Enhancer of Zeste Homolog 2 (EZH2) Is a Marker of High-Grade Neuroendocrine Neoplasia in Gastroenteropancreatic and Pulmonary Tract and Predicts Poor Prognosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patient Enrollment for Immunohistochemistry
2.3. Immunohistochemical Staining
2.4. Statistics
3. Results
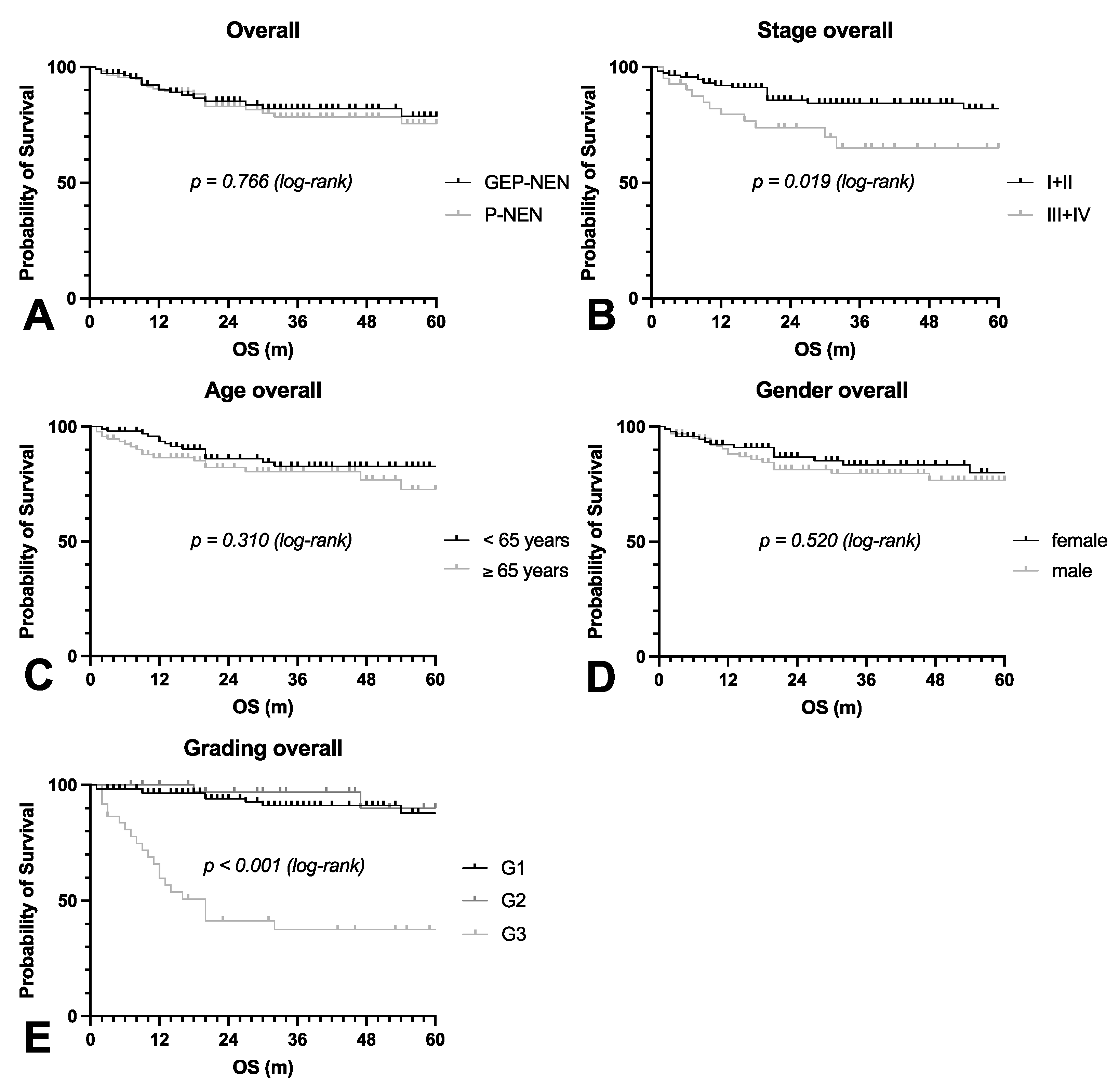
3.1. Patient Characteristics
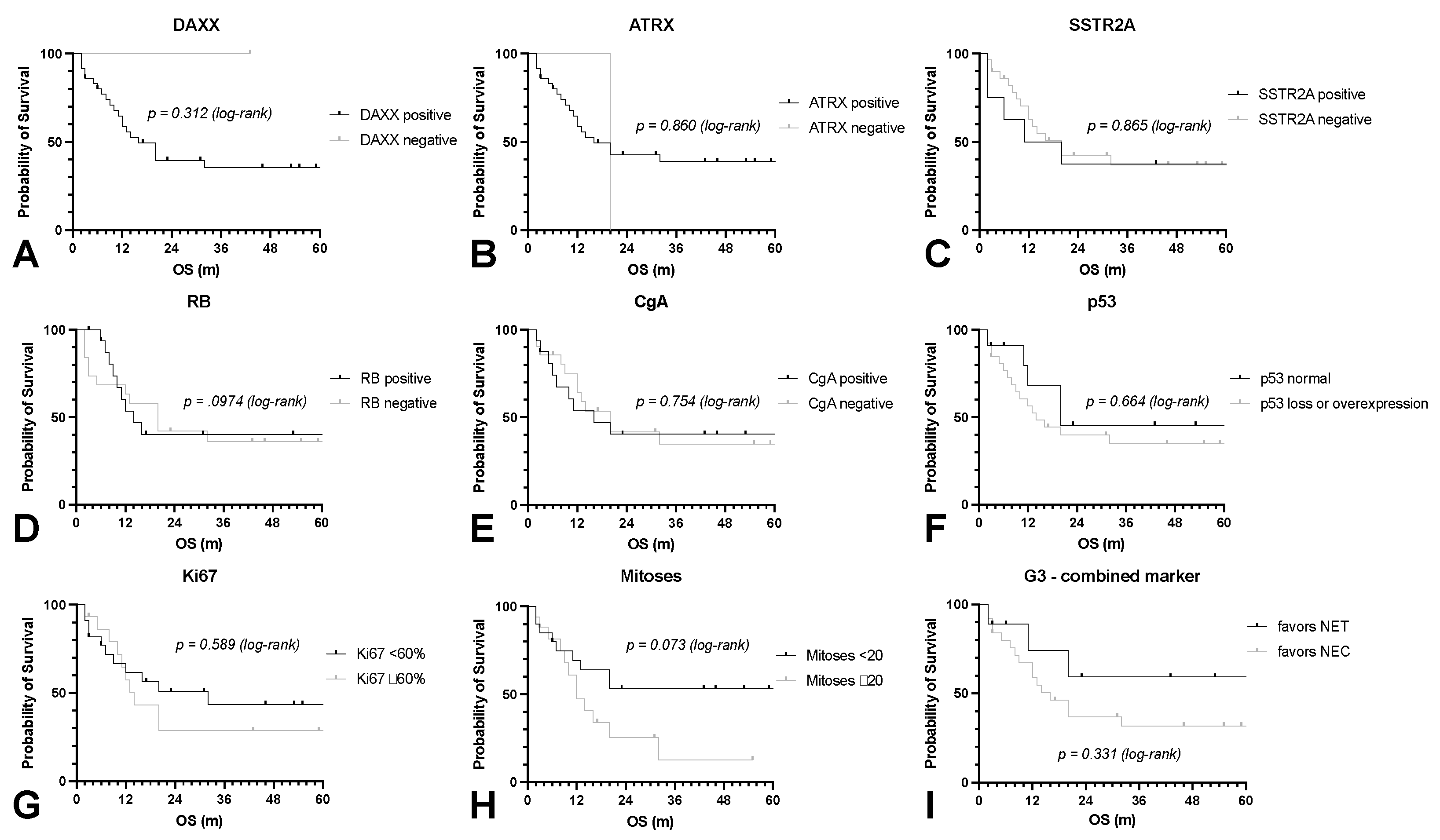
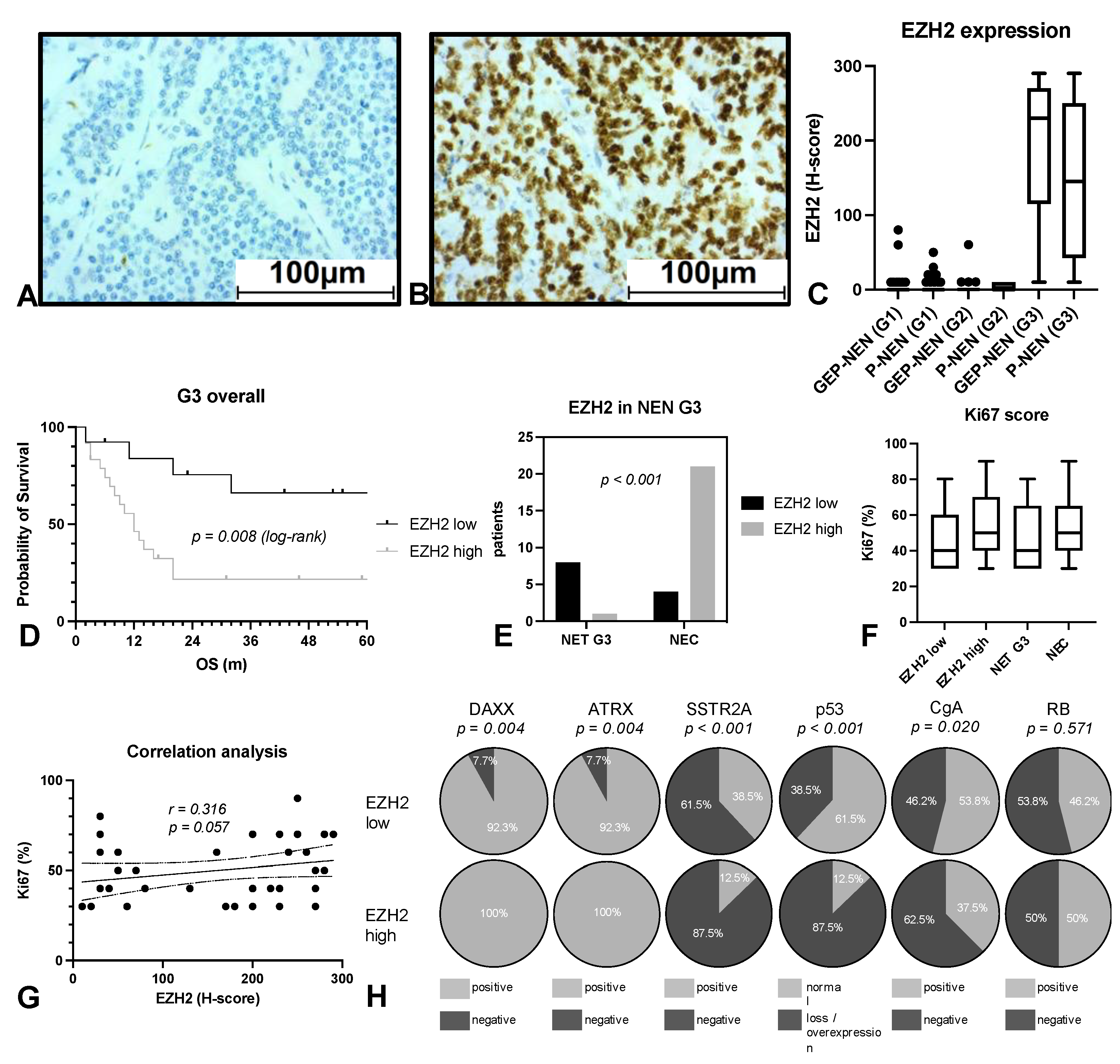
3.2. EZH2 Expression Strongly Predicts Patient’s Survival in NEN G3
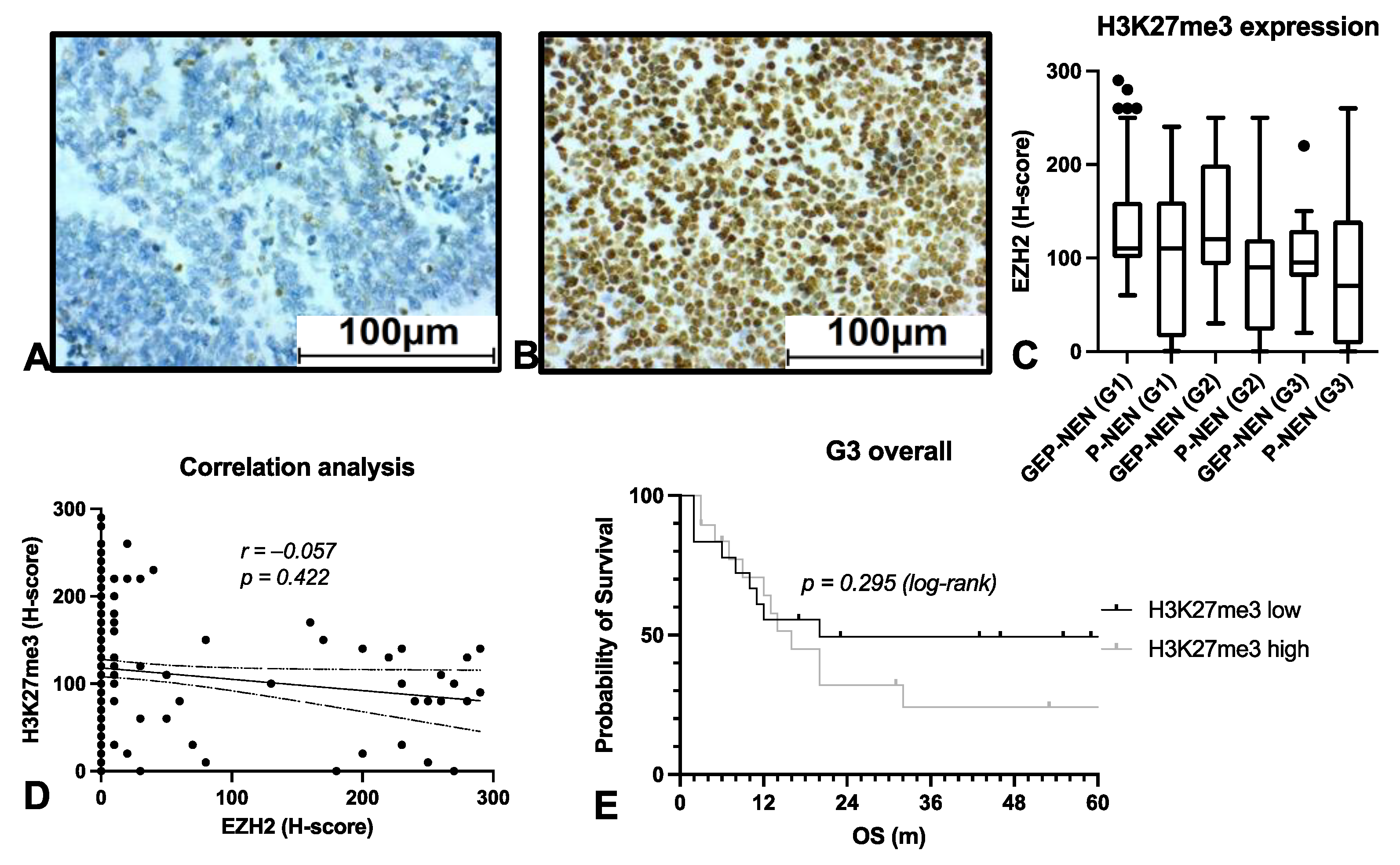
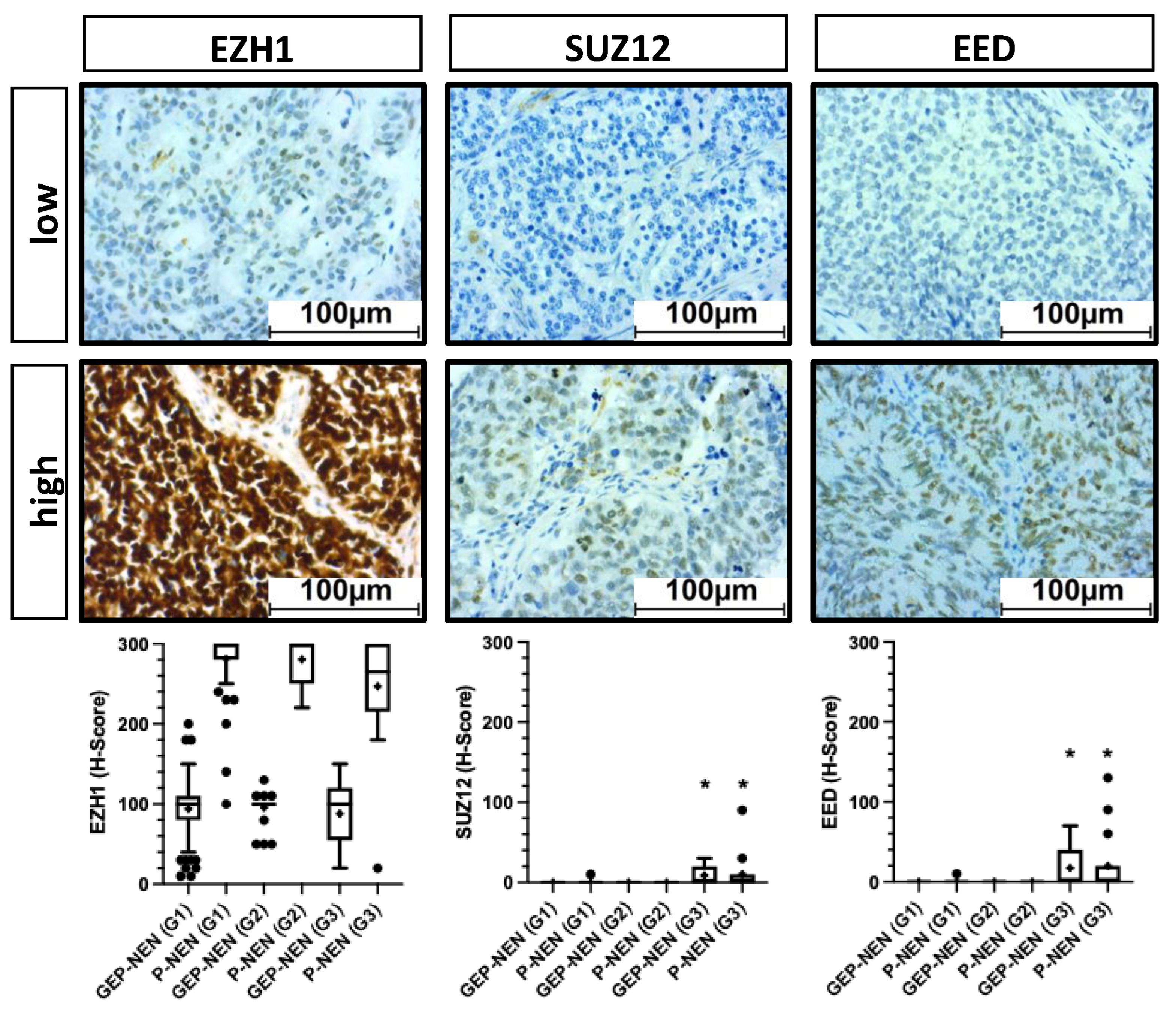
3.3. EZH2 Influences Survival on NEN Patients Independently of PRC2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Mehta, K.; Byers, L.A.; Sorbye, H.; Yao, J.C. Comparative Study of Lung and Extrapulmonary Poorly Differentiated Neuroendocrine Carcinomas: A SEER Database Analysis of 162,983 Cases: Lung Versus Extrapulmonary NECs. Cancer 2018, 124, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. WHO Classification of Tumours Editorial Board the 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A Common Classification Framework for Neuroendocrine Neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) Expert Consensus Proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary Neuroendocrine (Carcinoid) Tumors: European Neuroendocrine Tumor Society Expert Consensus and Recommendations for Best Practice for Typical and Atypical Pulmonary Carcinoids. Ann. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef]
- Perren, A.; Couvelard, A.; Scoazec, J.-Y.; Costa, F.; Borbath, I.; Delle Fave, G.; Gorbounova, V.; Gross, D.; Grossman, A.; Jensen, R.T.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology—Diagnosis and Prognostic Stratification. Neuroendocrinology 2017, 105, 196–200. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef]
