Electroporation and Immunotherapy—Unleashing the Abscopal Effect
Abstract
Simple Summary
Abstract
1. Introduction
The Abscopal Effect
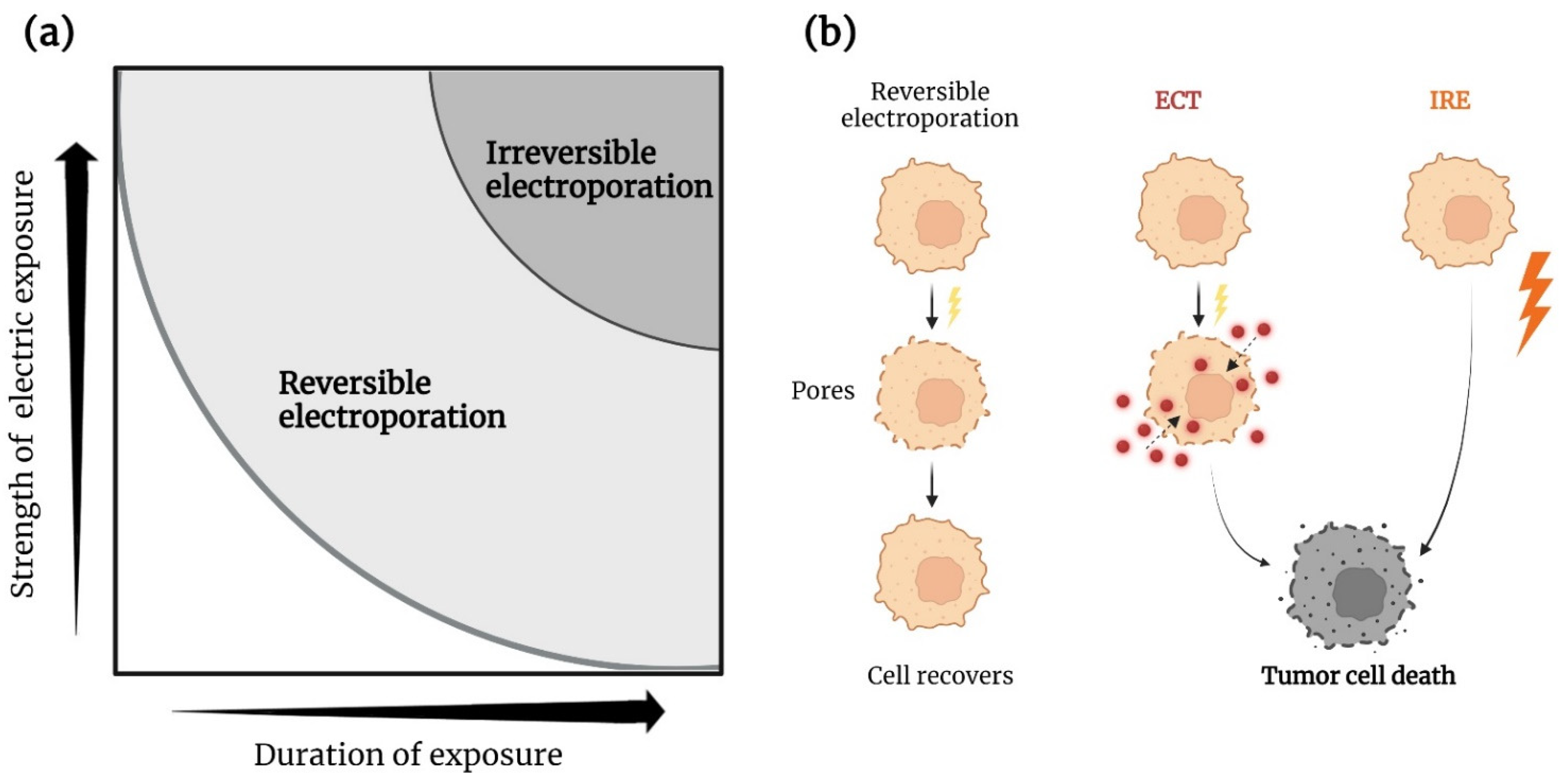
2. Electroporation
2.1. Electrochemotherapy
2.2. Irreversible Electroporation
3. Modulation of the Immune System
3.1. The Interplay between Cancer and Immune Cells
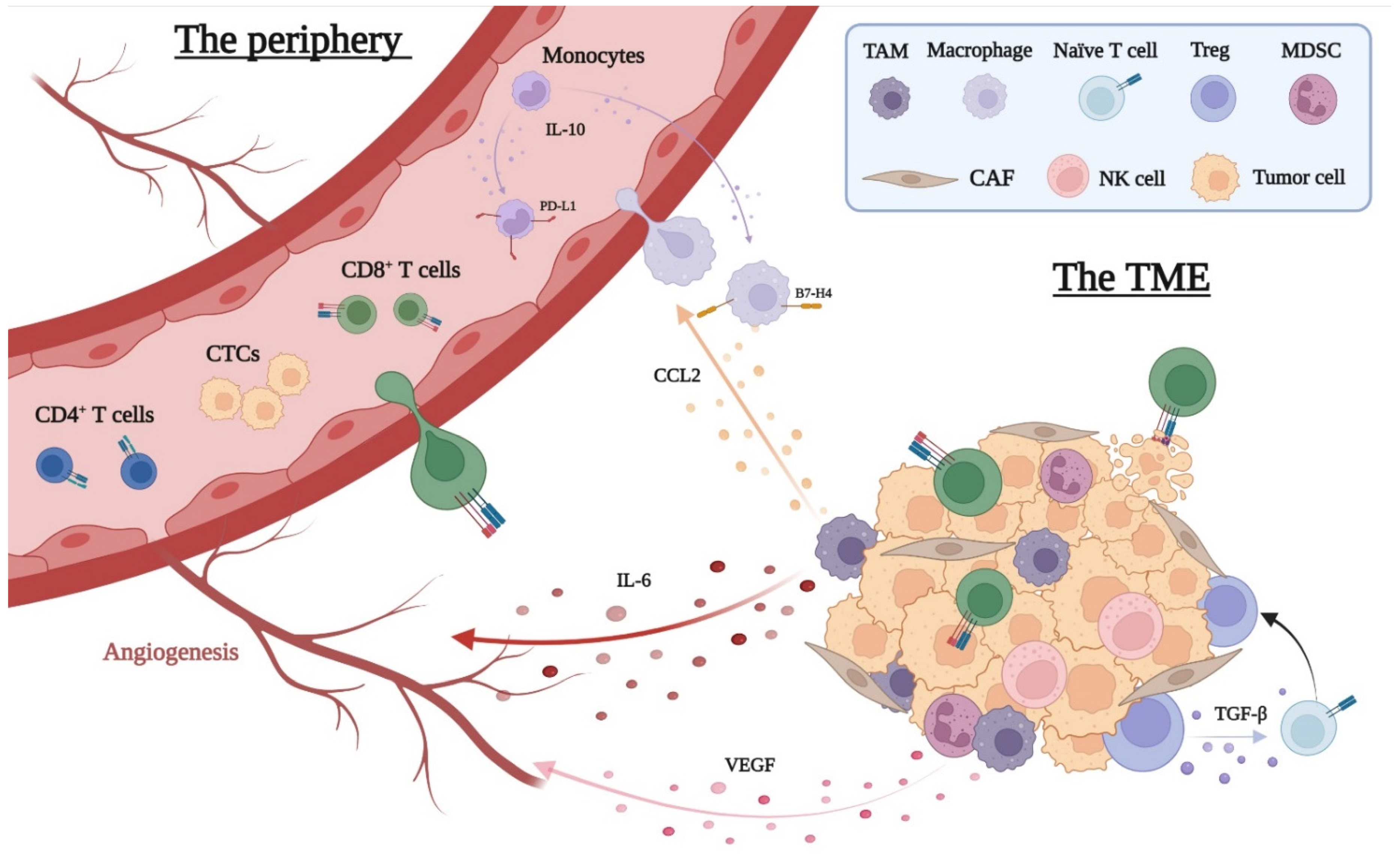
3.2. The Local Immune Response to Cancer
3.3. The Peripheral Immune Response to Cancer
3.4. ECT and the Immune System
3.5. IRE and its Effects on the Immune System
| Species | Authors | Interventions (n) | Cancer Types | Key Findings |
|---|---|---|---|---|
| Human | Guo et al., 2021 [112] | IRE (11) | Hepatocellular carcinoma | The peripheral neutrophils and monocytes increased by day 1 after IRE and returned to baseline at day 7, while CD4+ T cells decreased by day 1 followed by an increase in the next days. CD8+ T cells remained unchanged. Treg cells decreased from day 3 to 14 followed by an increase at one month. |
| He et al., 2019 [104] | IRE (34) | Locally advanced pancreatic cancer | The peripheral CD4+ T cells, CD8+ T cells, and NK cells decreased by day 3 after IRE followed by an increase at day 7, while a reverse trend was shown for Treg cells. IL-6 and IL-10 levels increased at day 3 after IRE followed by a decrease at day 7. IL-2 increased from day 3 to day 7. Concentrations of IFN-γ and TNF did not significantly change. Increased numbers of CD4+ T cells, CD8+ T cells, and NK cells or decreased Treg cells were associated with longer OS. | |
| Pandit et al., 2019 [97] | IRE/pancreatectomy (11/4) | Locally advanced pancreatic cancer | The peripheral Treg populations increased day 1 to 3 and decreased from day 3 to 5 in the IRE group compared to increases on day 1 to 3 as well as increases on day 3 to 5 in the pancreatectomy group. | |
| Scheffer et al., 2019 [113] | IRE (10) | Locally advanced pancreatic cancer | Pre- and post-IRE peripheral levels of CD4+ and CD8+ T cells did not change. At 2 weeks following IRE, a decrease in total Tregs was observed, as well as in aTregs and in resting Tregs, accompanied by a transient increase in both peripheral CD4+PD-1+ and CD8+PD-1+ T cell numbers. | |
| Beitel-White et al., 2019 [114] | IRE (8) | Pancreatic cancer (stage III) | An increase in current change during IRE treatment was associated with decreases in Treg populations 24 h after IRE. Changes in current above 20A induced decreased Treg populations. Further, a trend was shown towards increased survival for the group of patients with a >2% decrease in Treg cells. | |
| Swine | Fujimori et al., 2021 [115] | IRE/microwave ablation | Normal lung | Fifty percent of blood vessels and collagen were intact 2 days after IRE compared to 0% after microwave ablation. Further, the number of CD3+ T cells increased more after IRE than after microwave ablation. |
| Rabbit | Lee et al., 2012 [98] | IRE | Hepatocellular carcinoma | Examinations of non-IRE treated organs, e.g., the lungs, showed no metastases in the IRE group, while all 15 rabbits in the control group had lung metastases. IRE-treated tumors showed increased levels of CD30-positive cells, mainly in the zone between viable and dead tumor. |
| Mouse | Dai et al., 2021 [99] | IRE | Hepatocellular carcinoma | IRE increased the percentage of IFN-γ+ CD8+ T cells in splenocytes and increased tumor infiltration of CD8+ T cells. On day 7, reductions of both peripheral and intratumoral Treg cells and PD-1+ T cells were shown. Mice rejected the tumor re-challenge with hepatocellular carcinoma cells following IRE. |
| He et al., 2020 [95] | IRE | Pancreatic cancer | IRE resulted in longer survival and more proliferating CD8+ T cells in the tumor and spleen. Both memory and effector CD8+ T cells were increased in the tumor and the tumor-draining lymph node regions. The viable region showed increased microvessel density and softening of the extracellular matrix. Mice that were re-challenged with pancreatic cancer cells after IRE rejected the tumor challenge. | |
| Chen et al., 2017 [116] | IRE | Hepatocellular carcinoma | IRE induced a change in the T helper 1/T helper 2 cell ratio towards T helper 1 dominance, an increase in macrophage tumor infiltration, and an increase in IFN-γ and IL-2 compared to controls. | |
| White et al., 2018 [117] | IRE or cryoablation | Pancreatic cancer | IRE induced a higher number of tumor-infiltrating T cells and macrophages at 12 and 24 h after treatment. | |
| Bulvik et al., 2016 [94] | IRE/radiofrequency ablation (82/82) | Normal liver Hepatocellular carcinoma | The tumor infiltration of neutrophils and macrophages was increased in both groups; however, it was greater in the radiofrequency ablation group. In the IRE group, the infiltration of the neutrophils and macrophages extended along the preserved vessels within the ablation zone. At 72 h, persistent vessels in the ablation zone were seen for IRE-treated mice only. IL-6 levels peaked after 6 h, 3 and 10 times higher than controls (radiofrequency ablation and IRE, respectively). By 24 h, no elevations were seen. Radiofrequency ablation of the liver slowed the growth of an untreated tumor, while IRE resulted in greater reduction in tumor growth. Three days after treatment, the number of CD3+ cells was elevated in the untreated tumor in both groups. | |
| Neal et al., 2013 [100] | IRE | Renal carcinoma | IRE-treated immunocompetent mice showed robust T-cell infiltration at the zone between viable and dead tumors. Further, IRE-treated immunocompetent mice showed a greater treatment response than did immunodeficient mice. | |
| José et al., 2012 [93] | IRE | Pancreatic cancer | IRE was not found to activate apoptotic cell death measured by caspase-3 positive cells in the tumors. The vascular architecture of the tumor was disrupted from day 1 after IRE and onward. | |
| Al-sakere et al., 2007 [101] | IRE | Sarcoma | No tumor infiltration of CD4+ or CD8+ T lymphocytes, macrophages, APCs, dendritic cells were observed 2 and 6 h after IRE. | |
| Li et al., 2012 [105] | IRE/sham surgery/resection/control (28/28/28/28) | Osteosarcoma | IRE and resection increased the percentages of the peripheral CD3+ and CD4+ cells, as well as the CD4+/CD8+ ratio 7 days after treatment. A more rapid and prolonged increase was seen in the IRE group. IRE and resection caused decreases in IL-10 from day 3 to 21. The percentage of INF-γ-positive splenocytes was higher in the IRE group. | |
| Rat | He et al., 2021 [88] | IRE | Pancreatic cancer | IRE caused increased levels of HMGB1, HSP70, and calreticulin. Seven days after IRE, higher frequencies of M1 macrophages in the tumor and a regional lymph node were seen compared to controls, while a decrease in M2 macrophages was seen in the tumor. |
| Cell | He et al., 2021 [88] | IRE | Pancreatic cancer | HMGB1 were shown to induce M1 macrophage polarization via receptor of advanced glycation end-product. Further, HMGB1 could enhance the phagocytosis of dying tumor cells by macrophages. |
| Shao et al., 2019 [8] | IRE/thermal therapy/cryosurgery | Melanoma | IRE caused the greatest protein release, second lowest denaturation rate of the released protein (30%), the most TLR2 (a measure of the relative antigen content of the released protein) release, and the strongest T cell response. | |
| Zhao et al., 2019 [87] | IRE/radiotherapy | Pancreatic cancer Melanoma | IRE increased the ATP and HMGB1 levels by 11 and 13 fold, respectively, compared to radiotherapy, which did not cause the release of ATP and HMGB1. IRE: Cells increased the expression of makers for DC activation/maturation by 51–72%, compared to non-IRE treated cells. Radiotherapy: Cells did not increase the expression of makers for DC activation/maturation, compared to non-radiotherapy treated cells. IRE increased the ATP and HMGB1 levels by 8 and 9 fold, respectively. | |
| Goswami et al., 2017 [118] | IRE/thermal shock/chemical poration | Triple negative breast cancer | IRE caused upregulation of IL-6 and TNF, while thymic stromal lymphopoietin was down-regulated. Cancer cells treated with thermal shock or chemical poration showed no down-regulation of thymic stromal lymphopoietin. |
