Psychological Impact of TP53-Variant-Carrier Newborns and Counselling on Mothers: A Pediatric Surveillance Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
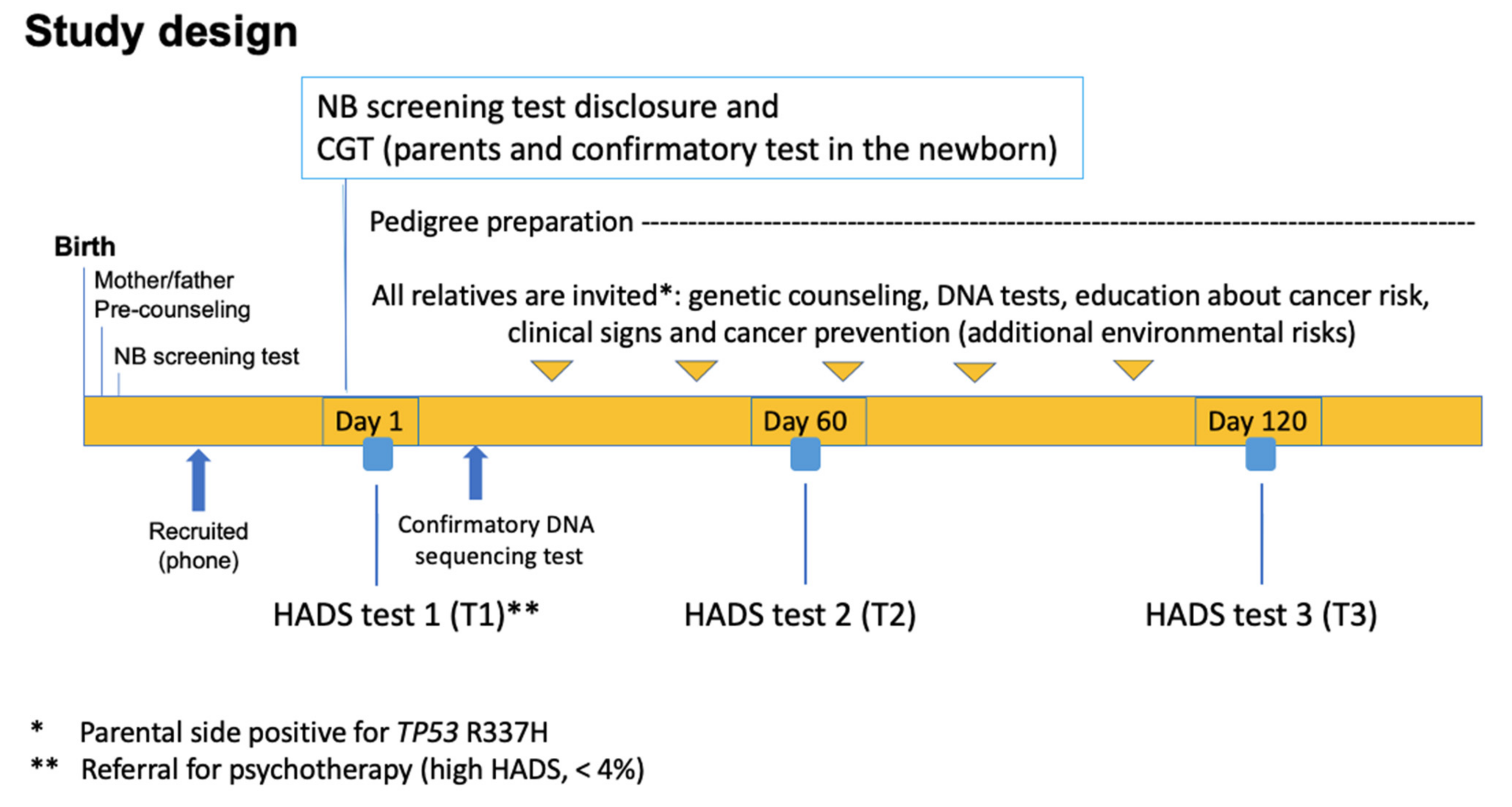
2.1. Design and Participants
2.2. Recruitment of p.R337H-Carrier Newborns through Neonatal Screening
2.3. Longitudinal Analysis—Assessment of the Psychological Impact of the TP53 p.R337H Mutation Carrier Status of Newborns on Mothers
2.4. Data Analysis
3. Results
3.1. Cross-Sectional Analysis in Women Close to Term of Pregnancy—Assessment of the Sociodemographic Covariates, Such as Marital Status, Number of Children, Employment, Education Level, and Family Income
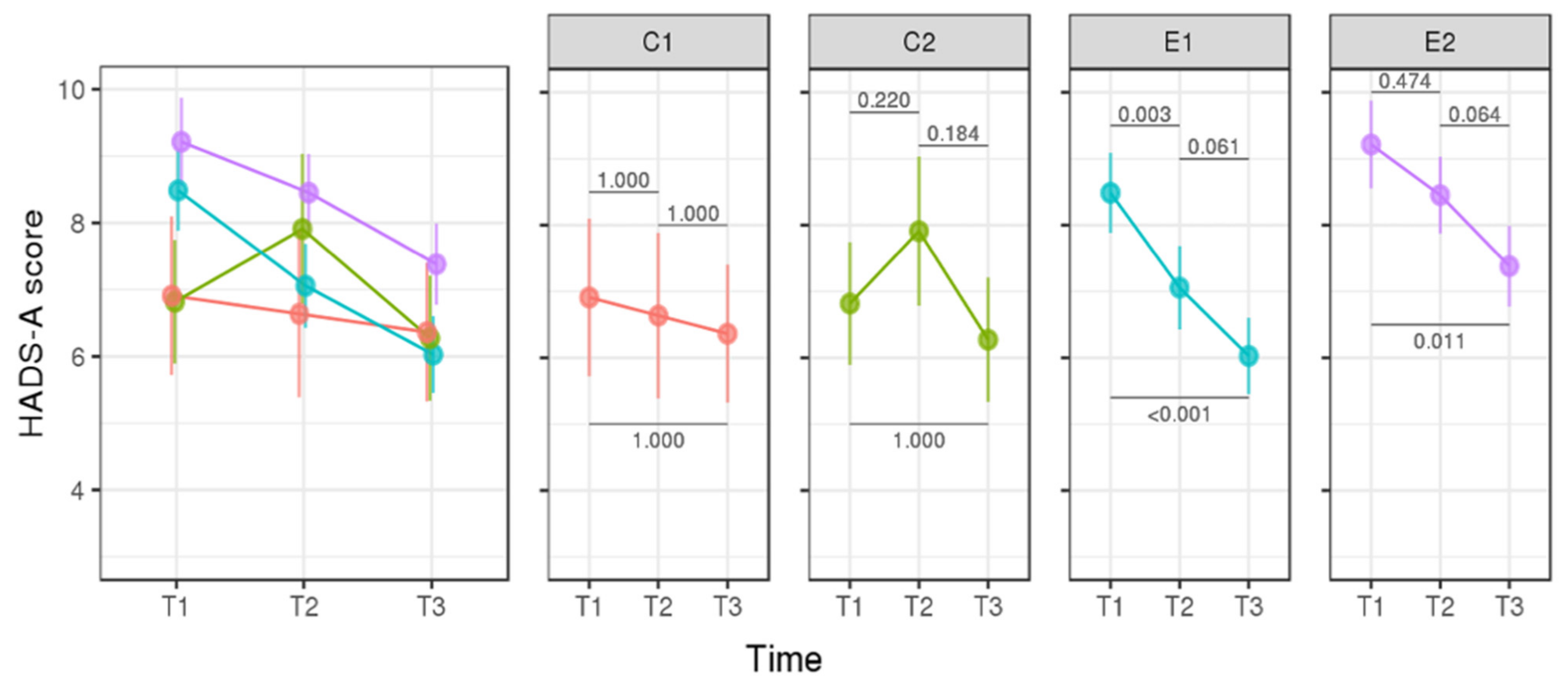
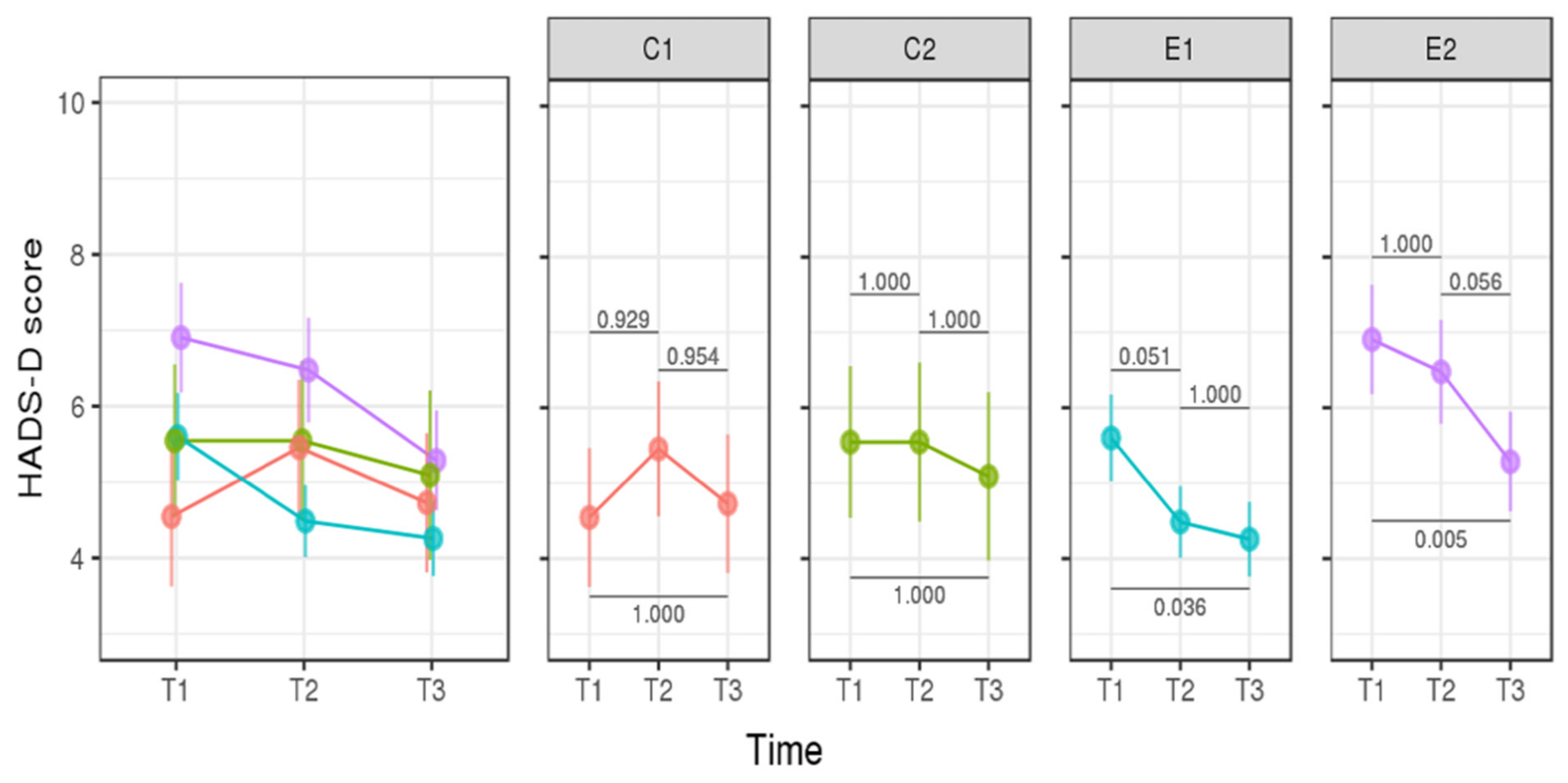
3.2. Longitudinal Analysis—Assessment of the Psychological Impact of p.R337H Mutation Carrier Status of Newborns on Mothers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latronico, A.C.; Pinto, E.M.; Domenice, S.; Fragoso, M.C.; Martin, R.M.; Zerbini, M.C.; Lucon, A.M.; Mendonca, B.B. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 2001, 86, 4970–4973. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.E.J.; Gerber, V.K.Q.; Ibañez, H.C.; Melanda, V.S.; Parise, I.Z.S.; Watanabe, F.M.; Pianovski, M.A.D.; Fiori, C.M.C.M.; Fabro, A.L.M.R.; da Silva, D.B.; et al. Penetrance of the TP53 R337H mutation and pediatric adrenocortical carcinoma incidence associated with environmental influences in a 12-year observational cohort in Southern Brazil. Cancers 2019, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Seidinger, A.L.; Mastellaro, M.J.; Paschoal Fortes, F.; Godoy Assumpção, J.; Aparecida Cardinalli, I.; Aparecida Ganazza, M.; Correa Ribeiro, R.; Brandalise, S.R.; Dos Santos Aguiar, S.; Yunes, J.A. Association of the highly prevalent TP53 P.R337H mutation with pediatric choroid plexus carcinoma and osteosarcoma in southeast Brazil. Cancer 2011, 117, 2228–2235. [Google Scholar] [CrossRef]
- Seidinger, A.L.; Fortes, F.P.; Mastellaro, M.J.; Cardinalli, I.A.; Zambaldi, L.G.; Aguiar, S.S.; Yunes, J.A. Occurrence of neuroblastoma among TP53 p.R337H carriers. PLoS ONE 2015, 10, e0140356. [Google Scholar] [CrossRef] [PubMed]