- Yachida, S.; Vakiani, E.; White, C.M.; Zhong, Y.; Saunders, T.; Morgan, R.; de Wilde, R.F.; Maitra, A.; Hicks, J.; Demarzo, A.M.; et al. Small Cell and Large Cell Neuroendocrine Carcinomas of the Pancreas Are Genetically Similar and Distinct from Well-Differentiated Pancreatic Neuroendocrine Tumors. Am. J. Surg. Pathol. 2012, 36, 173–184. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 Protein: From the Known and the Unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Pelosi, G.; Bresaola, E.; Bogina, G.; Pasini, F.; Rodella, S.; Castelli, P.; Iacono, C.; Serio, G.; Zamboni, G. Endocrine Tumors of the Pancreas: Ki-67 Immunoreactivity on Paraffin Sections Is an Independent Predictor for Malignancy: A Comparative Study with Proliferating-Cell Nuclear Antigen and Progesterone Receptor Protein Immunostaining, Mitotic Index, and Other Clinicopathologic Variables. Hum. Pathol. 1996, 27, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Pellat, A.; Cottereau, A.S.; Terris, B.; Coriat, R. Neuroendocrine Carcinomas of the Digestive Tract: What Is New? Cancers 2021, 13, 3766. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Meeker, A.; García-Carbonero, R.; Pietras, K.; Astudillo, A.; Casanovas, O.; Scarpa, A. Molecular Biology of Neuroendocrine Tumors: From Pathways to Biomarkers and Targets. Cancer Metastasis Rev. 2014, 33, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Caplin, M.E. The Use of Biomarkers in Neuroendocrine Tumours. Frontline Gastroenterol. 2013, 4, 175–181. [Google Scholar] [CrossRef]
- Taupenot, L.; Harper, K.L.; O’Connor, D.T. The Chromogranin–Secretogranin Family. N. Engl. J. Med. 2003, 348, 1134–1149. [Google Scholar] [CrossRef]
- Amorim, J.P.; Santos, G.; Vinagre, J.; Soares, P. The Role of ATRX in the Alternative Lengthening of Telomeres (ALT) Phenotype. Genes 2016, 7, 66. [Google Scholar] [CrossRef]
- Arakelyan, J.; Zohrabyan, D.; Philip, P.A. Molecular Profile of Pancreatic Neuroendocrine Neoplasms (PanNENs): Opportunities for Personalized Therapies. Cancer 2021, 127, 345–353. [Google Scholar] [CrossRef]
- Bellizzi, A.M. Immunohistochemistry in the Diagnosis and Classification of Neuroendocrine Neoplasms: What Can Brown Do for You? Hum. Pathol. 2020, 96, 8–33. [Google Scholar] [CrossRef]
