Basal Cell Carcinoma: Pathology, Current Clinical Treatment, and Potential Use of Lipid Nanoparticles
Abstract
:Simple Summary
Abstract
1. Introduction
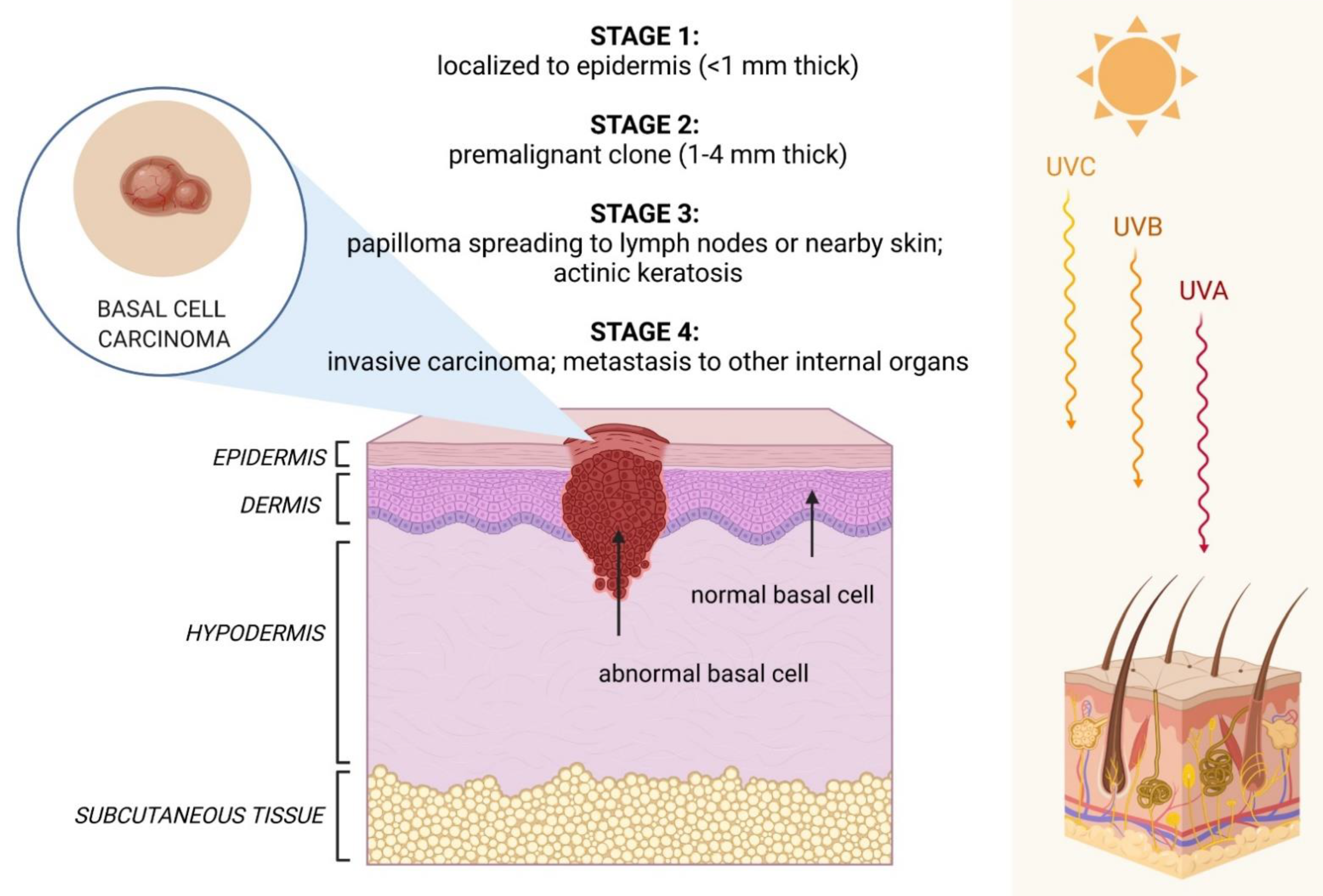
2. Clinical Features of BCC
3. Available Therapies
3.1. Treatment of Locally Advanced/Metastatic BCC
3.2. Aesthetic Expectations of Patients
4. Nanoparticle-Related Therapies for BCC
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. Author Correction: The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 15705. [Google Scholar] [CrossRef] [PubMed]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef] [PubMed]
- Derebaşınlıoğlu, H. Distribution of skin cancers of the head and neck according to anatomical subunit. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowska, M.; Koziej, M.; Antoszewski, B. Detailed head localization and incidence of skin cancers. Sci. Rep. 2021, 11, 12391. [Google Scholar] [CrossRef]
- Seidl-Philipp, M.; Frischhut, N.; Höllweger, N.; Schmuth, M.; Nguyen, V.A. Known and new facts on basal cell carcinoma. J. Dtsch. Dermatol. Ges. 2021, 19, 1021–1041. [Google Scholar] [CrossRef]
- Gold, N.B.; Campbell, I.M.; Sheppard, S.E.; Tan, W.-H. Proposed criteria for nevoid basal cell carcinoma syndrome in children assessed using statistical optimization. Sci. Rep. 2021, 11, 19791. [Google Scholar] [CrossRef]
- Fontanillas, P.; Alipanahi, B.; Furlotte, N.A.; Johnson, M.; Wilson, C.H.; Pitts, S.J.; Gentleman, R.; Auton, A. Disease risk scores for skin cancers. Nat. Commun. 2021, 12, 160. [Google Scholar] [CrossRef]
- Knox, S.; O’Boyle, N.M. Skin lipids in health and disease: A review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef]
- Swaney, M.H.; Kalan, L.R. Living in Your Skin: Microbes, Molecules, and Mechanisms. Infect. Immun. 2021, 89, e00695-20. [Google Scholar] [CrossRef]
- Jiang, J. Hedgehog signaling mechanism and role in cancer. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef]
- Guerrini-Rousseau, L.; Smith, M.J.; Kratz, C.P.; Doergeloh, B.; Hirsch, S.; Hopman, S.M.J.; Jorgensen, M.; Kuhlen, M.; Michaeli, O.; Milde, T.; et al. Current recommendations for cancer surveillance in Gorlin syndrome: A report from the SIOPE host genome working group (SIOPE HGWG). Fam. Cancer 2021, 20, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Verkouteren, B.J.A.; Cosgun, B.; Reinders, M.; Kessler, P.; Vermeulen, R.J.; Klaassens, M.; Lambrechts, S.; van Rheenen, J.R.; van Geel, M.; Vreeburg, M.; et al. A guideline for the clinical management of basal cell naevus syndrome (Gorlin-Goltz syndrome). Br. J. Dermatol. 2022, 186, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Stockfleth, E. Light and Skin. Curr. Probl. Dermatol. 2021, 55, 53–61. [Google Scholar] [CrossRef]