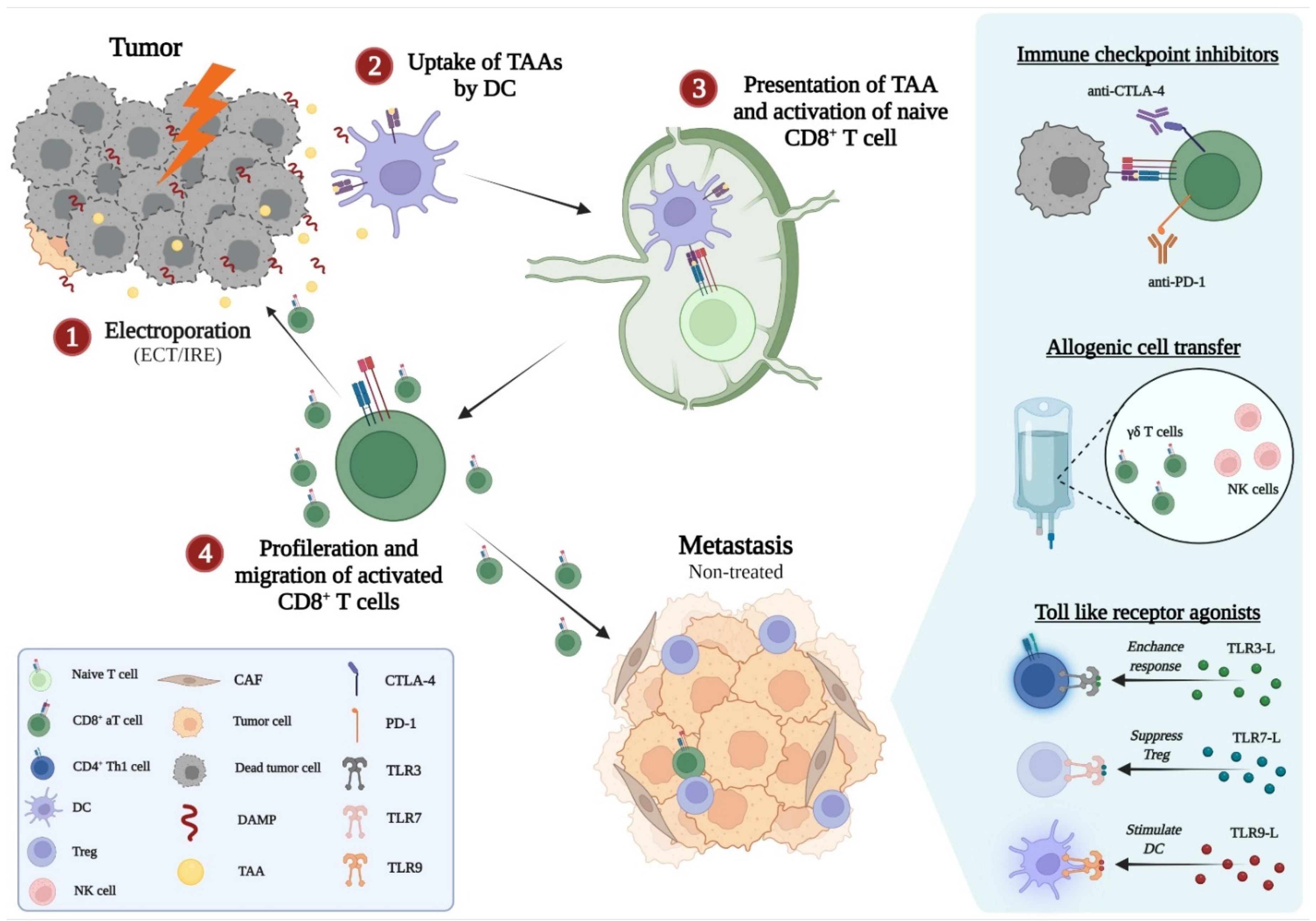
4. The Synergy of Electroporation and Immunotherapy
4.1. Immunotherapy
4.2. ECT and the Synergy with Immunotherapy
4.3. IRE Plus Immunotherapy
| Species | Authors | Interventions (n, Study Design) | Cancer Types | Key Findings |
|---|---|---|---|---|
| Human | He et al., 2021 [141] | IRE/IRE + toripalimab (70/15) ** | Locally advanced pancreatic cancer | Median OS: 1, 2, and 3 year OS rates: IRE: 23.4 months: 91%, 45%, and 12%. IRE + toripalimab: 44.3 months: 100%, 100%, and 33.3%. Increased CD4+ and CD8+ T cells, while CD8+ Treg cells decreased compared to IRE. Further the levels of IL-4, IL-6, TNF, and IFN-γ increased markedly more than in the IRE group. |
| Pan et al., 2020 [142] | IRE/IRE + allogenic NK cell transfer (46/46) **** | Locally advanced pancreatic cancer | Median OS: Response rates: IRE: 11.8 months: 15% CR, 41% PR. IRE + NK cells: 12.4 months: 30% CR, 41% PR. Increased CD4+, CD8+ T cells, NK cells, and B cells compared to IRE alone. Further, the levels of IL-2, TNF-β, and IFN-γ increased markedly more than in the IRE group. | |
| Lin et al., 2020 [144] | IRE/IRE + allogenic γδ T cell transfer (32/30) **** | Locally advanced pancreatic cancer | Median OS: IRE: 11.0 months. IRE + T cells: 14.5 months. Twenty-five incidences of grade 3/4 adverse events equally distributed in both groups. | |
| O’Neill et al., 2020 [140] | IRE + nivolumab (10) *** | Locally advanced pancreatic cancer | Median OS: 18 months; 1 year OS: 67%. Adverse events ≥ grade 3: 70% of patients. By day 90, T effector memory cells were increased two fold from baseline. | |
| Yang et al., 2019 [147] | IRE/IRE + allogenic NK cell transfer (22/18) **** | Unresectable Intrahepatic cholangiocarcinoma/hepatocellular carcinoma | Median OS: Response rates: IRE: 17.9 months: 5% CR, 64% PR IRE + NK cells: 23.2 months: 17% CR, 72% PR Higher lymphocyte count and IL-2, TNF-β, IFN-γ levels post-treatment compared to IRE alone. No serious adverse events. | |
| Alnaggar et al., 2018 [148] | IRE/IRE + allogenic NK cell transfer (20/20) ** | Metastatic hepatocellular carcinoma | Median OS: IRE: 8.9 months. IRE + NK cells: 10.1 months. Lower number of circulating tumor cells in the IRE + NK cell group at 7 and 30 days after treatment. No serious adverse events and no differences in lymphocyte subsets between the two groups after treatment. | |
| Lin et al., 2017 [143] | IRE/IRE + allogenic NK cell transfer (39/32) *** | Pancreatic cancer (stage III/IV) | Median OS: IRE: 11.4 months (stage III), 8.7 months (stage IV). IRE + NK cells: 13.2 months (stage III), 9.8 months (stage IV). No serious adverse events. | |
| Lin et al., 2017 [155] | IRE/IRE + allogenic NK cell transfer (19/20) *** | Metastatic pancreatic cancer | IRE: 16% CR, 47% PR IRE + NK cells: 30% CR, 50% PR | |
| Mouse | Burbach et al., 2021 [149] | IRE + anti-CTLA-4 + anti-PD-1 | Prostate cancer | IRE/anti-CTLA-4: 0%/15% CR. IRE + anti-CTLA-4: 46% CR Increased number of CD8+ T cells both locally and systemically compared to IRE or anti-CTLA-4. IRE + anti-CTLA-4, and subsequent anti-PD-1: Sustained tumor regression after CR. |
| Shi et al., 2021 [150] | IRE + anti-PD-L1 | Hepatocellular carcinoma | IRE + anti-PD-L1-induced necrosis, T cell and inflammatory cell infiltration in both treated and non-treated tumors. | |
| Babikr et al., 2021 [96] | IRE + anti-PD-L1 + TLR3 + TLR9 agonists | Lymphoma Breast cancer | IRE: 0% CR. IRE + TLR3 + TLR9: Superior primary tumor growth inhibition and CD8+ T cell response compared to IRE and IRE + anti-PD-1. IRE + anti-PD-1 + TLR3 + TLR9: 100% CR of treated and non-treated tumors. Increased the tumor infiltration of CD8+ and CD4+ T cells and the CD8+ T cell response compared to IRE alone. Induced a M1/M2 macrophage balance towards the anti-tumor M1 and reduced Tregs and MDSCs. IRE + anti-PD-1 + TLR3 + TLR9: 100% CR of treated tumors. | |
| Zhang et al., 2021 [156] | IRE + anti-OX40 | Pancreatic cancer Metastatic pancreatic cancer | Median survival: Control/anti-OX40/IRE: 22/24/51 days. IRE + anti-OX40: 80% were alive at 120 days (median survival not reached). Increased tumor infiltration of CD8+ T cells and decreased MDSCs, as well as higher levels of IFN-γ and TNF-α compared to IRE alone. Secondary, non-treated tumor, median survival: Control/anti-OX40/IRE: 21/21/31 days, respectively. IRE + anti-OX40: 44 days. | |
| Sun et al., 2021 [90] | IRE + M1 oncolytic virus | Pancreatic cancer | Median survival: Control/M1 virus/IRE: 31/34/46 days. IRE + M1: 58 days. Increased tumor infiltration of CD4+ and CD8+ T cells. | |
| Yang et al., 2021 [157] | IRE + DC vaccine | Pancreatic cancer | Median survival: Control/IRE/DC vaccine: 35/44/49 days. IRE + anti-PD-1: 77 days. Twice as high mean number of tumor-infiltrating CD8+ T cells compared to IRE alone. | |
| Lasarte-Cia et al., 2021 [152] | IRE + STING agonist | Melanoma Hepatocellular carcinoma | Control/IRE/STING: 0% CR. IRE + STING: 13% CR. Control/IRE/STING: 0%/17%/20% CR. IRE + STING: 67% CR. | |
| Go et al., 2020 [153] | IRE + STING agonist | Lewis lung carcinoma | IRE + STING: Reduced the tumor volume, induced a M1/M2 macrophage balance towards the anti-tumor M1 phenotype, and increased the tumor infiltration of CD8+ and CD4+ T cells compared to IRE or STING alone. | |
| Yu et al., 2020 [158] | IRE + indoleamine 2,3-dioxygenase inhibitor loaded electric pulse responsive iron-oxide-nanocube clusters | Prostate cancer | Combination treatment induced higher calreticulin tumor exposure, increased frequency of tumor-infiltrating CD3+ T cells, and higher CD8+ T cell-to-Tregs ratio compared to IRE alone. Further, it reduced the tumor growth of both treated and non-treated tumors more than IRE alone. | |
| Narayanan et al., 2019 [151] | IRE + TLR7 agonist/anti-PD-1 | Pancreatic cancer | IRE: 20–35% CR in immunocompetent mice; 0% CR in immunodeficient mice. Generated tumor antigen-specific T cell responses. IRE + TLR7/anti-PD-1 were not superior to IRE alone in survival and tumor growth reduction. | |
| Zhao et al., 2019 [87] | IRE + anti-PD-1 + anti-CTLA-4/radiotherapy + anti-PD-1 | Pancreatic cancer Melanoma | Median survival: Control/anti-PD-1/IRE: 6/8/12 days. Radiotherapy + anti-PD-1: 30 days; 0% were alive at 120 days. IRE + anti-PD-1: 32 days; 36% were alive at 120 days. IRE + anti-PD-1 + anti-CTLA-4: 41 days. However, not significantly different from IRE + anti-PD-1, and weight loss suggested considerable toxicity. Median survival: Control/anti-PD-1/IRE: 5/6/8 days. IRE + anti-PD-1: 23 days | |
| Vivas et al., 2019 [159] | IRE + polyinosinic-polycytidylic acid and poly-L-lysine | Hepatocellular carcinoma | Control/IRE/polyinosinic-polycytidylic acid and poly-L-lysine: 0%/27%/30% CR. IRE + polyinosinic-polycytidylic acid and poly-L-lysine: 71% CR. | |
| Pasquet et al., 2019 [160] | IRE + IL-12 GET | Melanoma | Control/IRE/IL-12 GET: 0% CR. IRE + IL-12 GET: 42% CR. |
5. Perspectives
5.1. Ongoing Trials
5.2. Intertumoral Heterogeneity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| anti-CTLA-4 | cytotoxic T-lymphocyte-associated antigen 4 inhibitor |
| anti-PD-1 | programmed death-1 receptor inhibitor |
| anti-PD-L1 | programmed death-1 receptor ligand inhibitor |
| APC | antigen-presenting cell |
| aTreg | activated Treg |
| CAF | cancer-associated fibroblast |
| CCL | CC chemokine ligand |
| CR | complete response |
| DAMP | danger-associated molecular pattern |
| DC | dendritic cell |
| ECT | electrochemotherapy |
| GET | gene electrotransfer |
| HMGB1 | high mobility group box 1 |
| ICD | immunogenic cell death |
| ICI | immune checkpoint inhibitor |
| IFN | interferon |
| IL | interleukin |
| M | macrophage |
| MDSC | myeloid-derived suppressor cell |
| MHC | major histocompatibility complex |
| NK cell | natural killer cell |
| NK cell transfer | NK cell transfer therapy |
| OS | overall survival |
| PD | progressive disease |
| PR | partial response |
| RCT | randomized controlled trial |
| STING | stimulator of interferon genes |
| TAA | tumor-associated antigen |
| TAM | tumor-associated macrophage |
| TGF | transforming growth-factor |