- Duan, R.; Du, W.; Guo, W. EZH2: A Novel Target for Cancer Treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef]
- Bohnenberger, H.; Ströbel, P. Recent Advances and Conceptual Changes in the Classification of Neuroendocrine Tumors of the Thymus. Virchows Arch. 2021, 478, 129–135. [Google Scholar] [CrossRef]
- Kirmizis, A.; Bartley, S.M.; Kuzmichev, A.; Margueron, R.; Reinberg, D.; Green, R.; Farnham, P.J. Silencing of Human Polycomb Target Genes Is Associated with Methylation of Histone H3 Lys 27. Genes Dev. 2004, 18, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Margueron, R.; Li, G.; Sarma, K.; Blais, A.; Zavadil, J.; Woodcock, C.L.; Dynlacht, B.D.; Reinberg, D. Ezh1 and Ezh2 Maintain Repressive Chromatin through Different Mechanisms. Mol. Cell 2008, 32, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Kuzmichev, A. Histone Methyltransferase Activity Associated with a Human Multiprotein Complex Containing the Enhancer of Zeste Protein. Genes Dev. 2002, 16, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Kuzmichev, A.; Jenuwein, T.; Tempst, P.; Reinberg, D. Different EZH2-Containing Complexes Target Methylation of Histone H1 or Nucleosomal Histone H3. Mol. Cell 2004, 14, 183–193. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Jun, S.; Lee, M.; Kim, H.-C.; Wang, X.; Ji, H.; McCrea, P.D.; Park, J.-I. PAF and EZH2 Induce Wnt/β-Catenin Signaling Hyperactivation. Mol. Cell 2013, 52, 193–205. [Google Scholar] [CrossRef]
- Xu, K.; Wu, Z.J.; Groner, A.C.; He, H.H.; Cai, C.; Lis, R.T.; Wu, X.; Stack, E.C.; Loda, M.; Liu, T.; et al. EZH2 Oncogenic Activity in Castration-Resistant Prostate Cancer Cells Is Polycomb-Independent. Science 2012, 338, 1465–1469. [Google Scholar] [CrossRef]
- Fouse, S.D.; Costello, J.F. Cancer Stem Cells Activate STAT3 the EZ Way. Cancer Cell 2013, 23, 711–713. [Google Scholar] [CrossRef][Green Version]
- Kim, E.; Kim, M.; Woo, D.-H.; Shin, Y.; Shin, J.; Chang, N.; Oh, Y.T.; Kim, H.; Rheey, J.; Nakano, I.; et al. Phosphorylation of EZH2 Activates STAT3 Signaling via STAT3 Methylation and Promotes Tumorigenicity of Glioblastoma Stem-like Cells. Cancer Cell 2013, 23, 839–852. [Google Scholar] [CrossRef]