- Tse, B.C.Y.; Ireland, R.A.; Lee, J.Y.; Marsh-Wakefield, F.; Kok, L.F.; Don, A.S.; Byrne, S.N. Exposure to Systemic Immunosuppressive Ultraviolet Radiation Alters T Cell Recirculation through Sphingosine-1-Phosphate. J. Immunol. 2021, 207, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Georgescu, S.R.; Mitran, M.I.; Mitran, C.I.; Matei, C.; Caruntu, A.; Scheau, C.; Nicolae, I.; Matei, A.; Caruntu, C.; et al. Current Perspectives on the Role of Matrix Metalloproteinases in the Pathogenesis of Basal Cell Carcinoma. Biomolecules 2021, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, M.B.; Ford, A.E.; Liu, Y.; Zhang, J.Y. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riihilä, P.; Nissinen, L.; Kähäri, V.M. Matrix metalloproteinases in keratinocyte carcinomas. Exp. Dermatol. 2021, 30, 50–61. [Google Scholar] [CrossRef]
- Pietrobono, S.; Gagliardi, S.; Stecca, B. Non-canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors Beyond Smoothened. Front. Genet. 2019, 10, 556. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Dhanyamraju, P.K.; Lauth, M. DYRK1B blocks canonical and promotes non-canonical Hedgehog signaling through activation of the mTOR/AKT pathway. Oncotarget 2017, 8, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Dasgeb, B.; Pajouhanfar, S.; Jazayeri, A.; Schoenberg, E.; Kumar, G.; Fortina, P.; Berger, A.C.; Uitto, J. Novel PTCH1 and concurrent TP53 mutations in four patients with numerous non-syndromic basal cell carcinomas: The paradigm of oncogenic synergy. Exp. Dermatol. 2021, 31, 736–742. [Google Scholar] [CrossRef]
- Moujaess, E.; Merhy, R.; Kattan, J.; Sarkis, A.S.; Tomb, R. Immune checkpoint inhibitors for advanced or metastatic basal cell carcinoma: How much evidence do we need? Immunotherapy 2021, 13, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Crow, L.D.; Jambusaria-Pahlajani, A.; Chung, C.L.; Baran, D.A.; Lowenstein, S.E.; Abdelmalek, M.; Ahmed, R.L.; Anadkat, M.J.; Arcasoy, S.M.; Berg, D.; et al. Initial skin cancer screening for solid organ transplant recipients in the United States: Delphi method development of expert consensus guidelines. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2019, 32, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Seviiri, M.; Law, M.H.; Ong, J.S.; Gharahkhani, P.; Nyholt, D.R.; Olsen, C.M.; Whiteman, D.C.; MacGregor, S. Polygenic Risk Scores Allow Risk Stratification for Keratinocyte Cancer in Organ-Transplant Recipients. J. Investig. Dermatol. 2021, 141, 325–333.e326. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.P.; Kus, K.J.B.; Ruiz, E. Basal Cell Carcinoma Review. Hematol./Oncol. Clin. N. Am. 2019, 33, 13–24. [Google Scholar] [CrossRef]
- Galindo-Ferreiro, A.; Sanchez-Tocino, H.; Diez-Montero, C.; Belani-Raju, M.; García-Sanz, R.; Diego-Alonso, M.; Llorente-Gonzalez, I.; Perez, P.C.; Khandekar, R.; Schellini, S. Characteristics and Recurrence of Primary Eyelid Basal Cell Carcinoma in Central Spain. J. Curr. Ophthalmol. 2020, 32, 183–188. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.D.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Vornicescu, C.; Șenilă, S.C.; Bejinariu, N.I.; Vesa, Ș.C.; Boșca, A.B.; Chirilă, D.N.; Melincovici, C.S.; Sorițău, O.; Mihu, C.M. Predictive factors for the recurrence of surgically excised basal cell carcinomas: A retrospective clinical and immunopathological pilot study. Exp. Ther. Med. 2021, 22, 1336. [Google Scholar] [CrossRef]
- Tanese, K. Diagnosis and Management of Basal Cell Carcinoma. Curr. Treat. Options Oncol. 2019, 20, 13. [Google Scholar] [CrossRef]
- Jia, H.X.; He, Y.L. Efficacy and safety of imiquimod 5% cream for basal cell carcinoma: A meta-analysis of randomized controlled trial. J. Dermatol. Treat. 2020, 31, 831–838. [Google Scholar] [CrossRef]
- Hendel, K.; Jemec, G.B.E.; Haedersdal, M.; Wiegell, S.R. Electrochemotherapy with bleomycin for basal cell carcinomas: A systematic review. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Piccioni, A.; Gutiérrez Garcìa-Rodrigo, C.; Sacco, G.; Pellegrini, C.; Fargnoli, M.C. Sequential treatment with calcitriol and methyl aminolevulinate-daylight photodynamic therapy for patients with multiple actinic keratoses of the upper extremities. Photodiagnosis Photodyn. Ther. 2021, 34, 102325. [Google Scholar] [CrossRef]
- Food and Drug Administration. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203388lbl.pdf (accessed on 10 December 2021).
- Food and Drug Administration. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205266s004lbl.pdf (accessed on 10 December 2021).