| TLR | toll-like receptor |
| TME | tumor microenvironment |
| TNF | tumor necrosis factor |
| Treg | regulatory T cell |
References
- Sale, A.J.; Hamilton, W.A. Effects of High Electric Fields on Micro-Organisms: III. Lysis of Erythrocytes and Protoplasts. Biochim. Biophys. Acta 1968, 163, 37–43. [Google Scholar] [CrossRef]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene Transfer into Mouse Lyoma Cells by Electroporation in High Electric Fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Belehradek, M.; Domenge, C.; Luboinski, B.; Orlowski, S.; Belehradek, J.J.; Mir, L.M. Electrochemotherapy, a New Antitumor Treatment. First Clinical Phase I-II Trial. Cancer 1993, 72, 3694–3700. [Google Scholar] [CrossRef]
- Onik, G.; Rubinsky, B. Irreversible Electroporation: First Patient Experience Focal Therapy of Prostate Cancer. In Irreversible Electroporation; Springer: Berlin/Heidelberg, Germany, 2010; pp. 235–247. [Google Scholar] [CrossRef]
- Mole, R.H. Whole Body Irradiation—Radiobiology or Medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic Review of Case Reports on the Abscopal Effect. Curr. Probl. Cancer 2021, 40, 25–37. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using Immunotherapy to Boost the Abscopal Effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Shao, Q.; Flanagan, S.O.; Lam, T.; Roy, P.; Pelaez, F.; Burbach, B.J.; Azarin, S.M.; Shimizu, Y.; Bischof, J.C. Engineering T Cell Response to Cancer Antigens by Choice of Focal Therapeutic Conditions. Int. J. Hyperth. 2019, 36, 130–138. [Google Scholar] [CrossRef]
- Calvet, C.Y.; Mir, L.M. The Promising Alliance of Anti-Cancer Electrochemotherapy with Immunotherapy. Cancer Metastasis Rev. 2016, 35, 165–177. [Google Scholar] [CrossRef]
- Goggins, C.A.; Khachemoune, A. The Use of Electrochemotherapy in Combination with Immunotherapy in the Treatment of Metastatic Melanoma: A Focused Review. Int. J. Dermatol. 2019, 58, 865–870. [Google Scholar] [CrossRef]
- Rai, Z.L.; Feakins, R.; Pallett, L.J.; Manas, D.; Davidson, B.R. Irreversible Electroporation (IRE) in Locally Advanced Pancreatic Cancer: A Review of Current Clinical Outcomes, Mechanism of Action and Opportunities for Synergistic Therapy. J. Clin. Med. 2021, 10, 1609. [Google Scholar] [CrossRef]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; Tol, P.M.; Davalos, R.V.; Rubinsky, B.; Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Miklavčič, D.; Mali, B.; Kos, B.; Heller, R.; Serša, G. Electrochemotherapy: From the Drawing Board into Medical Practice. Biomed. Eng. Online 2014, 13, 29. [Google Scholar] [CrossRef]
- Sersa, G.; Miklavcic, D.; Cemazar, M.; Rudolf, Z.; Pucihar, G.; Snoj, M. Electrochemotherapy in Treatment of Tumours. Eur. J. Surg. Oncol. 2008, 34, 232–240. [Google Scholar] [CrossRef]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef]
- Aycock, K.N.; Davalos, R.V. Irreversible Electroporation: Background, Theory, and Review of Recent Developments in Clinical Oncology. Bioelectricity 2019, 1, 214–234. [Google Scholar] [CrossRef]
- Gissel, H.; Lee, R.C.; Gehl, J. Electroporation and Cellular Physiology. In Clinical Aspects of Electroporation; Springer: Berlin, Germany, 2011; pp. 9–17. [Google Scholar] [CrossRef]
- Brock, R.M.; Beitel-white, N.; Davalos, R.V.; Allen, I.C.; Allen, I.C. Starting a Fire Without Flame: The Induction of Cell Death and Inflammation in Electroporation-Based Tumor Ablation Strategies. Front. Oncol. 2020, 10, 1235. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Aymeric, L.; Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Martins, I. Tumor Cell Death and ATP Release Prime Dendritic Cells and Efficient Anticancer Immunity. Cancer Res. 2010, 70, 855–858. [Google Scholar] [CrossRef]
- Kepp, O.; Tesniere, A.; Zitvogel, L.; Kroemer, G. The Immunogenicity of Tumor Cell Death. Curr. Opin. Oncol. 2009, 21, 71–76. [Google Scholar] [CrossRef]
- Gardai, S.J.; Mcphillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-ullrich, J.E.; Bratton, D.L.; Oldenborg, P.; Michalak, M.; Henson, P.M.; et al. Cell-Surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through Trans-Activation of LRP on the Phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-stubbs, S.; Obeid, M.; et al. Caspase-Dependent Immunogenicity of Doxorubicin-Induced Tumor Cell Death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Guss, K.A.; Nelson, C.E.; Hudson, A.; Kraus, M.E.; Carroll, S.B.; Barreira, R.; Reis, C.; Green, D.R. RIPK1 and NF-KB Signaling in Dying Cells Determines Cross-Priming of CD8+ T Cells. Science 2015, 350, 1720–1723. [Google Scholar] [CrossRef]
- Walsh, M.P.; Duncan, B.; Larabee, S.; Krauss, A.; Davis, J.P.E.; Cui, Y.; Kim, S.Y.; Guimond, M.; Bachovchin, W.; Fry, T.J. Val-BoroPro Accelerates T Cell Priming via Modulation of Dendritic Cell Trafficking Resulting in Complete Regression of Established Murine Tumors. PLoS ONE 2013, 8, e58860. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.B.; Davalos, R.V. Advances in Therapeutic Electroporation to Mitigate Muscle Contractions. Membr. Sci. Technol. 2012, 2, 1000e102. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, S.; Zhu, L.; Zhang, L.; Liu, J.; Xu, D.; Tian, G.; Jiang, T. Antitumor Effect and Immune Response of Nanosecond Pulsed Electric Fields in Pancreatic Cancer. Front. Oncol. 2020, 10, 621092. [Google Scholar] [CrossRef] [PubMed]
- Wasson, E.M.; Ivey, J.W.; Verbridge, S.S.; Davalos, R.V.; Tech, V.; Building, S.E.; Tech, V. The Feasibility of Enhancing Susceptibility of Glioblastoma Cells to IRE Using a Calcium Adjuvant. Ann. Biomed. Eng. 2017, 45, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.-M.; Rols, M.-P. Electrochemotherapy: Progress and Prospects. Curr. Pharm. Des. 2012, 18, 3406–3415. [Google Scholar] [CrossRef]
- Poddevin, B.; Orlowski, S.; Belehradek, J.J.; Mir, L.M. Very High Cytotoxicity of Bleomycin Introduced into the Cytosol of Cells in Culture. Biochem. Pharmacol. 1991, 42, 67–75. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard Operating Procedures of the Electrochemotherapy: Instructions for the Use of Bleomycin or Cisplatin Administered Either Systemically or Locally and Electric Pulses Delivered by the CliniporatorTM by Means of Invasive or Non-Invasive Electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Tarantino, L.; Busto, G.; Nasto, A.; Fristachi, R.; Cacace, L.; Talamo, M.; Accardo, C.; Bortone, S.; Gallo, P.; Tarantino, P.; et al. Percutaneous Electrochemotherapy in the Treatment of Portal Vein Tumor Thrombosis at Hepatic Hilum in Patients with Hepatocellular Carcinoma in Cirrhosis: A Feasibility Study. World J. Gastroenterol. 2017, 23, 906–918. [Google Scholar] [CrossRef]
- Edhemovic, I.; Brecelj, E.; Cemazar, M.; Boc, N.; Trotovsek, B.; Djokic, M.; Dezman, R.; Ivanecz, A.; Potrc, S.; Bosnjak, M.; et al. Intraoperative Electrochemotherapy of Colorectal Liver Metastases: A Prospective Phase II Study. Eur. J. Surg. Oncol. 2020, 46, 1628–1633. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Fusco, R.; D’alessio, V.; Petrillo, A.; Lastoria, S.; Piccirillo, M.; Albino, V.; Belli, A.; Tafuto, S.; et al. Clinical Phase I/II Study: Local Disease Control and Survival in Locally Advanced Pancreatic Cancer Treated with Electrochemotherapy. J. Clin. Med. 2021, 10, 1305. [Google Scholar] [CrossRef]
- Sersa, G. The State-of-the-Art of Electrochemotherapy before the ESOPE Study; Advantages and Clinical Uses. Eur. J. Cancer Suppl. 2006, 4, 52–59. [Google Scholar] [CrossRef]
- Clover, A.J.P.; de Terlizzi, F.; Bertino, G.; Curatolo, P.; Odili, J.; Campana, L.G.; Kunte, C.; Muir, T.; Brizio, M.; Sersa, G.; et al. Electrochemotherapy in the Treatment of Cutaneous Malignancy: Outcomes and Subgroup Analysis from the Cumulative Results from the Pan-European International Network for Sharing Practice in Electrochemotherapy Database for 2482 Lesions in 987 Patients (2008–2019). Eur. J. Cancer 2020, 138, 30–40. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Bertino, G.; McCaul, J.A.; Grau, J.J.; de Bree, R.; Sersa, G.; Occhini, A.; Groselj, A.; Langdon, C.; Heuveling, D.A.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) Project: Results from the Treatment of Mucosal Cancers. Eur. J. Cancer 2017, 87, 172–181. [Google Scholar] [CrossRef]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Bastholt, L.; Grob, J.-J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.J.; et al. Diagnosis and Treatment of Melanoma. European Consensus-Based Interdisciplinary Guideline—Update 2016. Eur. J. Cancer 2016, 63, 201–217. [Google Scholar] [CrossRef]