- Mastellaro, M.J.; Seidinger, A.L.; Kang, G.; Abrahão, R.; Miranda, E.C.M.; Pounds, S.B.; Cardinalli, I.A.; Aguiar, S.S.; Figueiredo, B.C.; Rodriguez-Galindo, C.; et al. Contribution of the TP53 R337H mutation to the cancer burden in southern Brazil: Insights from the study of 55 families of children with adrenocortical tumors. Cancer 2017, 123, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Palmero, E.I.; Schüler-Faccini, L.; Caleffi, M.; Achatz, M.I.W.; Olivier, M.; Martel-Planche, G.; Marcel, V.; Aguiar, E.; Giacomazzi, J.; Ewald, I.P.; et al. Detection of R337H, a germline TP53 mutation predisposing to multiple cancers, in asymptomatic women participating in a breast cancer screening program in Southern Brazil. Cancer Lett. 2008, 261, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Li, F.P.; Fraumeni, J.F., Jr. Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann. Inter. Med. 1969, 71, 747–752. [Google Scholar] [CrossRef]
- Birch, J.M.; Hartley, A.L.; Tricker, K.J.; Prosser, J.; Condie, A.; Kelsey, A.M.; Harris, M.; Jones, P.H.; Binchy, A.; Crowther, D. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res. 1994, 54, 1298–1304. [Google Scholar]
- Frebourg, T.; Abel, A.; Bonaiti-Pellie, C.; Brugières, L.; Berthet, P.; Bressac-de Paillerets, B.; Chevrier, A.; Chompret, A.; Cohen-Haguenauer, O.; Delattre, O.; et al. Li-Fraumeni syndrome: Update, new data and guidelines for clinical management. Bull. Cancer 2001, 88, 581–587. [Google Scholar]
- Tinat, J.; Bougeard, G.; Baert-Desurmont, S.; Vasseur, S.; Martin, C.; Bouvignies, E.; Caron, O.; Bressac-de Paillerets, B.; Berthet, P.; Dugast, C.; et al. 2009 version of the Chompret criteria for Li Fraumeni syndrome. J. Clin. Oncol. 2009, 27, e108–e109. [Google Scholar] [CrossRef]
- Tosin, K.C.F.; Legal, E.F.; Pianovski, M.A.D.; Ibañez, H.C.; Custódio, G.; Carvalho, D.S.; Figueiredo, M.M.O.; Filho, A.H.; Fiori, C.M.C.M.; Rodrigues, A.L.M.; et al. Newborn screening for the detection of the TP53 R337H variant and surveillance for early diagnosis of pediatric adrenocortical tumors: Lessons learned and way forward. Cancers 2021, 13, 6111. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.L.; Best, A.F.; Peters, J.A.; DeCastro, R.M.; Khincha, P.P.; Loud, J.T.; Bremer, R.C.; Rosenberg, P.S.; Savage, S.A. Risks of first and subsequent cancers among TP53 mutation-carriers in the NCI LFS cohort. Cancer 2016, 122, 3673–3681. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.M.; Billerbeck, A.E.C.; Villares, M.C.B.F.; Domenice, S.; Mendonça, B.B.; Latronico, A.C. Founder Effect for the Highly Prevalent R337H Mutation of Tumor Suppressor p53 in Brazilian Patients with Adrenocortical Tumors. Arq. Bras. Endocrinol. Metab. 2004, 48, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.M.; Zambetti, G. What 20 years of research has taught us about the TP53 p.R337H mutation. Cancer 2020, 21, 4678–4686. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 tumor suppressor gene: Important milestones at the various steps of tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Guha, T.; Malkin, D. Inherited TP53 mutations and the Li-Fraumeni syndrome. Cold Spring Harb. Perspect. Med. 2017, 7, a026187. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Menichini, P.; Speciale, A.; Cutrona, G.; Fais, F.; Taiana, E.; Neri, A.; Bomben, R.; Gentile, M.; Gattei, V.; et al. Heterogeneity of TP53 Mutations and P53 Protein Residual Function in Cancer: Does It Matter? Front. Oncol. 2020, 10, 593383. [Google Scholar] [CrossRef]
- Pinto, E.M.; Figueiredo, B.C.; Chen, W.; Galvao, H.C.R.; Formiga, M.N.; Fragoso, M.C.B.V.; Ashton-Prolla, P.; Ribeiro, E.M.S.F.; Felix, G.; Costa, T.E.B.; et al. XAF1 as a Modifier of p53 Function and Cancer Susceptibility. Sci. Adv. 2020, 6, eaba3231. [Google Scholar] [CrossRef]
- Watson, I.R.; Takahashi, K.; Futreal, P.A.; Chin, L. Emerging patterns of somatic mutations in cancer. Nat. Rev. Genet. 2013, 14, 703–718. [Google Scholar] [CrossRef]
- Wu, C.C.; Shete, S.; Amos, C.I.; Strong, L.C. Joint effects of germ-line p53 mutation and sex on cancer risk in Li–Fraumeni syndrome. Cancer Res. 2006, 66, 8287. [Google Scholar] [CrossRef]
- Croyle, R.T.; Smith, K.R.; Botkin, J.R.; Baty, B.; Nash, J. Psychological responses to BRCA1 mutation testing: Preliminary findings. Health Psychol. 1997, 16, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Heshka, J.T.; Palleschi, C.; Howley, H.; Wilson, B.; Wells, P.S. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet. Med. 2008, 10, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Ashida, S.; Hadley, D.W.; Vaughn, B.K.; Kuhn, N.R.; Jenkins, J.F.; Koehly, L.M. The impact of familial environment on depression scores after genetic testing for cancer susceptibility. Clin. Genet. 2009, 75, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.G.; Lobel, M.; Moyer, A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: A meta-analytic review. Health Psychol. 2009, 28, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Lloyd, S.; Davidson, J.; Meyer, L.; Eeles, R.; Ebbs, S.; Murday, V. The impact of genetic counselling on risk perception and mental health in women with a family history of breast cancer. Br. J. Cancer 1999, 79, 868–874. [Google Scholar] [CrossRef]
- Patenaude, A.F.; Guttmacher, A.E.; Collins, F.S. Genetic testing and psychology. New roles, new responsibilities. Am. Psychol. 2002, 57, 271–286. [Google Scholar] [CrossRef]