- Sekulic, A.; Migden, M.R.; Basset-Seguin, N.; Garbe, C.; Gesierich, A.; Lao, C.D.; Miller, C.; Mortier, L.; Murrell, D.F.; Hamid, O.; et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017, 17, 332. [Google Scholar] [CrossRef] [PubMed]
- Basset-Séguin, N.; Hauschild, A.; Kunstfeld, R.; Grob, J.; Dréno, B.; Mortier, L.; Ascierto, P.A.; Licitra, L.; Dutriaux, C.; Thomas, L.; et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur. J. Cancer 2017, 86, 334–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzavara Pinton, P.; Licitra, L.; Peris, K.; Santoro, A.; Ascierto, P.A. Vismodegib in the treatment of basal cell carcinoma: Indications for clinical practice. Future Oncol. 2015, 11, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Schulze, H.J.; Hauschild, A.; Leiter, U.; Meier, F.; Haferkamp, S.; Ulrich, C.; Wahl, R.U.; Berking, C.; Herbst, R.; et al. Effectiveness, safety and utilization of vismodegib in locally advanced basal cell carcinoma under real-world conditions in Germany—The non-interventional study NIELS. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Słowińska, M.; Dudzisz-Śledź, M.; Sobczuk, P.; Łasińska, I.; Pieruszka, A.; Cybulska-Stopa, B.; Kowalczyk, A.; Świtaj, T.; Czarnecka, I.; Koseła-Paterczyk, H.; et al. Analysis of efficacy and safety of vismodegib therapy in patients with advanced basal cell carcinoma—Real world multicenter cohort study. Acad. Dermatol. Venereol. JEADV 2022. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Guminksi, A.; Gutzmer, R.; Lear, J.T.; Lewis, K.D.; Chang, A.L.S.; Combemale, P.; Dirix, L.; Kaatz, M.; Kudchadkar, R.; et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br. J. Dermatol. 2020, 182, 1369–1378. [Google Scholar] [CrossRef]
- Xie, P.; Lefrançois, P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2018, 79, 1089–1100.e1017. [Google Scholar] [CrossRef]
- Mistretta, C.M.; Kumari, A. Tongue and Taste Organ Biology and Function: Homeostasis Maintained by Hedgehog Signaling. Annu. Rev. Physiol. 2017, 79, 335–356. [Google Scholar] [CrossRef] [Green Version]
- Abe, Y.; Tanaka, N. Roles of the Hedgehog Signaling Pathway in Epidermal and Hair Follicle Development, Homeostasis, and Cancer. J. Dev. Biol. 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, H.Q.; Chen, L.; Nawas, Z.; Lee, H.H.; Silapunt, S.; Migden, M. Switching Hedgehog inhibitors and other strategies to address resistance when treating advanced basal cell carcinoma. Oncotarget 2021, 12, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Büttner, R.; Longshore, J.W.; López-Ríos, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB measurement in clinical practice: Considerations on assay requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.L.S.; Dalle, S.; et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet. Oncol. 2021, 22, 848–857. [Google Scholar] [CrossRef]
- Thomson, J.; Hogan, S.; Leonardi-Bee, J.; Williams, H.C.; Bath-Hextall, F.J. Interventions for basal cell carcinoma: Abridged Cochrane systematic review and GRADE assessments. Br. J. Dermatol. 2021, 185, 499–511. [Google Scholar] [CrossRef]
- Herms, F.; Basset-Seguin, N. Emerging drugs for the treatment of basal cell carcinoma. Expert Opin. Emerg. Drugs 2021, 26, 17–26. [Google Scholar] [CrossRef]
- Bertrand, N.; Guerreschi, P.; Basset-Seguin, N.; Saiag, P.; Dupuy, A.; Dalac-Rat, S.; Dziwniel, V.; Depoortère, C.; Duhamel, A.; Mortier, L. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: First results of a multicenter, open-label, phase 2 trial (VISMONEO study): Neoadjuvant Vismodegib in Locally Advanced Basal Cell Carcinoma. EClinicalMedicine 2021, 35, 100844. [Google Scholar] [CrossRef]
- Epstein, E.H.; Lear, J.; Saldanha, G.; Tang, J.Y.; Harwood, C. Hedgehog pathway inhibition by topical patidegib to reduce BCC burden in patients with basal cell nevus (Gorlin) syndrome. J. Clin. Oncol. 2018, 36, e21626. [Google Scholar] [CrossRef]
- Barry, B.W. Drug delivery routes in skin: A novel approach. Adv. Drug Deliv. Rev. 2002, 54, S31–S40. [Google Scholar] [CrossRef]