- Michielin, O.; van Akkooi, A.; Lorigan, P.; Ascierto, P.; Dummer, R.; Robert, C.; Arance, A.; Blank, C.; Sileni, V.C.; Donia, M.; et al. ESMO Consensus Conference Recommendations on the Management of Locoregional Melanoma: Under the Auspices of the ESMO Guidelines Committee. Ann. Oncol. 2020, 31, 1449–1461. [Google Scholar] [CrossRef]
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.C.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and Treatment of Invasive Squamous Cell Carcinoma of the Skin: European Consensus-Based Interdisciplinary Guideline. Eur. J. Cancer 2015, 51, 1989–2007. [Google Scholar] [CrossRef]
- Capanna, R.; Piccioli, A.; Gasbarrini, A.; Di Martino, A.; Biagini, R.; Denaro, V.; Ghermandi, R.; Girolami, M.; Spinelli, M.S.; Zoccali, C.; et al. Algoritmo Terapeutico per Il Trattamento Delle Metastasi Del Sacro. Raccomandazioni Del Gruppo Di Studio SIOT Sulle Metastasi Ossee. G. Ital. Ortop. Traumatol. 2016, 42, 242–250. [Google Scholar]
- Rudno-Rudzińska, J.; Kielan, W.; Guziński, M.; Płochocki, M.; Antończyk, A.; Kulbacka, J. New Therapeutic Strategy: Personalization of Pancreatic Cancer Treatment-Irreversible Electroporation (IRE), Electrochemotherapy (ECT) and Calcium Electroporation (CaEP)—A Pilot Preclinical Study. Surg. Oncol. 2021, 38, 101634. [Google Scholar] [CrossRef] [PubMed]
- Ruarus, A.H.; Vroomen, L.G.P.H.; Geboers, B.; van Veldhuisen, E.; Puijk, R.S.; Nieuwenhuizen, S.; Besselink, M.G.; Zonderhuis, B.M.; Kazemier, G.; de Gruijl, T.D.; et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology 2020, 294, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.R.; Ruarus, A.H.; Vroomen, L.G.P.H.; Puijk, R.S.; Geboers, B.; Nieuwenhuizen, S.; van den Bemd, B.A.T.; Nielsen, K.; de Vries, J.J.J.; van Lienden, K.P.; et al. Irreversible Electroporation to Treat Unresectable Colorectal Liver Metastases (COLDFIRE-2): A Phase II, Two-Center, Single-Arm Clinical Trial. Radiology 2021, 299, 470–480. [Google Scholar] [CrossRef] [PubMed]
- van den Bos, W.; Scheltema, M.J.; Siriwardana, A.R.; Kalsbeek, A.M.F.; Thompson, J.E.; Ting, F.; Böhm, M.; Haynes, A.M.; Shnier, R.; Delprado, W.; et al. Focal Irreversible Electroporation as Primary Treatment for Localized Prostate Cancer. BJU Int. 2018, 121, 716–724. [Google Scholar] [CrossRef]
- Guenther, E.; Klein, N.; Zapf, S.; Weil, S.; Schlosser, C.; Rubinsky, B.; Stehling, M.K. Prostate Cancer Treatment with Irreversible Electroporation (IRE): Safety, Efficacy and Clinical Experience in 471 Treatments. PLoS ONE 2019, 14, e0215093. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Sun, S.; Zhang, Y.; Lin, X.; Lao, X.; Cui, B. Irreversible Electroporation versus Radiotherapy after Induction Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer: A Propensity Score Analysis. BMC Cancer 2019, 19, 394. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Barone, A.; Hazarika, M.; Theoret, M.R.; Mishra-Kalyani, P.; Chen, H.; He, K.; Sridhara, R.; Subramaniam, S.; Pfuma, E.; Wang, Y.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Patients with Unresectable or Metastatic Melanoma. Clin. Cancer Res. 2017, 23, 5661–5665. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and Its Ligands Are Important Immune Checkpoints in Cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in Colorectal Cancer: Rationale, Challenges and Potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef]
- Chretien, A.; Le Roy, A.; Vey, N.; Prebet, T.; Blaise, D.; Fauriat, C.; Olive, D. Cancer-Induced Alterations of NK-Mediated Target Recognition: Current and Investigational Pharmacological Strategies Aiming at Restoring NK-Mediated Anti-Tumor Activity. Front. Immunol. 2014, 5, 122. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic Immunity in Cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Galon, J.; Fridman, W.H.; Smyth, M.J. From Mice to Humans: Developments in Cancer Immunoediting. J. Clin. Invest. 2015, 125, 3338–3346. [Google Scholar] [CrossRef]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef]
- Orhan, A.; Vogelsang, R.P.; Andersen, M.B.; Madsen, M.T.; Hölmich, E.R.; Raskov, H.; Gögenur, I. The Prognostic Value of Tumour-Infiltrating Lymphocytes in Pancreatic Cancer: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2020, 132, 71–84. [Google Scholar] [CrossRef]
- Gao, Z.-H.; Li, C.-X.; Liu, M.; Jiang, J.-Y. Predictive and Prognostic Role of Tumour-Infiltrating Lymphocytes in Breast Cancer Patients with Different Molecular Subtypes: A Meta-Analysis. BMC Cancer 2020, 20, 1150. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic Value of Tumor-Infiltrating FoxP3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef]
- Frydrychowicz, M.; Boruczkowski, M.; Kolecka-Bednarczyk, A.; Dworacki, G. The Dual Role of Treg in Cancer. Scand. J. Immunol. 2017, 86, 436–443. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Keep, R.F.; Mostarica-Stojkovic, M.; Andjelkovic, A.V. CCL2 Regulates Angiogenesis via Activation of Ets-1 Transcription Factor. J. Immunol. 2006, 177, 2651–2661. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, X.; Wu, Y.; Wang, X. Interaction between Treg Cells and Tumor-Associated Macrophages in the Tumor Microenvironment of Epithelial Ovarian Cancer. Oncol. Rep. 2016, 36, 3472–3478. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Gaggar, S.; Gögenur, I. Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in Cancer and Cancer Immunotherapy. Front. Oncol. 2021, 11, 668731. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Rus Bakarurraini, N.A.A.; Nasir, S.N. Extracellular Vesicles and DAMPs in Cancer: A Mini-Review. Front. Immunol. 2021, 12, 740548. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G.; MikIavcic, D.; Cemazar, M.; Jean Belehradek, J.; Jarm, T.; Mir, L.M. Electrochemotherapy with CDDP on LPB Sarcoma: Comparison of the Anti-Tumor Effectiveness in Immunocompetent and Immunodeficient Mice. Bioelectrochem. Bioenerg. 1997, 43, 279–283. [Google Scholar] [CrossRef]
- Calvet, C.Y.; Famin, D.; André, F.M.; Mir, L.M. Electrochemotherapy with Bleomycin Induces Hallmarks of Immunogenic Cell Death in Murine Colon Cancer Cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef]
- Ursic, K.; Kos, S.; Kamensek, U.; Cemazar, M.; Scancar, J.; Bucek, S.; Kranjc, S.; Staresinic, B.; Sersa, G. Comparable Effectiveness and Immunomodulatory Actions of Oxaliplatin and Cisplatin in Electrochemotherapy of Murine Melanoma. Bioelectrochemistry 2018, 119, 161–171. [Google Scholar] [CrossRef]
- Roux, S.; Bernat, C.; François, B.A.; Opolon, P.; Carpentier, A.F.; Zitvogel, L.; Mir, L.M.; Robert, C. Tumor Destruction Using Electrochemotherapy Followed by CpG Oligodeoxynucleotide Injection Induces Distant Tumor Responses. Cancer Immunol. Res. Immunother. 2008, 57, 1291–1300. [Google Scholar] [CrossRef]
- Bigi, L.; Galdo, G.; Cesinaro, A.M.; Vaschieri, C.; Marconi, A.; Fantini, F. Electrochemotherapy Induces Apoptotic Death in Melanoma Metastases: A Histologic and Immunohistochemical Investigation. Clin. Cosmet. Investig. Dermatol. 2016, 9, 451–459. [Google Scholar] [CrossRef]
- Tremble, L.F.; O’Brien, M.A.; Soden, D.M.; Forde, P.F. Electrochemotherapy with Cisplatin Increases Survival and Induces Immunogenic Responses in Murine Models of Lung Cancer and Colorectal Cancer. Cancer Lett. 2019, 442, 475–482. [Google Scholar] [CrossRef]
- Zmuc, J.; Gasljevic, G.; Sersa, G.; Edhemovic, I.; Boc, N.; Seliskar, A.; Plavec, T.; Brloznik, M.; Milevoj, N.; Brecelj, E.; et al. Large Liver Blood Vessels and Bile Ducts Are Not Damaged by Electrochemotherapy with Bleomycin in Pigs. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Gasljevic, G.; Edhemovic, I.; Cemazar, M.; Brecelj, E.; Gadzijev, E.M.; Music, M.M.; Sersa, G. Histopathological Findings in Colorectal Liver Metastases after Electrochemotherapy. PLoS ONE 2017, 12, e0180709. [Google Scholar] [CrossRef]