- Lee, S.T.; Li, Z.; Wu, Z.; Aau, M.; Guan, P.; Karuturi, R.K.M.; Liou, Y.C.; Yu, Q. Context-Specific Regulation of NF-ΚB Target Gene Expression by EZH2 in Breast Cancers. Mol. Cell 2011, 43, 798–810. [Google Scholar] [CrossRef]
- Shi, B.; Liang, J.; Yang, X.; Wang, Y.; Zhao, Y.; Wu, H.; Sun, L.; Zhang, Y.; Chen, Y.; Li, R.; et al. Integration of Estrogen and Wnt Signaling Circuits by the Polycomb Group Protein EZH2 in Breast Cancer Cells. Mol. Cell. Biol. 2007, 27, 5105–5119. [Google Scholar] [CrossRef]
- Morin, R.D.; Arthur, S.E.; Assouline, S. Treating Lymphoma Is Now a Bit EZ-Er. Blood Adv. 2021, 5, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- April-Monn, S.L.; Andreasi, V.; Schiavo Lena, M.; Sadowski, M.C.; Kim-Fuchs, C.; Buri, M.C.; Ketkar, A.; Maire, R.; Di Domenico, A.; Schrader, J.; et al. EZH2 Inhibition as New Epigenetic Treatment Option for Pancreatic Neuroendocrine Neoplasms (PanNENs). Cancers 2021, 13, 5014. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Roberts, C.W.M. Targeting EZH2 in Cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hou, P.; Fan, D.; Dong, M.; Ma, M.; Li, H.; Yao, R.; Li, Y.; Wang, G.; Geng, P.; et al. The Degradation of EZH2 Mediated by LncRNA ANCR Attenuated the Invasion and Metastasis of Breast Cancer. Cell Death Differ. 2017, 24, 59–71. [Google Scholar] [CrossRef]
- Lv, Y.-F.; Yan, G.-N.; Meng, G.; Zhang, X.; Guo, Q.-N. Enhancer of Zeste Homolog 2 Silencing Inhibits Tumor Growth and Lung Metastasis in Osteosarcoma. Sci. Rep. 2015, 5, 12999. [Google Scholar] [CrossRef]
- Chen, S.; Huang, L.; Sun, K.; Wu, D.; Li, M.; Li, M.; Zhong, B.; Chen, M.; Zhang, S. Enhancer of Zeste Homolog 2 as an Independent Prognostic Marker for Cancer: A Meta-Analysis. PLoS ONE 2015, 10, e0125480. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Q.; Li, L. Functional and Therapeutic Significance of EZH2 in Urological Cancers. Oncotarget 2017, 8, 38044–38055. [Google Scholar] [CrossRef]