- Souto, E.B.; Macedo, A.S.; Dias-Ferreira, J.; Cano, A.; Zielińska, A.; Matos, C.M. Elastic and Ultradeformable Liposomes for Transdermal Delivery of Active Pharmaceutical Ingredients (APIs). Int. J. Mol. Sci. 2021, 22, 9743. [Google Scholar] [CrossRef] [PubMed]
- de Melo Barbosa, R.; Cano, A.; Zielińska, A.; da Silva, C.F.; Severino, P.; Souto, E.B. Chapter 4—Combination drug delivery system to enhance the transdermal drug delivery of bioactive molecules, In Combination Drug Delivery Approach as an Effective Therapy for Various Diseases; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2022; pp. 65–80. [Google Scholar] [CrossRef]
- Severino, P.; Fangueiro, J.F.; Ferreira, S.V.; Basso, R.; Chaud, M.V.; Santana, M.H.; Rosmaninho, A.; Souto, E.B. Nanoemulsions and nanoparticles for non-melanoma skin cancer: Effects of lipid materials. Clin. Transl. Oncol. 2013, 15, 417–424. [Google Scholar] [CrossRef]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Souto, S.B.; Zielinska, A.; Durazzo, A.; Lucarini, M.; Santini, A.; Horbanczuk, O.K.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. Perillaldehyde 1,2-epoxide Loaded SLN-Tailored mAb: Production, Physicochemical Characterization and In Vitro Cytotoxicity Profile in MCF-7 Cell Lines. Pharmaceutics 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.B.; Doktorovova, S.; Campos, J.R.; Martins-Lopes, P.; Silva, A.M. Surface-tailored anti-HER2/neu-solid lipid nanoparticles for site-specific targeting MCF-7 and BT-474 breast cancer cells. Eur. J. Pharm. Sci. 2019, 128, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Jager, E.; Jager, A.; Stepanek, P.; Cano, A.; Viseras, C.; de Melo Barbosa, R.; Chorilli, M.; Zielinska, A.; Severino, P.; et al. Lipid Nanomaterials for Targeted Delivery of Dermocosmetic Ingredients: Advances in Photoprotection and Skin Anti-Aging. Nanomaterials 2022, 12, 377. [Google Scholar] [CrossRef]
- Zielińska, A.; Szalata, M.; Gorczyński, A.; Karczewski, J.; Eder, P.; Severino, P.; Cabeda, J.M.; Souto, E.B.; Słomski, R. Cancer Nanopharmaceuticals: Physicochemical Characterization and In Vitro/In Vivo Applications. Cancers 2021, 13, 1896. [Google Scholar] [CrossRef]
- Andreani, T.; Fangueiro, J.F.; Jose, S.; Santini, A.; Silva, A.M.; Souto, E.B. Hydrophilic Polymers for Modified-Release Nanoparticles: A Review of Mathematical Modelling for Pharmacokinetic Analysis. Curr. Pharm. Des. 2015, 21, 3090–3096. [Google Scholar] [CrossRef]
- Craciun, A.-M.; Barhalescu, M.L.; Agop, M.; Ochiuz, L. Theoretical Modeling of Long-Time Drug Release from Nitrosalicyl-Imine-Chitosan Hydrogels through Multifractal Logistic Type Laws. Comput. Math. Methods Med. 2019, 2019, 4091464. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wei, T.; Zhao, J.; Huang, Y.; Deng, H.; Kumar, A.; Wang, C.; Liang, Z.; Ma, X.; Liang, X.-J. Multifunctional aptamer-based nanoparticles for targeted drug delivery to circumvent cancer resistance. Biomaterials 2016, 91, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Musumeci, D.; Russo Krauss, I.; Piccolo, M.; Irace, C.; Paduano, L.; Montesarchio, D. Exploring the conformational behaviour and aggregation properties of lipid-conjugated AS1411 aptamers. Int. J. Biol. Macromol. 2018, 118, 1384–1399. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood–brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, M. Review of the effects of manufactured nanoparticles on mammalian target organs. J. Appl. Toxicol. JAT 2018, 38, 25–40. [Google Scholar] [CrossRef]