- Campana, L.G.; Mocellin, S.; Basso, M.; Puccetti, O.; De Salvo, G.L.; Chiarion-Sileni, V.; Vecchiato, A.; Corti, L.; Rossi, C.R.; Nitti, D. Bleomycin-Based Electrochemotherapy: Clinical Outcome from a Single Institution’s Experience with 52 Patients. Ann. Surg. Oncol. 2009, 16, 191–199. [Google Scholar] [CrossRef]
- Gerlini, G.; Sestini, S. Dendritic Cells Recruitment in Melanoma Metastasis Treated by Electrochemotherapy. Clin. Exp. Metastasis 2013, 30, 37–45. [Google Scholar] [CrossRef]
- Markelc, B.; Sersa, G.; Cemazar, M. Differential Mechanisms Associated with Vascular Disrupting Action of Electrochemotherapy: Intravital Microscopy on the Level of Single Normal and Tumor Blood Vessels. PLoS ONE 2013, 8, e59557. [Google Scholar] [CrossRef]
- Torrero, M.N.; Henk, W.G.; Li, S. Regression of High-Grade Malignancy in Mice by Bleomycin and Interleukin-12 Electrochemogenetherapy. Clin. Cancer Res. 2006, 12, 257–263. [Google Scholar] [CrossRef]
- Mekid, H.; Tounekti, O.; Spatz, A.; Cemazar, M.; El Kebir, F.Z.; Mir, L.M. In Vivo Evolution of Tumour Cells after the Generation of Double-Strand DNA Breaks. Br. J. Cancer 2003, 88, 1763–1771. [Google Scholar] [CrossRef][Green Version]
- Fernandes, P.; Donovan, T.R.O.; Mckenna, S.L.; Forde, P.F. Electrochemotherapy Causes Caspase-Independent Necrotic-Like Death in Pancreatic Cancer Cells. Cancers 2019, 11, 1177. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Gill, K.S.; Saglio, G.; Cilloni, D.; Soden, D.M.; Forde, P.F. Expressional Changes in Stemness Markers Post Electrochemotherapy in Pancreatic Cancer Cells. Bioelectrochemistry 2018, 122, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-B.; Sung, C.-K.; Baik, K.Y.; Moon, K.-W.; Kim, H.-S.; Yi, J.-H.; Jung, J.-H.; Moon, M.-H.; Choi, O.-K. Changes of Apoptosis in Tumor Tissues with Time after Irreversible Electroporation. Biochem. Biophys. Res. Commun. 2013, 435, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Bakos, G.; Shires, P.K.; Gritter, L.; Crissman, J.W.; Harris, J.L.; Clymer, J.W. Histological and Finite Element Analysis of Cell Death Due to Irreversible Electroporation. Technol. Cancer Res. Treat. 2014, 13, 561–569. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible Electroporation Reverses Resistance to Immune Checkpoint Blockade in Pancreatic Cancer. Nat. Commun. 2019, 10, 899. [Google Scholar] [CrossRef]
- He, C.; Sun, S.; Zhang, Y.; Xie, F.; Li, S. The Role of Irreversible Electroporation in Promoting M1 Macrophage Polarization via Regulating the HMGB1-RAGE-MAPK Axis in Pancreatic Cancer. Oncoimmunology 2021, 10, 1897295. [Google Scholar] [CrossRef]
- Ichikawa, M.L.; Vu, N.K.; Nijagal, A.; Rubinsky, B.; Chang, T.T. Neutrophils Are Important for the Development of pro - Reparative Macrophages after Irreversible Electroporation of the Liver in Mice. Sci. Rep. 2021, 11, 14986. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Y.; He, C.; Hu, W.; Liu, W.; Huang, X.; Wu, J.; Xie, F.; Chen, C.; Wang, J.; et al. Combining NanoKnife with M1 Oncolytic Virus Enhances Anticancer Activity in Pancreatic Cancer. Cancer Lett. 2021, 502, 9–24. [Google Scholar] [CrossRef]
- Ringel-scaia, V.M.; Beitel-white, N.; Lorenzo, M.F.; Brock, R.M.; Huie, K.E.; Coutermarsh-ott, S.; Eden, K.; Mcdaniel, D.K.; Verbridge, S.S.; Rossmeisl, J.H.; et al. High-Frequency Irreversible Electroporation Is an Effective Tumor Ablation Strategy That Induces Immunologic Cell Death and Promotes Systemic Anti-Tumor Immunity. EBioMedicine 2019, 44, 112–125. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Klein, R.; Nijm, G.M.; Alan, V.; Omary, R.A.; Yang, G.; Andrew, C. Liver-Directed Irreversible Electroporation Therapy: Longitudinal Efficacy Studies in a Rat Model of Hepatocellular Carcinoma. Cancer Res. 2011, 70, 1555. [Google Scholar] [CrossRef]
- José, A.; Sobrevals, L.; Ivorra, A.; Fillat, C. Irreversible Electroporation Shows Efficacy against Pancreatic Carcinoma without Systemic Toxicity in Mouse Models. Cancer Lett. 2012, 317, 16–23. [Google Scholar] [CrossRef]
- Bulvik, B.E.; Ahmed, M.; Andriyanov, A.V.; Goldberg, S.N. Irreversible Electroporation versus Radiofrequency Ablation: A Comparison of Local and Systemic. Radiology 2016, 280, 413–424. [Google Scholar] [CrossRef]
- He, C.; Lin, X.; Zhang, Y.; Lin, X.; Li, S. T-Cell Activation and Immune Memory Enhancement Induced by Irreversible Electroporation in Pancreatic Cancer. Clin. Transl. Med. 2020, 10, e39. [Google Scholar] [CrossRef]
- Babikr, F.; Wan, J.; Xu, A.; Wu, Z.; Ahmed, S.; Freywald, A.; Chibbar, R.; Wu, Y.; Moser, M.; Groot, G.; et al. Distinct Roles but Cooperative Effect of TLR3/9 Agonists and PD-1 Blockade in Concerting the Immunotolerant Microinvironment of Irreversible Electroporation-Ablated Tumors. Nat. Cellluar Mol. Immunol. 2021, 18, 2632–2647. [Google Scholar] [CrossRef]
- Pandit, H.; Hong, Y.K.; Li, Y.; Rostas, J.; Pulliam, Z. Evaluating the Regulatory Immunomodulation Effect of Irreversible Electroporation (IRE) in Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 800–806. [Google Scholar] [CrossRef]
- Lee, E.W.; Wong, D.; Tafti, B.A.; Prieto, V.; Totonchy, M.; Hilton, J.; Dry, S.; Cho, S.; Loh, C.T. Irreversible Electroporation in Eradication of Rabbit VX2 Liver Tumor. JVIR 2012, 23, 833–840. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, Z.; Lei, K.; Liao, J.; Peng, Z.; Lin, M.; Liang, P.; Yu, J.; Peng, S.; Chen, S.; et al. Irreversible Electroporation Induces CD8 + T Cell Immune Response against Post-Ablation Hepatocellular Carcinoma Growth. Cancer Lett. 2021, 503, 1–10. [Google Scholar] [CrossRef]
- Neal, R.E.; Jr, J.H.R.; Robertson, J.L.; Arena, C.B.; Davis, E.M.; Singh, R.N.; Stallings, J.; Davalos, R.V. Improved Local and Systemic Anti-Tumor Efficacy for Irreversible Electroporation in Immunocompetent versus Immunodeficient Mice. PLoS ONE 2013, 8, e64559. [Google Scholar] [CrossRef]
- Al-Sakere, B.; Bernat, C.; André, F.; Connault, E.; Opolon, P.; Davalos, R.V.; Mir, L.M. A Study of the Immunological Response to Tumor Ablation with Irreversible Electroporation. Technol. Cancer Res. Treat. 2007, 6, 301–306. [Google Scholar] [CrossRef]
- Martinez, O.M.; Villanueva, J.; Abtahi, S.; Beatty, P.R.; Esquivel, C.O.; Krams, S.M. CD30 Expression Identifies a Functional Alloreactive Human T-Lymphocyte Subset. Transplantation 1998, 65, 1240–1247. [Google Scholar] [CrossRef]
- Mateizel, I.; Spits, C.; Verloes, A.; Mertzanidou, A.; Liebaers, I.; Sermon, K. Characterization of CD30 Expression in Human Embryonic Stem Cell Lines Cultured in Serum-Free Media and Passaged Mechanically. Hum. Reprod. 2009, 24, 2477–2489. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, J.; Sun, S.; Zhang, Y.; Li, S. Immunomodulatory Effect after Irreversible Electroporation in Patients with Locally Advanced Pancreatic Cancer. J. Oncol. 2019, 2019, 9346017. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, K.; Li, W.; Qiu, X.; Ma, B.; Fan, Q.; Li, Z. Immunologic Response to Tumor Ablation with Irreversible Electroporation. PLoS ONE 2012, 7, e48749. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.H.; Gemlo, B.T.; Myers, W.W.; Rayner, A.A.; Lanier, L.L. In Vivo and in Vitro Activation of Natural Killer Cells in Advanced Cancer Patients Undergoing Combined Recombinant Interleukin-2 and LAK Cell Therapy. J. Clin. Oncol. 1987, 5, 1933–1941. [Google Scholar] [CrossRef]
- Wrangle, J.M.; Patterson, A.; Johnson, C.B.; Neitzke, D.J.; Mehrotra, S.; Denlinger, C.E.; Paulos, C.M.; Li, Z.; Cole, D.J.; Rubinstein, M.P. IL-2 and beyond in Cancer Immunotherapy. J. Interf. Cytokine Res. 2018, 38, 45–68. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and Related Cytokines as the Critical Lynchpins between Inflammation and Cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Kryczek, I.; Zou, L.; Rodriguez, P.; Zhu, G.; Wei, S.; Mottram, P.; Brumlik, M.; Cheng, P.; Curiel, T.; Myers, L.; et al. B7-H4 Expression Identifi Es a Novel Suppressive Macrophage Population in Human Ovarian Carcinoma. J. Exp. Med. 2006, 203, 871–881. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, W.P. B7-H4, a Promising Target for Immunotherapy. Cell. Immunol. 2019, 347, 104008. [Google Scholar] [CrossRef]