- Hessmann, E.; Johnsen, S.A.; Siveke, J.T.; Ellenrieder, V. Epigenetic Treatment of Pancreatic Cancer: Is There a Therapeutic Perspective on the Horizon? Gut 2017, 66, 168–179. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, W.; Howard, T.P.; Vazquez, F.; Tsherniak, A.; Wu, J.N.; Wang, W.; Haswell, J.R.; Walensky, L.D.; Hahn, W.C.; et al. SWI/SNF-Mutant Cancers Depend on Catalytic and Non-Catalytic Activity of EZH2. Nat. Med. 2015, 21, 1491–1496. [Google Scholar] [CrossRef]
- Bremer, S.C.B.; Conradi, L.-C.; Mechie, N.-C.; Amanzada, A.; Mavropoulou, E.; Kitz, J.; Ghadimi, M.; Ellenrieder, V.; Ströbel, P.; Hessmann, E.; et al. Enhancer of Zeste Homolog 2 in Colorectal Cancer Development and Progression. Digestion 2021, 102, 227–235. [Google Scholar] [CrossRef]
- Bohnenberger, H.; Kaderali, L.; Ströbel, P.; Yepes, D.; Plessmann, U.; Dharia, N.V.; Yao, S.; Heydt, C.; Merkelbach-Bruse, S.; Emmert, A.; et al. Comparative Proteomics Reveals a Diagnostic Signature for Pulmonary Head-and-neck Cancer Metastasis. EMBO Mol. Med. 2018, 10, e8428. [Google Scholar] [CrossRef] [PubMed]
- Dinter, H.; Bohnenberger, H.; Beck, J.; Bornemann-Kolatzki, K.; Schütz, E.; Küffer, S.; Klein, L.; Franks, T.J.; Roden, A.; Emmert, A.; et al. Molecular Classification of Neuroendocrine Tumors of the Thymus. J. Thorac. Oncol. 2019, 14, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Marchiò, C.; Gatti, G.; Massa, F.; Bertero, L.; Filosso, P.; Pelosi, G.; Cassoni, P.; Volante, M.; Papotti, M. Distinctive Pathological and Clinical Features of Lung Carcinoids with High Proliferation Index. Virchows Arch. 2017, 471, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Quinn, A.M.; Chaturvedi, A.; Nonaka, D. High-Grade Neuroendocrine Carcinoma of the Lung with Carcinoid Morphology: A Study of 12 Cases. Am. J. Surg. Pathol. 2017, 41, 263–270. [Google Scholar] [CrossRef]
- Man, D.; Wu, J.; Shen, Z.; Zhu, X. Prognosis of Patients with Neuroendocrine Tumor: A SEER Database Analysis. Cancer Manag. Res. 2018, 10, 5629–5638. [Google Scholar] [CrossRef]