- Botega, N.J.; Bio, M.R.; Zomignani, M.A.; Garcia, C., Jr.; Pereira, W.A.B. Mood disorders among medical in-patients: A validation study of the hospital anxiety and depression scale (HAD). Rev. Saúde Pública 1995, 29, 355–363. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1973; p. 848. [Google Scholar]
- Rigby, R.A.; Stasinopoulos, D.M. Generalized additive models for location, scale and shape (with discussion). J. R. Stat. Soc. Ser. C Appl. Stat. 2005, 54, 507–554. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2017. Available online: https://www.R-project.org/ (accessed on 12 March 2021).
- Højsgaard, S.; Halekoh, U.; Yan, J. The R package geepack for generalized estimating equations. J. Stat. Softw. 2005, 15, 1. [Google Scholar] [CrossRef]
- Bosch, N.; Junyent, N.; Gadea, N.; Brunet, J.; Cajal, T.R.Y.; Torres, A.; Graña, B.; Velasco, A.; Darder, E.; Mensa, I.; et al. What factors may influence psychological wellbeing at three months and one-year post BRCA genetic result disclosure? Breast 2012, 21, 755–760. [Google Scholar] [CrossRef]
- Kasparian, N.A.; Meiser, B.; Butow, P.N.; Simpson, J.M.; Mann, G.J. Genetic testing for melanoma risk: A prospective cohort study of uptake and outcomes among Australian families. Genet. Med. 2009, 11, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Aktan-Collan, K.; Kääriäinen, H.; Järvinen, H.; Peltomäki, P.; Pylvänäinen, K.; Mecklin, J.P.; Haukkala, A. Psychosocial consequences of predictive genetic testing for Lynch syndrome and associations to surveillance behaviour in a 7-year follow-up study. Fam. Cancer 2013, 12, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Collins, V.R.; Meiser, B.; Ukoumunne, O.C.; Gaff, C.; St John, D.J.; Halliday, J.L. The impact of predictive genetic testing for hereditary nonpolyposis colorectal cancer: Three years after testing. Genet. Med. 2007, 9, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Van Oostrom, I.; Meijers-Heijboer, H.; Duivenvoorden, H.J.; Bröcker-Vriends, A.H.J.T.; van Asperen, C.J.; Sijmons, R.H.; Seynaeve, C.; Van Gool, A.R.; Klijn, J.G.M.; Tibben, A. The commonsense model of self-regulation and psychological adjustment to predictive genetic testing: A prospective study. Psychooncology 2007, 16, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Dougall, A.L.; Smith, A.W.; Somers, T.J.; Posluszny, D.M.; Rubinstein, W.S.; Baum, A. Coping with genetic testing for breast cancer susceptibility. Psychosom. Med. 2009, 71, 98–105. [Google Scholar] [CrossRef]
- Graves, K.D.; Gatammah, R.; Peshkin, B.N.; Krieger, A.; Gell, C.; Valdimarsdottir, H.B.; Schwartz, M.D. BRCA1/2 genetic testing uptake and psychosocial outcomes in men. Fam. Cancer 2011, 10, 213–223. [Google Scholar] [CrossRef]