- Kuang, D.; Zhao, Q.; Peng, C.; Xu, J.; Zhang, J.; Wu, C.; Zheng, L. Activated Monocytes in Peritumoral Stroma of Hepatocellular Carcinoma Foster Immune Privilege and Disease Progression through PD-L1. J. Exp. Med. 2009, 206, 1327–1337. [Google Scholar] [CrossRef]
- Guo, X.; Du, F.; Liu, Q.; Guo, Y.; Wang, Q.; Huang, W.; Wang, Z. Immunological Effect of Irreversible Electroporation on Hepatocellular Carcinoma. BMC Cancer 2021, 21, 443. [Google Scholar] [CrossRef]
- Scheffer, H.J.; Stam, A.G.M.; Geboers, B.; Vroomen, L.G.P.H.; Ruarus, A.; de Bruijn, B.; van den Tol, M.P.; Kazemier, G.; Meijerink, M.R.; de Gruijl, T.D. Irreversible Electroporation of Locally Advanced Pancreatic Cancer Transiently Alleviates Immune Suppression and Creates a Window for Antitumor T Cell Activation. Oncoimmunology 2019, 8, 1652532. [Google Scholar] [CrossRef]
- Martin, R.C.G.; Li, Y.; Brock, R.M.; Allen, I.C.; Davalos, R.V. Real-Time Prediction of Patient Immune Cell Modulation during Irreversible Electroporation Therapy. Sci. Reports 2019, 9, 17739. [Google Scholar] [CrossRef]
- Fujimori, M.; Kimura, Y.; Ueshima, E.; Dupuy, D.E.; Adusumilli, P.S.; Solomon, S.B.; Srimathveeravalli, G. Lung Ablation with Irreversible Electroporation Promotes Immune Cell Infiltration by Sparing Extracellular Matrix Proteins and Vasculature: Implications for Immunotherapy. Bioelectricity 2021, 3, 204–214. [Google Scholar] [CrossRef]
- Chen, X.; Ren, Z.; Yin, S.; Xu, Y.; Guo, D.; Xie, H.; Zhou, L.; Wu, L.; Jiang, J.; Li, H.; et al. The Local Liver Ablation with Pulsed Electric Field Stimulate Systemic Immune Reaction against Hepatocellular Carcinoma (HCC) with Time-Dependent Cytokine Profile. Cytokine 2017, 93, 44–50. [Google Scholar] [CrossRef]
- White, S.B.; Zhang, Z.; Chen, J.; Gogineni, V.R.; Larson, A.C. Early Immunologic Response of Irreversible Electroporation versus Cryoablation in a Rodent Model of Pancreatic Cancer. J. Vasc. Interv. Radiol. 2018, 29, 1764–1769. [Google Scholar] [CrossRef]
- Goswami, I.; Coutermarsh-Ott, S.; Morrison, R.G.; Allen, I.; Davalos, R.V.; Verbridge, S.S.; Bickford, L.R. Irreversible Electroporation Inhibits Pro-Cancer Inflammatory Signaling in Triple Negative Breast Cancer Cells. Bioelectrochemistry 2017, 113, 42–50. [Google Scholar] [CrossRef]
- Cameron, F.; Whiteside, G.; Perry, C. Ipilimumab. Drugs 2011, 71, 1093–1104. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Williams, R.; Cooley, S.; Bachanova, V.; Waldmann, T.A.; Blazar, B.R.; Weisdorf, D.J.; Miller, J.S.; Verneris, M.R. Role of Recipient CD8+ T Cell Exhaustion in the Rejection of Adoptively Transferred Haploidentical NK Cells. Blood 2016, 128, 503. [Google Scholar] [CrossRef]
- Andersen, M.H.; Gehl, J.; Reker, S.; Pedersen, L.; Becker, J.C.; Geertsen, P.; Thor Straten, P. Dynamic Changes of Specific T Cell Responses to Melanoma Correlate with IL-2 Administration. Semin. Cancer Biol. 2003, 13, 449–459. [Google Scholar] [CrossRef]
- Theurich, S.; Rothschild, S.I.; Hoffmann, M.; Fabri, M.; Sommer, A.; Garcia-Marquez, M.; Thelen, M.; Schill, C.; Merki, R.; Schmid, T.; et al. Local Tumor Treatment in Combination with Systemic Ipilimumab Immunotherapy Prolongs Overall Survival in Patients with Advanced Malignant Melanoma. Cancer Immunol. Res. 2016, 4, 744–754. [Google Scholar] [CrossRef]
- Campana, L.G.; Peric, B.; Mascherini, M.; Spina, R.; Kunte, C.; Kis, E.; Rozsa, P.; Quaglino, P.; Jones, R.P.; Clover, A.J.P.; et al. Combination of Pembrolizumab with Electrochemotherapy in Cutaneous Metastases from Melanoma: A Comparative Retrospective Study from the InspECT and Slovenian Cancer Registry. Cancers 2021, 13, 4289. [Google Scholar] [CrossRef]
- Heppt, M.V.; Eigentler, T.K.; Kähler, K.C.; Herbst, R.A.; Göppner, D.; Gambichler, T.; Ulrich, J.; Dippel, E. Immune Checkpoint Blockade with Concurrent Electrochemotherapy in Advanced Melanoma: A Retrospective Multicenter Analysis. Cancer Immunol. Immunother. 2016, 65, 951–959. [Google Scholar] [CrossRef]
- Hribernik, A.; Cemazar, M.; Sersa, G.; Bosnjak, M.; Snoj, M. Effectiveness of Electrochemotherapy after IFN-α Adjuvant Therapy of Melanoma Patients. Radiol. Oncol. 2016, 50, 21–27. [Google Scholar] [CrossRef]
- Ursic, K.; Kos, S.; Kamensek, U.; Cemazar, M.; Miceska, S.; Markelc, B.; Bucek, S.; Staresinic, B.; Kloboves, V.; Heller, R.; et al. Potentiation of Electrochemotherapy Effectiveness by Immunostimulation with IL-12 Gene Electrotransfer in Mice Is Dependent on Tumor Immune Status. J. Control. Release 2021, 332, 623–635. [Google Scholar] [CrossRef]
- Sedlar, A.; Dolinsek, T.; Markelc, B.; Prosen, L.; Kranjc, S.; Bosnjak, M.; Blagus, T.; Cemazar, M.; Sersa, G. Potentiation of Electrochemotherapy by Intramuscular IL-12 Gene Electrotransfer in Murine Sarcoma and Carcinoma with Different Immunogenicity. Radiol. Oncol. 2012, 46, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Kishida, T.; Asada, H.; Itokawa, Y.; Yasutomi, K.; Shin-ya, M. Electrochemo-Gene Therapy of Cancer: Intratumoral Delivery of Interleukin-12 Gene and Bleomycin Synergistically Induced Therapeutic Immunity and Suppressed Subcutaneous and Metastatic Melanomas in Mice. Mol. Ther. 2003, 8, 739–745. [Google Scholar] [CrossRef]
- Tremble, L.F.; O’Brien, M.A.; Forde, P.F.; Soden, D.M. ICOS Activation in Combination with Electrochemotherapy Generates Effective Anti-Cancer Immunological Responses in Murine Models of Primary, Secondary and Metastatic Disease. Cancer Lett. 2018, 420, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Cemazar, M.; Todorovic, V.; Scancar, J.; Lampreht, U.; Stimac, M.; Kamensek, U.; Kranjc, S.; Coer, A.; Sersa, G. Adjuvant TNF-α Therapy to Electrochemotherapy with Intravenous Cisplatin in Murine Sarcoma Exerts Synergistic Antitumor Effectiveness. Radiol. Oncol. 2015, 49, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G.; Cemazar, M.; Menart, V. Anti-Tumor Effectiveness of Electrochemotherapy with Bleomycin Is Increased by TNF-a on SA-l Tumors in Mice. Cancer Lett. 1997, 116, 85–92. [Google Scholar] [CrossRef]
- Quaresmini, D.; Di Lauro, A.; Fucci, L.; Strippoli, S.; De Risi, I.; Sciacovelli, A.M.; Albano, A.; Achille, G.; Montepara, M.; Russo, S.; et al. Electrochemotherapy as a Trigger to Overcome Primary Resistance to Anti-PD-1 Treatment: A Case Report of Melanoma of the Scalp. Front. Oncol. 2021, 11, 742666. [Google Scholar] [CrossRef]
- Karaca, B.; Yayla, G.; Erdem, M.; Gürler, T. Electrochemotherapy with Anti-PD-1 Treatment Induced Durable Complete Response in Heavily Pretreated Metastatic Melanoma Patient. Anticancer. Drugs 2018, 29, 190–196. [Google Scholar] [CrossRef]
- Mozzillo, N.; Simeone, E.; Benedetto, L.; Curvietto, M.; Giannarelli, D.; Gentilcore, G.; Camerlingo, R.; Capone, M.; Madonna, G.; Festino, L.; et al. Assessing a Novel Immuno-Oncology-Based Combination Therapy: Ipilimumab plus Electrochemotherapy. Oncoimmunology 2015, 4, e1008842. [Google Scholar] [CrossRef]
- Brizio, M.; Fava, P.; Astrua, C.; Cavaliere, G.; Savoia, P. Complete Regression of Melanoma Skin Metastases after Electrochemotherapy plus Ipilimumab Treatment: An Unusual Clinical Presentation. Eur. J. Dermatol. 2015, 25, 271–272. [Google Scholar] [CrossRef]
- Salvadori, C.; Svara, T.; Rocchigiani, G.; Millanta, F.; Pavlin, D.; Cemazar, M.; Tratar, U.L.; Sersa, G.; Tozon, N.; Poli, A. Effects of Electrochemotherapy with Cisplatin and Peritumoral IL-12 Gene Electrotransfer on Canine Mast Cell Tumors: A Histopathologic and Immunohistochemical Study. Radiol. Oncol. 2017, 51, 286–294. [Google Scholar] [CrossRef]
- Ramirez, L.H.; Orlowski, S.; An, D.; Bindoula, G.; Dzodic, R.; Ardouin, P.; Bognel, C.; Belehradek, J.; Munck, J.N.; Mir, L.M. Electrochemotherapy on Liver Tumours in Rabbits. Br. J. Cancer 1998, 77, 2104–2111. [Google Scholar] [CrossRef]
- Mir, L.M.; Roth, C.; Stéphane, O.; Françoise, Q.C.; Fradelizi, D.; Belehradek, J.; Kourilsky, P. Systemic Antitumor Effects of Electrochemotherapy Combined with Histoincompatible Cells Secreting Interleukin-2. J. Immunother. 1995, 17, 30–38. [Google Scholar] [CrossRef]