- Clift, A.K.; Faiz, O.; Goldin, R.; Martin, J.; Wasan, H.; Liedke, M.-O.; Schloericke, E.; Malczewska, A.; Rindi, G.; Kidd, M.; et al. Predicting the Survival of Patients with Small Bowel Neuroendocrine Tumours: Comparison of 3 Systems. Endocr. Connect. 2017, 6, 71–81. [Google Scholar] [CrossRef]
- Scoazec, J.-Y.; Couvelard, A.; Monges, G.; Guyétant, S.; Bisot-Locard, S.; Parot, X.; Lepage, C.; PRONET Study Group Professional Practices and Diagnostic Issues in Neuroendocrine Tumour Pathology. Results of a Prospective One-Year Survey among French Pathologists (the PRONET Study). Neuroendocrinology 2017, 105, 67–76. [Google Scholar] [CrossRef]
- Faviana, P.; Marconcini, R.; Ricci, S.; Galli, L.; Lippolis, P.; Farci, F.; Castagna, M.; Boldrini, L. EZH2 Expression in Intestinal Neuroendocrine Tumors. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 689–693. [Google Scholar] [CrossRef]
- Bondgaard, A.-L.R.Ø.; Poulsen, T.T.; Poulsen, H.S.; Skov, B.G. Different Expression of EZH2, BMI1 and Ki67 in Low and High Grade Neuroendocrine Tumors of the Lung. Cancer Biomark. 2012, 11, 123–128. [Google Scholar] [CrossRef]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; MacDonald, T.; et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016, 30, 563–577. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, D.; Zhou, T.; Song, H.; Hulsurkar, M.; Su, N.; Liu, Y.; Wang, Z.; Shao, L.; Ittmann, M.; et al. Androgen Deprivation Promotes Neuroendocrine Differentiation and Angiogenesis through CREB-EZH2-TSP1 Pathway in Prostate Cancers. Nat. Commun. 2018, 9, 4080. [Google Scholar] [CrossRef] [PubMed]
- Sperling, F.; Misiak, D.; Hüttelmaier, S.; Michl, P.; Griesmann, H. IGF2BP1 Promotes Proliferation of Neuroendocrine Neoplasms by Post-Transcriptional Enhancement of EZH2. Cancers 2022, 14, 2121. [Google Scholar] [CrossRef] [PubMed]
- Barazeghi, E.; Hellman, P.; Norlén, O.; Westin, G.; Stålberg, P. EZH2 Presents a Therapeutic Target for Neuroendocrine Tumors of the Small Intestine. Sci. Rep. 2021, 11, 22733. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Lange, C.A. Roles of the EZH2 Histone Methyltransferase in Cancer Epigenetics. Mutat. Res. 2008, 647, 21–29. [Google Scholar] [CrossRef]