- Broadstock, M.; Michie, S.; Marteau, T. Psychological consequences of predictive genetic testing: A systematic review. Eur. J. Hum. Genet. 2000, 8, 731–738. [Google Scholar] [CrossRef]
- Foster, C.; Watson, M.; Eeles, R.; Eccles, D.; Ashley, S.; Davidson, R.; Mackay, J.; Morrison, P.J.; Hopwood, P.; Evans, D.G.R.; et al. Predictive genetic testing for BRCA1/2 in a UK clinical cohort: Three-year follow-up. Br. J. Cancer 2007, 96, 718–724. [Google Scholar] [CrossRef][Green Version]
- Condello, C.; Gesuita, R.; Pensabene, M.; Spagnoletti, I.; Capuano, I.; Baldi, C.; Carle, F.; Contegiacomo, A. Distress and family functioning in oncogenetic counselling for hereditary and familial breast and/or ovarian cancers. J. Genet. Counsel. 2007, 16, 625–634. [Google Scholar] [CrossRef]
- Esplen, M.J.; Wong, J.; Aronson, M.; Butler, K.; Rothenmund, H.; Semotiuk, K.; Madlensky, L.; Way, C.; Dicks, E.; Green, J.; et al. Long-term psychosocial and behavioral adjustment in individuals receiving genetic test results in Lynch syndrome. Clin. Genet. 2015, 87, 525–532. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Poll, A.; Llacuachaqui, M.; Nanda, S.; Tulman, A.; Mian, N.; Sun, P.; Narod, S.A. Patient satisfaction and cancer-related distress among unselected Jewish women undergoing genetic testing for BRCA1 and BRCA2. Clin. Genet. 2010, 78, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Bergenmar, M.; Hansson, J.; Brandberg, Y. Family members’ perceptions of genetic testing for malignant melanoma-a prospective interview study. Eur. J. Oncol. Nurs. 2009, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Stump, T.K.; Aspinwall, L.G.; Kohlmann, W.; Champine, M.; Hauglid, J.; Wu, Y.P.; Scott, E.; Cassidy, P.; Leachman, S.A. Genetic test reporting and counseling for melanoma risk in minors may improve sun protection without inducing distress. J. Genet. Counsel. 2018, 27, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Low, C.A.; Bower, J.E.; Kwan, L.; Seldon, J. Benefit finding in response to BRCA1/2 testing. Ann. Behav. Med. 2008, 35, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.K.; Pentz, R.D.; Marani, S.K.; Ward, P.A.; Blanco, A.M.; LaRue, D.; Vogel, K.; Solomon, T.; Strong, L.C. Psychological functioning in persons considering genetic counselling and testing for Li–Fraumeni syndrome. Psychooncology 2008, 17, 783–789. [Google Scholar] [CrossRef]