- O’Neill, C.; Hayat, T.; Hamm, J.; Healey, M.; Zheng, Q.; Li, Y.; Martin, R.C.G. A Phase 1b Trial of Concurrent Immunotherapy and Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Adenocarcinoma. Surgery 2020, 168, 610–616. [Google Scholar] [CrossRef]
- He, C.; Sun, S.; Zhang, Y.; Li, S. Irreversible Electroporation Plus Anti-PD-1 Antibody versus Irreversible Electroporation Alone for Patients with Locally Advanced Pancreatic Cancer. J. Inflamm. Res. 2021, 14, 4795–4807. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, C.; Fan, Y.; Wang, Y.; Li, R.; Hu, X. Efficacy of Irreversible Electroporation Ablation Combined with Natural Killer Cells in Treating Locally Advanced Pancreatic Cancer. JBUON 2020, 25, 1643–1649. [Google Scholar]
- Lin, M.; Alnaggar, M.; Liang, S.; Wang, X.; Liang, Y.; Zhang, M.; Chen, J.; Niu, L.; Xu, K. An Important Discovery on Combination of Irreversible Electroporation and Allogeneic Natural Killer Cell Immunotherapy for Unresectable Pancreatic Cancer. Oncotarget 2017, 8, 101795–101807. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhang, X.; Liang, S.; Luo, H.; Alnaggar, M.; Liu, A.; Yin, Z.; Chen, J.; Niu, L.; Jiang, Y. Irreversible Electroporation plus Allogenic Vγ9Vδ2 T Cells Enhances Antitumor Effect for Locally Advanced Pancreatic Cancer Patients. Nat. Signal Transduct. Target. Ther. 2020, 5, 215. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, T.; Li, J.; Mai, F.; Li, J.; Chen, Y.; Jing, Y.; Dong, X.; Lin, L.; He, J.; et al. Selenium Nanoparticles as New Strategy to Potentiate Γδ T Cell Anti-Tumor Cytotoxicity through Upregulation of Tubulin-α Acetylation. Biomaterials 2019, 222, 119397. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer Immunotherapy with Γδ T Cells: Many Paths Ahead of Us. Cell. Mol. Immunol. 2020, 17, 925–939. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Z.; Du, D.; Wu, Y.; Qiu, S.; Mu, F.; Xu, K.; Chen, J. Safety and Short-Term Efficacy of Irreversible Electroporation and Allogenic Natural Killer Cell Immunotherapy Combination in the Treatment of Patients with Unresectable Primary Liver Cancer. Cardiovasc. Intervent. Radiol. 2019, 42, 48–59. [Google Scholar] [CrossRef]
- Alnaggar, M.; Lin, M.; Mesmare, A.; Liang, S.; Qaid, A.; Xu, K.; Chen, J.; Niu, L.; Yin, Z. Allogenic Natural Killer Cell Immunotherapy Combined with Irreversible Electroporation for Stage IV Hepatocellular Carcinoma: Survival Outcome. Cell. Physiol. Biochem. 2018, 48, 1882–1893. [Google Scholar] [CrossRef]
- Burbach, B.J.; Flanagan, S.D.O.; Shao, Q.; Young, K.M.; Slaughter, J.R.; Rollins, M.R.; Street, T.J.L.; Granger, V.E.; Beura, L.K.; Azarin, S.M.; et al. Irreversible Electroporation Augments Checkpoint Immunotherapy in Prostate Cancer and Promotes Tumor Antigen-Specific Tissue-Resident Memory CD8+ T Cells. Nat. Commun. 2021, 12, 3862. [Google Scholar] [CrossRef]
- Shi, X.; Neill, C.O.; Wang, X.; Chen, Y.; Yu, Y.; Tan, M.; Lv, G.; Li, Y.; Martin, R.C. Irreversible Electroporation Enhances Immunotherapeutic Effect in the Off-Target Tumor in a Murine Model of Orthotopic HCC. Am. J. Cancer Res. 2021, 11, 3304–3319. [Google Scholar]
- Narayanan, J.S.S.; Ray, P.; Hayashi, T.; Whisenant, T.C.; Vicente, D.; Carson, D.A.; Miller, A.M.; Schoenberger, S.P.; White, R.R. Irreversible Electroporation Combined With Checkpoint Blockade and TLR7 Stimulation Induces Antitumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol. Res. 2019, 7, 1714–1726. [Google Scholar] [CrossRef]
- Lasarte-Cia, A.; Lozano, T.; Cano, D.; Martín-otal, C.; Navarro, F.; Gorraiz, M.; Casares, N.; Vivas, I.; Lasarte, J.J. Intratumoral STING Agonist Injection Combined with Irreversible Electroporation Delays Tumor Growth in a Model of Hepatocarcinoma. Biomed Res. Int. 2021, 2021, 8852233. [Google Scholar] [CrossRef]
- Go, E.; Yang, H.; Chon, H.J.; Yang, D.; Ryu, W. Combination of Irreversible Electroporation and STING Agonist for Effective Cancer Immunotherapy. Cancers 2020, 12, 3123. [Google Scholar] [CrossRef]
- Geboers, B.; Timmer, F.E.F.; Ruarus, A.H.; Pouw, J.E.E.; Schouten, E.A.C.; Bakker, J.; Puijk, R.S.; Nieuwenhuizen, S.; Dijkstra, M.; Tol, M.P.; et al. Irreversible Electroporation and Nivolumab Combined with Intratumoral Administration of a Toll-Like Receptor Ligand, as a Means of In Vivo Vaccination for Metastatic Pancreatic Ductal Adenocarcinoma (PANFIRE-III). A Phase-I Study Protocol. Cancers 2021, 13, 3902. [Google Scholar] [CrossRef]
- Lin, M.; Liang, S.; Wang, X.; Liang, Y.; Zhang, M.; Chen, J.; Niu, L.; Xu, K. Short-Term Clinical Efficacy of Percutaneous Irreversible Electroporation Combined with Allogeneic Natural Killer Cell for Treating Metastatic Pancreatic Cancer. Immunol. Lett. 2017, 186, 20–27. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Zhou, Y.; Wang, Q.; Liu, Q.; Wu, Z.; Ding, X. OX40 Agonist Combined with Irreversible Electroporation Synergistically Eradicates Established Tumors and Drives Systemic Antitumor Immune Response in a Syngeneic Pancreatic Cancer Model. Am. J. Cancer Res. 2021, 11, 2782–2801. [Google Scholar]
- Yang, J.; Eresen, A.; Shangguan, J.; Ma, Q.; Yaghmai, V. Irreversible Electroporation Ablation Overcomes Tumor-Associated Immunosuppression to Improve the Efficacy of DC Vaccination in a Mice Model of Pancreatic Cancer. Oncoimmunology 2021, 10, 1875638. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, W.; Kwak, K.; Choi, H.; Kim, D.H. Electric Pulse Responsive Magnetic Nanoclusters Loaded with Indoleamine 2,3-Dioxygenase Inhibitor for Synergistic Immuno-Ablation Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 54415–54425. [Google Scholar] [CrossRef]
- Vivas, I.; Iribarren, K.; Lozano, T.; Cano, D.; Lasarte-cia, A.; Chocarro, S.; Gorraiz, M.; Sarobe, P.; Herv, S.; Bilbao, I.; et al. Therapeutic Effect of Irreversible Electroporation in Combination with Poly-ICLC Adjuvant in Preclinical Models of Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2019, 30, 1098–1105. [Google Scholar] [CrossRef]
- Pasquet, L.; Bellard, E.; Chabot, S.; Markelc, B.; Rols, M.; Teissie, J.; Golzio, M. Pre-Clinical Investigation of the Synergy Effect of Interleukin-12 Gene-Electro-Transfer during Partially Irreversible Electropermeabilization against Melanoma. J. Immunother. Cancer 2019, 7, 161. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Østrup, O.; Dagenborg, V.J.; Rødland, E.A.; Skarpeteig, V.; Silwal-Pandit, L.; Grzyb, K.; Berstad, A.E.; Fretland, Å.A.; Mælandsmo, G.M.; Børresen-Dale, A.L.; et al. Molecular Signatures Reflecting Microenvironmental Metabolism and Chemotherapy-Induced Immunogenic Cell Death in Colorectal Liver Metastases. Oncotarget 2017, 8, 76290–76304. [Google Scholar] [CrossRef]
- Dagenborg, V.J.; Marshall, S.E.; Grzyb, K.; Fretland, Å.A.; Lund-Iversen, M.; Mælandsmo, G.M.; Ree, A.H.; Edwin, B.; Yaqub, S.; Flatmark, K. Low Concordance Between T-Cell Densities in Matched Primary Tumors and Liver Metastases in Microsatellite Stable Colorectal Cancer. Front. Oncol. 2021, 11, 671629. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; Velculescu, V.E.; et al. Distant Metastasis Occurs Late during the Genetic Evolution of Pancreatic Cancer. Nature 2011, 467, 1114–1117. [Google Scholar] [CrossRef]
- Campbell, P.J.; Yachida, S.; Mudie, L.J.; Stephens, P.J.; Erin, D.; Stebbings, L.A.; Morsberger, L.A.; Latimer, C.; Mclaren, S.; Lin, M.; et al. The Patterns and Dynamics of Genomic Instability in Metastatic Pancreatic Cancer. Nature 2010, 467, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.D.; Chang, J.Y. Time to Abandon Single-Site Irradiation for Inducing Abscopal Effects. Nat. Rev. Clin. Oncol. 2019, 16, 123–135. [Google Scholar] [CrossRef] [PubMed]
| Species | Authors | Interventions (Type, n) | Cancer Types | Key Findings |
|---|---|---|---|---|
| Human | Gasljevic et al., 2017 [77] | ECT (bleomycin, 7) | Colorectal cancer | ECT induced coagulation necrosis. The majority of vessels >5 mm in diameter remained functional. |
| Bigi et al., 2016 [74] | ECT (bleomycin, 2) | Cutaneous melanoma | High prevalence of tumor-infiltrating CD8+ T cells and foci of NK cells 3 h to 1 month after ECT. Apoptotic cell death was followed by necrosis 48–72 h after ECT. | |