- Luo, J.; Wang, K.; Yeh, S.; Sun, Y.; Liang, L.; Xiao, Y.; Xu, W.; Niu, Y.; Cheng, L.; Maity, S.N.; et al. LncRNA-P21 Alters the Antiandrogen Enzalutamide-Induced Prostate Cancer Neuroendocrine Differentiation via Modulating the EZH2/STAT3 Signaling. Nat. Commun. 2019, 10, 2571. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 219) | GEP-NEN (n = 126) | P-NEN (n = 93) | p-Value |
|---|---|---|---|---|
| Age median (range) (years) | 64 (18–87) | 65 (18–87) | 62 (20–81) | |
| Age No. (%) (years) | ||||
| <65 | 115 | 62 (49.2) | 53 (57.0) | 0.254 |
| ≥65 | 104 | 64 (50.8) | 40 (43.0) | |
| Gender No. (%) | ||||
| Female | 106 | 49 (38.9) | 57 (61.3) | 0.001 * |
| Male | 113 | 77 (61.1) | 36 (38.7) | |
| pT No. (%) | ||||
| 1+2 | 127 | 50 (54.9) | 77 (89.5) | <0.001 * |
| 3+4 | 50 | 41 (45.1) | 9 (10.5) | |
| pN No. (%) | ||||
| 0 | 85 | 27 (40.9) | 58 (77.3) | <0.001 * |
| + | 56 | 39 (59.1) | 17 (22.7) | |
| G No. (%) | ||||
| 1+2 | 182 | 109 (86.5) | 73 (78.5) | 0.118 |
| 3 | 37 | 17 (13.5) | 20 (21.5) | |
| UICC stage No. (%) | ||||
| I+II | 130 | 53 (58.2) | 77 (89.5) | <0.001 * |
| III+IV | 47 | 38 (41.8) | 9 (10.5) |
| Characteristic | EZH2 Low H-Score < 100 (n = 195) | EZH2 High H-Score > 100 (n = 24) | p-Value |
|---|---|---|---|
| Age median (range) (years) | 63 (18–87) | 68.5 (42–84) | |
| Age No. (%) (years) | |||
| <65 | 105 (53.8) | 10 (41.7) | 0.260 |
| ≥65 | 90 (46.2) | 14 (58.3) | |
| Gender No. (%) | |||
| Female | 99 (50.8) | 7 (29.2) | 0.046 * |
| Male | 96 (49.2) | 17 (70.8) | |
| Localization No. (%) | |||
| P-NEN | 82 (42.1) | 11 (45.8) | |
| GEP-NEN | 113 (57.9) | 13 (54.2) | 0.724 |
| -intestinal | 91 (46.7) | 11 (45.8) | |
| -pancreatic | 22 (11.3) | 2 (8.3) | |
| pT No. (%) | |||
| 1+2 | 117 (75.5) | 10 (45.5) | 0.003 * |
| 3+4 | 38 (24.5) | 12 (54.5) | |
| pN No. (%) | |||
| 0 | 74 (61.7) | 11 (52.4) | 0.422 |
| + | 46 (38.3) | 10 (47.6) | |
| G No. (%) | |||
| 1+2 | 182 (93.3) | 0 (0.0) | <0.001 * |
| 3 | 13 (6.7) | 24 (100.0) | |
| UICC stage No. (%) | |||