- Robson, M.; Storm, C.D.; Weitzel, J.; Wollins, D.S.; Offit, K. American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J. Clin. Oncol. 2010, 28, 893–901. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Sandrini, F.; Figueiredo, B.; Zambetti, G.P.; Michalkiewicz, E.; Lafferty, A.R.; DeLacerda, L.; Rabin, M.; Cadwell, C.; Sampaio, G.; et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc. Natl. Acad. Sci. USA 2001, 98, 9330–9335. [Google Scholar] [CrossRef]
- Pereira, R.M.; Michalkiewicz, E.; Sandrini, F.; Figueiredo, B.C.; Pianovski, M.; França, S.N.; Boguszewski, M.C.; Costa, O.; Cat, I.; Filho, L.D.L.; et al. Tumores do córtex adrenal na infância. Arq. Bras. Endocrinol. Metabol. 2004, 48, 651–658. [Google Scholar] [CrossRef]
- Custódio, G.; Parise, G.A.; Filho, N.K.; Komechen, H.; Sabbaga, C.C.; Rosati, R.; Grisa, L.; Parise, I.Z.; Pianovski, M.A.; Fiori, C.M.; et al. Impact of Neonatal Screening and Surveillance for the TP53 R337H Mutation on Early Detection of Childhood Adrenocortical Tumors. J. Clin. Oncol. 2013, 31, 2619–2626. [Google Scholar] [CrossRef]
- Custodio, G.; Taques, G.R.; Figueiredo, B.C.; Gugelmin, E.S.; Oliveira Figueiredo, M.M.; Watanabe, F.; Pontarolo, R.; Lalli, E.; Bleggi Torres, L.F. Increased Incidence of Choroid Plexus Carcinoma Due to the Germline TP53 R337H Mutation in Southern Brazil. PLoS ONE 2011, 6, e18015. [Google Scholar] [CrossRef]
- Figueiredo, B.C.; Sandrini, R.; Zambetti, G.P.; Pereira, R.M.; Cheng, C.; Liu, W.; Lacerda, L.; Pianovski, M.A.; Michalkiewicz, E.; Jenkins, J.; et al. Penetrance of adrenocortical tumours associated with the germline TP53 R337H mutation. J. Med. Genet. 2006, 43, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Giacomazzi, J.; Graudenz, M.S.; Osório, C.B.D.T.; Koehler-Santos, P.; Palmero, E.; de Oliveira, M.Z.; Michelli, R.A.D.; Scapulatempo-Neto, C.; Fernandes, G.C.; Achatz, M.I.; et al. Prevalence of the TP53 p.R337H Mutation in Breast Cancer Patients in Brazil. PLoS ONE 2014, 9, e99893. [Google Scholar] [CrossRef] [PubMed]
- Cury, N.M.; Ferraz, V.E.; A Silva, W. TP53 p.R337H prevalence in a series of Brazilian hereditary breast cancer families. Hered. Cancer Clin. Pract. 2014, 12, 8. [Google Scholar] [CrossRef]
- Couto, P.P.; Bastos-Rodrigues, L.; Schayek, H.; Melo, F.M.; Lisboa, R.G.C.; Miranda, D.M.; Vilhena, A.; Bale, A.E.; Friedman, E.; De Marco, L. Spectrum of germline mutations in smokers and non-smokers in Brazilian non-small-cell lung cancer (NSCLC) patients. Carcinogenesis 2017, 38, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Michalkiewicz, E.; Sandrini, R.; Figueiredo, B.; Miranda, E.C.; Caran, E.; Oliveira-Filho, A.G.; Marques, R.; Pianovski, M.A.; Lacerda, L.; Cristofani, L.M.; et al. Clinical and Outcome Characteristics of Children with Adrenocortical Tumors: A Report from the International Pediatric Adrenocortical Tumor Registry. J. Clin. Oncol. 