| Gerlini et al., 2013 [79] | ECT (bleomycin, 9) | Metastatic melanoma | ECT promoted Langerhans cell migration from the tumor to draining lymph nodes and DC recruitment to the tumor. Further, DCs found in low number before ECT greatly increased at day 7 to 14. | |
| Mouse | Tremble et al., 2019 [75] | ECT (cisplatin) | Colorectal cancer | ECT increased tumor infiltration of macrophages, neutrophils, B, NK, natural killer T cells, and DCs. Further, it decreased tumor growth of both treated and distal non-treated tumors. |
| Ursic et al., 2018 [72] | ECT (cisplatin/oxaliplatin) | Melanoma | ECT induced a 4-fold increase in tumor infiltration of NK cells and CD8+ T cells. | |
| Calvet et al., 2014 [71] | ECT (bleomycin) | Colon cancer | ECT induced ICD through the liberation of ATP and HMGB1 and the translocation of calreticulin to the cell surface. Seven out of 8 immunocompetent mice were disease-free 24 days after ECT treatment, whereas all immunodeficient mice presented PD. | |
| Markelc et al., 2013 [80] | ECT (bleomycin) | Colorectal cancer | ECT induced a complete stop of the tumor blood vessels for up to 24 h. No damage to peritumoral normal blood vessels. | |
| Roux et al., 2008 [73] | ECT (bleomycin) | Sarcoma | ECT induced recruitment of tumor-infiltrating DCs and CD8+ T cells after 48–96 h, while the presence of CD4+ T cells remained stable. | |
| Torrero et al., 2006 [81] | ECT (bleomycin) | Breast cancer | ECT induced inhibition of angiogenesis in tumors but did not increase CD8+ T cell activity. | |
| Mekid et al., 2003 [82] | ECT (bleomycin) | Sarcoma | ECT increased the tumor infiltration of lymphocytes after 25, 50, and 75 h, in particular in the vicinity of apoptotic cells. | |
| Sersa et al., 1997 [70] | ECT (cisplatin) | Sarcoma | The tumor growth delay in immunocompetent mice was twice as long as in immunodeficient mice. Further, a high percentage of tumor cures was achieved in immunocompetent mice but none in immunodeficient mice. Of the mice cured after ECT, 75% rejected the tumor challenge, while none of the control mice did. | |
| Cell | Fernandes et al., 2019 [83] | ECT (bleomycin/cisplatin/oxaliplatin) | Pancreatic cancer | ECT led to necroptosis. |
| Ali et al., 2018 [84] | ECT (bleomycin/cisplatin/oxaliplatin) | Pancreatic cancer | The ECT treatments induced changes in stemness inducing factors related to cancer stem cells. |
| Species | Authors | Interventions (n) | Cancer Types | Key Findings |
|---|---|---|---|---|
| Human | Campana et al., 2021 [124] | ECT (bleomycin)/pembrolizumab/ECT + pembrolizumab (41/44/45) ** | Metastatic melanoma | Local response: ECT/pembrolizumab: 44%/32 CR, 37%/7% PR ECT + pembrolizumab: 49% CR, 29% PR Systemic response: Pembrolizumab: 21% CR, 4% PR ECT + pembrolizumab: 11% CR, 13% PR Two-year OS: Pembrolizumab: 43% ECT + pembrolizumab: 70% |
| Quaresmini et al., 2021 [133] | ECT (bleomycin) + nivolumab (1) * | Metastatic melanoma | Durable CR (>1 year) | |
| Karaca et al., 2018 [134] | ECT (bleomycin) + nivolumab (1) * | Metastatic melanoma | Durable CR (>1 year) locally and systemic | |
| Hribernok et al., 2016 [126] | ECT (bleomycin/cisplatin) + INF-α (5) ** | Advanced melanoma | Three patients with CR (1–23 lesions), 1 patient with CR of >85% of lesions (80 lesions), 1 patient with PR (5 lesions) | |
| Theurich et al., 2016 [123] | (ECT/radiotherapy) + ipilimumab/ipilimumab (45/82) *** | Advanced melanoma | Local response: Ipilimumab: 0% CR, 18% PR Ipilimumab + (ECT/radiotherapy): 7% CR, 31% PR Median OS: Ipilimumab: 42 weeks Ipilimumab + (ECT/radiotherapy): 93 weeks (hazard ratio 0.46) | |
| Heppt et al., 2016 [125] | ECT (bleomycin) + ICI (ipilimumab/pembrolizumab/nivolumab, 33) ** | Metastatic melanoma | Local response: 15% CR, 52% PR Systemic response: 6% CR, 16% PR Median progression free survival: 2.5 months; median OS: not reached | |
| Mozzillo et al., 2015 [135] | ECT (bleomycin) + ipilimumab (15) ** | Metastatic melanoma | Local response: 27% CR, 40% PR Systemic response: 0% CR, 33% PR One-year OS: 86% At week 10 and 12, a decrease in the absolute Treg number was seen in responders compared to no responders | |
| Brizio et al., 2015 [136] | ECT (bleomycin) + ipilimumab (1) * | Metastatic melanoma | ECT: Multiple liver and adrenal glands metastases after 3 ECT treatments ECT + ipilimumab: Durable CR (1 year) locally and systemically | |
| Andersen et al., 2003 [122] | ECT (bleomycin) + IL-2 (6) *** | Metastatic melanoma | ECT + IL-2 induced a partial remission Antitumor cytotoxic T lymphocyte responses declined following IL-2 therapy | |
| Dog | Salvadori et al., 2017 [137] | ECT (cisplatin) + IL-12 GET | Mast cell tumor | Sixty-four percent CR Increased tumor infiltration of T lymphocytes at 4 weeks |
| Rabbit | Ramirez et al., 1998 [138] | ECT (bleomycin) + IL-2 secreting cells | Hepatocellular carcinoma | Median survival: Controls: 50 days (average number of metastases of 27) ECT: 82 days (average number of metastases of 18) ECT + IL-2: 80 days (average number of metastases of 3) |
| Mouse | Ursic et al., 2021 [127] | ECT (cisplatin/oxaliplatin/bleomycin) + IL-12 GET | Colorectal cancer Breast cancer Melanoma | ECT (cisplatin/oxaliplatin/bleomycin): 83%/83%/50% CR ECT + IL-12: 100%/100%/50% CR ECT: 50%/33%/17% CR ECT + IL-12: 67%/83%/33% CR ECT: 0%/0%/0% CR ECT + IL-12: 38%/0%/0% CR |
| Tremble et al., 2018 [130] | ECT (cisplatin) + inducible T-cell co-stimulator | Colorectal cancer Metastatic Lewis Lung Carcinoma | Median survival: Control/ECT: 12/24 days ECT + inducible T-cell co-stimulator: 80 days ECT + inducible T-cell co-stimulator reduced the tumor growth of secondary non-treated tumors and increased the survival One hundred-day survival of 33% compared to 0% in monotherapy groups | |
| Cemazar et al., 2015 [131] | ECT (cisplatin) + TNF-α | Fibrosarcoma | Control/ECT: 0% CR ECT + TNF-α: 36% CR | |
| Sedlar et al., 2012 [128] | ECT (cisplatin) + IL-12 GET | Fibrosarcoma | Control/ECT: 0%/17% CR ECT + IL-12: 60% CR | |
| Roux et al., 2008 [73] | ECT (bleomycin) + CpG oligodeoxynucleotides | Fibrosarcoma Melanoma | ECT: Recruitment of tumor-infiltrating CD8+ cells 48–96 h after ECT; CD4+ cells remained stable Forty-three percent CR in treated tumors, 0% CR in non-treated tumors ECT + CpG: 100% CR in treated tumors, 57% CR in non-treated tumors ECT + CpG: Superior efficacy in reducing tumor volume compared to ECT, both in treated and non-treated tumors; induced a functional and specific activation of T cells both regionally (draining lymph node) and peripherally | |
| Torrero et al., 2006 [81] | ECT (bleomycin) + IL-12 GET | Breast cancer | Median survival: Control/ECT: 34/46 days ECT + IL-12: 60 days | |
| Kishida et al., 2003 [129] | ECT (bleomycin) + IL-12 GET | Melanoma | Median survival: Control/ECT: 18/37 days ECT + IL-12: 62 days In a metastatic model, ECT + IL-12 reduced the number of metastatic foci and increased the survival compared to monotherapy | |
| Sersa et al., 1997 [132] | ECT (bleomycin) + TNF-α | Fibrosarcoma | Median survival: Control/ECT: 24/33 days. 0% CR ECT + TNF-α: 50 days. 33% CR | |
| Mir et al., 1995 [139] | ECT (bleomycin) + IL-2 secreting cells | Fibrosarcoma | ECT: 60% CR ECT + IL-2: 100% CR Fifty percent CR in non-treated tumors; increased infiltration of CD4+ and CD8+ T cells in both treated and non-treated tumors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Justesen, T.F.; Orhan, A.; Raskov, H.; Nolsoe, C.; Gögenur, I. Electroporation and Immunotherapy—Unleashing the Abscopal Effect. Cancers 2022, 14, 2876. https://doi.org/10.3390/cancers14122876
Justesen TF, Orhan A, Raskov H, Nolsoe C, Gögenur I. Electroporation and Immunotherapy—Unleashing the Abscopal Effect. Cancers. 2022; 14(12):2876. https://doi.org/10.3390/cancers14122876
Chicago/Turabian StyleJustesen, Tobias Freyberg, Adile Orhan, Hans Raskov, Christian Nolsoe, and Ismail Gögenur. 2022. "Electroporation and Immunotherapy—Unleashing the Abscopal Effect" Cancers 14, no. 12: 2876. https://doi.org/10.3390/cancers14122876
APA StyleJustesen, T. F., Orhan, A., Raskov, H., Nolsoe, C., & Gögenur, I. (2022). Electroporation and Immunotherapy—Unleashing the Abscopal Effect. Cancers, 14(12), 2876. https://doi.org/10.3390/cancers14122876








