI Resistance in Non-Small Cell Lung Cancer. Cancers. 2022; 14(12):2940. https://doi.org/10.3390/cancers14122940
Chicago/Turabian StyleYeo, Min-Kyung, Yoonjoo Kim, Da Hye Lee, Chaeuk Chung, and Go Eun Bae. 2022. "Cosuppression of NF-κB and AICDA Overcomes Acquired EGFR-TKI Resistance in Non-Small Cell Lung Cancer" Cancers 14, no. 12: 2940. https://doi.org/10.3390/cancers14122940
APA StyleYeo, M.-K., Kim, Y., Lee, D. H., Chung, C., & Bae, G. E. (2022). Cosuppression of NF-κB and AICDA Overcomes Acquired EGFR-TKI Resistance in Non-Small Cell Lung Cancer. Cancers, 14(12), 2940. https://doi.org/10.3390/cancers14122940