2004, 22, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Brain, K.; Sivell, S.; Bennert, K.; Howell, L.; France, L.; Jordan, S.; Rogers, M.; Gray, J.; Sampson, J. An exploratory comparison of genetic counselling protocols for HNPCC predictive testing. Clin. Genet. 2005, 68, 255–261. [Google Scholar] [CrossRef]
- Frebourg, T.; Genturis, T.E.R.N.; Lagercrantz, S.B.; Oliveira, C.; Magenheim, R.; Evans, D.G. Guidelines for the Li–Fraumeni and heritable TP53-related cancer syndromes. Eur. J. Hum. Genet. 2020, 28, 1379–1386. [Google Scholar] [CrossRef]
- Bougeard, G.; Renaux-Petel, M.; Flaman, J.M.; Charbonnier, C.; Fermey, P.; Belotti, M.; Gauthier-Villars, M.; Stoppa-Lyonnet, D.; Consolino, E.; Brugières, L.; et al. Revisiting Li–Fraumeni syndrome from TP53 mutation carriers. Clin. Oncol. 2015, 33, 2345. [Google Scholar] [CrossRef]
- Gomes, M.C.; Kotsopoulos, J.; de Almeida, G.L.; Costa, M.M.; Vieira, R.; de Ag Filho, F.; Pitombo, M.B.; Leal, P.R.F.; Royer, R.; Zhang, P.; et al. The R337H mutation in TP53 and breast cancer in Brazil. Hered. Cancer Clin. Pract. 2012, 10, 3. [Google Scholar] [CrossRef]
| Characteristics | Level | HADS-A Level | A p-Value | HADS-D Level | D p-Value |
|---|---|---|---|---|---|
| Number of children (N) 1 | p< 0.001 2 | p< 0.001 2 | |||
| 0 | 5.00 (0.19) | 3.56 (0.17) | |||
| 1 | 5.45 (0.14) | 4.14 (0.13) | |||
| 2 | 5.89 (0.20) | 4.72 (0.19) | |||
| 3 | 6.33 (0.31) | 5.30 (0.304) | |||
| 4 | 6.78 (0.44) | 5.88 (0.43) | |||
| 5 | 7.22 (0.58) | 6.46 (0.56) | |||
| Employment | p = 0.716 | p = 0.503 | |||
| No | 6 [3, 9] | 4 [2, 7] | |||
| Yes | 6 [3, 9] | 4 [2, 7] | |||
| Marital status | p < 0.001 | ||||
| Married | 5 [3, 9] | 4 [2, 6] | |||
| Not married | 7 [4, 1] | 6 [3, 9] | |||
| Education level | p < 0.006 | p < 0.003 | |||
| Elementary school | 7 [3, 1] | 5 [3, 9.8] | |||
| High school | 5 [3, 9] | 4 [2, 7] | |||
| Undergraduate | 5 [2, 8.5] | 3 [1.5, 8] | |||
| Graduated | 5 [3, 7] | 3.5 [2, 6] | |||
| Family income monthly | p = 0.249 | p = 0.254 | |||
| A (EUR < 221.79) * | 6 [3, 11] | 5 [3, 8] | |||
| B (EUR 221.79–443.58) * | 6 [3, 10] | 5 [2, 7] | |||
| C (EUR 443.58–665.37) * | 5 [3, 8] | 3.5 [2, 6] | |||
| D (EUR > 665.37) * | 5 [4, 7] | 4 [3, 6] |
| Level | Control N (%) | Experimental N (%) | p | |
|---|---|---|---|---|
| N | 22 | 77 | ||
|
Mean age (years ± SD) | 26.42 ± 5.30 | 27.03 ± 5.70 | >0.65 | |