| I+II | 118 (76.1) | 12 (54.5) | 0.032 * |
| III+IV | 37 (23.9) | 10 (45.5) |
| Characteristic | Regression Coefficient B | Standardized Beta Coefficient | p-Value | 95% CI |
|---|---|---|---|---|
| Grading | 77.172 | 0.214 | 0.013 | 3.900–32.215 |
| pT status | 18.058 | 0.745 | 0.000 | 64.938–89.406 |
| UICC stage | −21.549 | −0.256 | 0.003 | −35.758–−7.341 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bremer, S.C.B.; Bittner, G.; Elakad, O.; Dinter, H.; Gaedcke, J.; König, A.O.; Amanzada, A.; Ellenrieder, V.; Freiherr von Hammerstein-Equord, A.; Ströbel, P.; et al. Enhancer of Zeste Homolog 2 (EZH2) Is a Marker of High-Grade Neuroendocrine Neoplasia in Gastroenteropancreatic and Pulmonary Tract and Predicts Poor Prognosis. Cancers 2022, 14, 2828. https://doi.org/10.3390/cancers14122828
Bremer SCB, Bittner G, Elakad O, Dinter H, Gaedcke J, König AO, Amanzada A, Ellenrieder V, Freiherr von Hammerstein-Equord A, Ströbel P, et al. Enhancer of Zeste Homolog 2 (EZH2) Is a Marker of High-Grade Neuroendocrine Neoplasia in Gastroenteropancreatic and Pulmonary Tract and Predicts Poor Prognosis. Cancers. 2022; 14(12):2828. https://doi.org/10.3390/cancers14122828
Chicago/Turabian StyleBremer, Sebastian C. B., Gabi Bittner, Omar Elakad, Helen Dinter, Jochen Gaedcke, Alexander O. König, Ahmad Amanzada, Volker Ellenrieder, Alexander Freiherr von Hammerstein-Equord, Philipp Ströbel, and et al. 2022. "Enhancer of Zeste Homolog 2 (EZH2) Is a Marker of High-Grade Neuroendocrine Neoplasia in Gastroenteropancreatic and Pulmonary Tract and Predicts Poor Prognosis" Cancers 14, no. 12: 2828. https://doi.org/10.3390/cancers14122828
APA StyleBremer, S. C. B., Bittner, G., Elakad, O., Dinter, H., Gaedcke, J., König, A. O., Amanzada, A., Ellenrieder, V., Freiherr von Hammerstein-Equord, A., Ströbel, P., & Bohnenberger, H. (2022). Enhancer of Zeste Homolog 2 (EZH2) Is a Marker of High-Grade Neuroendocrine Neoplasia in Gastroenteropancreatic and Pulmonary Tract and Predicts Poor Prognosis. Cancers, 14(12), 2828. https://doi.org/10.3390/cancers14122828