- Sutunkova, M.P.; Katsnelson, B.A.; Privalova, L.I.; Gurvich, V.B.; Konysheva, L.K.; Shur, V.Y.; Shishkina, E.V.; Minigalieva, I.A.; Solovjeva, S.N.; Grebenkina, S.V.; et al. On the contribution of the phagocytosis and the solubilization to the iron oxide nanoparticles retention in and elimination from lungs under long-term inhalation exposure. Toxicology 2016, 363–364, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, R.; Dobrovolskaia, M.A.; Cheney, D.L.; Greish, K.F.; Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2019, 144, 112–132. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-By-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Pease, D.F.; Peterson, B.A.; Gilles, S.; Hordinsky, M.K.; Bohjanen, K.A.; Skubitz, K.M. Development of cutaneous squamous cell carcinoma after prolonged exposure to pegylated liposomal doxorubicin and hand-foot syndrome: A newly recognized toxicity. Cancer Chemother. Pharmacol. 2019, 84, 217–221. [Google Scholar] [CrossRef]
- Calienni, M.N.; Febres-Molina, C.; Llovera, R.E.; Zevallos-Delgado, C.; Tuttolomondo, M.E.; Paolino, D.; Fresta, M.; Barazorda-Ccahuana, H.L.; Gómez, B.; Alonso, S.D.V.; et al. Nanoformulation for potential topical delivery of Vismodegib in skin cancer treatment. Int. J. Pharm. 2019, 565, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Sakura, M.; Chiba, Y.; Kamiya, E.; Furukawa, A.; Kawamura, N.; Niwa, M.; Takeuchi, M.; Enokido, Y.; Hosokawa, M. Differences in the Histopathology and Cytokine Expression Pattern between Chronological Aging and Photoaging of Hairless Mice Skin. Mod. Res. Inflamm. 2014, 3, 82–89. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil shell-enriched solid lipid nanoparticles (SLN) for effective skin carcinoma treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhter, K.F.; Mumin, M.A.; Lui, E.M.K.; Charpentier, P.A. Transdermal nanotherapeutics: Panax quinquefolium polysaccharide nanoparticles attenuate UVB-induced skin cancer. Int. J. Biol. Macromol. 2021, 181, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Novak, B.; Hoeh, A.K.; Luebbert, H.; Dirschka, T. Epidermal penetration and protoporphyrin IX formation of two different 5-aminolevulinic acid formulations in ex vivo human skin. Photodiagnosis Photodyn. Ther. 2016, 14, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Triviño, F.J.; Ayén-Rodríguez, Á.; Llamas-Molina, J.M.; Saenz-Guirado, S.; Ruiz-Villaverde, R. Treatment of superficial basal cell carcinoma with 7.8% 5-aminolaevulinic acid nanoemulsion-based gel (BF-200 ALA) and photodynamic therapy: Results in clinical practice in a tertiary hospital. Dermatol. Ther. 2021, 34, e14558. [Google Scholar] [CrossRef]
- Arasi, M.B.; Pedini, F.; Valentini, S.; Felli, N.; Felicetti, F. Advances in Natural or Synthetic Nanoparticles for Metastatic Melanoma Therapy and Diagnosis. Cancers 2020, 12, 2893. [Google Scholar] [CrossRef]
- Rao, L.; Zhao, S.K.; Wen, C.; Tian, R.; Lin, L.; Cai, B.; Sun, Y.; Kang, F.; Yang, Z.; He, L.; et al. Activating Macrophage-Mediated Cancer Immunotherapy by Genetically Edited Nanoparticles. Adv. Mater. 2020, 32, e2004853. [Google Scholar] [CrossRef]
- Perry, J.; Minaei, E.; Engels, E.; Ashford, B.G.; McAlary, L.; Clark, J.R.; Gupta, R.; Tehei, M.; Corde, S.; Carolan, M.; et al. Thulium oxide nanoparticles as radioenhancers for the treatment of metastatic cutaneous squamous cell carcinoma. Phys. Med. Biol. 2020, 65, 215018. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łasińska, I.; Zielińska, A.; Mackiewicz, J.; Souto, E.B. Basal Cell Carcinoma: Pathology, Current Clinical Treatment, and Potential Use of Lipid Nanoparticles. Cancers 2022, 14, 2778. https://doi.org/10.3390/cancers14112778
Łasińska I, Zielińska A, Mackiewicz J, Souto EB. Basal Cell Carcinoma: Pathology, Current Clinical Treatment, and Potential Use of Lipid Nanoparticles. Cancers. 2022; 14(11):2778. https://doi.org/10.3390/cancers14112778
Chicago/Turabian StyleŁasińska, Izabela, Aleksandra Zielińska, Jacek Mackiewicz, and Eliana B. Souto. 2022. "Basal Cell Carcinoma: Pathology, Current Clinical Treatment, and Potential Use of Lipid Nanoparticles" Cancers 14, no. 11: 2778. https://doi.org/10.3390/cancers14112778
APA StyleŁasińska, I., Zielińska, A., Mackiewicz, J., & Souto, E. B. (2022). Basal Cell Carcinoma: Pathology, Current Clinical Treatment, and Potential Use of Lipid Nanoparticles. Cancers, 14(11), 2778. https://doi.org/10.3390/cancers14112778