| Number of children | 0 | 8 (36.4) | 33 (42.9) | 0.497 |
| 1 | 11 (50.0) | 27 (35.1) | ||
| 2 | 3 (13.6) | 7 (9.1) | ||
| 3 | 0 (0.0) | 6 (7.8) | ||
| 4 | 0 (0.0) | 2 (2.6) | ||
| 5 | 0 (0.0) | 2 (2.6) | ||
| Employment | No | 14 (63.6) | 45 (58.4) | 0.848 |
| Yes | 8 (36.4) | 32 (41.6) | ||
| Marital status | Married | 20 (90.9) | 62 (80.5) | 0.413 |
| Not married | 2 (9.1) | 15 (19.5) | ||
| Education level | Elementary school | 8 (36.4) | 29 (37.7) | 0.851 |
| High school | 10 (45.5) | 31 (40.3) | ||
| Undergraduate | 2 (9.1) | 5 (6.5) | ||
| Graduated | 2 (9.1) | 12 (15.6) | ||
| Family income (monthly) | A (EUR < 221.79) | 4 (18.2) | 17 (22.1) | 0.577 |
| B (EUR 221.79–443.58) | 11 (50.0) | 27 (35.1) | ||
| C (EUR 443.58–665.37) | 4 (18.2) | 23 (29.9) | ||
| D (EUR > 665.37) | 3 (13.6) | 10 (13.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozdziejewski, A.S.; Zotti, C.W.; de Carvalho, I.A.M.; dos Santos, T.C.; de Santi Walter, L.R.; Ogradowski, K.R.P.; Dammski, K.L.; Komechen, H.; Mendes, M.C.; de Souza, E.N.; et al. Psychological Impact of TP53-Variant-Carrier Newborns and Counselling on Mothers: A Pediatric Surveillance Cohort. Cancers 2022, 14, 2945. https://doi.org/10.3390/cancers14122945
Gozdziejewski AS, Zotti CW, de Carvalho IAM, dos Santos TC, de Santi Walter LR, Ogradowski KRP, Dammski KL, Komechen H, Mendes MC, de Souza EN, et al. Psychological Impact of TP53-Variant-Carrier Newborns and Counselling on Mothers: A Pediatric Surveillance Cohort. Cancers. 2022; 14(12):2945. https://doi.org/10.3390/cancers14122945
Chicago/Turabian StyleGozdziejewski, Amanda Scartezini, Clarice Wichinescki Zotti, Isabela Aparecida Moreira de Carvalho, Thairine Camargo dos Santos, Luana Rayana de Santi Walter, Karin Rosa Persegona Ogradowski, Karin Luiza Dammski, Heloisa Komechen, Monalisa Castilho Mendes, Emanuelle Nunes de Souza, and et al. 2022. "Psychological Impact of TP53-Variant-Carrier Newborns and Counselling on Mothers: A Pediatric Surveillance Cohort" Cancers 14, no. 12: 2945. https://doi.org/10.3390/cancers14122945
APA StyleGozdziejewski, A. S., Zotti, C. W., de Carvalho, I. A. M., dos Santos, T. C., de Santi Walter, L. R., Ogradowski, K. R. P., Dammski, K. L., Komechen, H., Mendes, M. C., de Souza, E. N., Paraizo, M. M., da Silva Parise, I. Z., Parise, G. A., Grion, A. L., Custódio, G., Mello, R. G., & Figueiredo, B. C. (2022). Psychological Impact of TP53-Variant-Carrier Newborns and Counselling on Mothers: A Pediatric Surveillance Cohort. Cancers, 14(12), 2945. https://doi.org/10.3390/cancers14122945






