Parental Sleep, Distress, and Quality of Life in Childhood Acute Lymphoblastic Leukemia: A Longitudinal Report from Diagnosis up to Three Years Later
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- To assess the longitudinal course of sleep problems, distress and QoL in parents of children with ALL, from diagnosis up to three years later.
- (2)
- To assess the influence of experiencing sleep problems and/or clinical distress levels on parental QoL over time.
- (3)
- To longitudinally explore determinants (sociodemographic, medical, and psychosocial) of experiencing sleep problems and/or distress over time.
2. Materials and Methods
2.1. Study Population
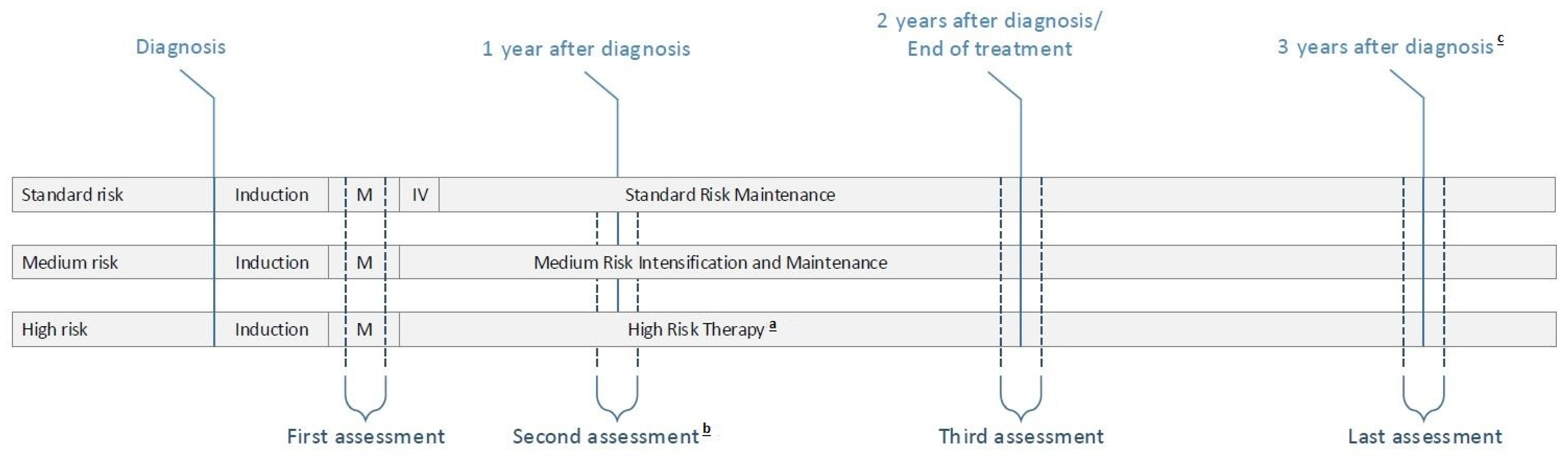
2.2. Overview of ALL11 Protocol and Study Measurements
2.3. Questionnaires
2.3.1. Sociodemographic Characteristics
2.3.2. Sleep
2.3.3. Distress and Psychosocial Factors
2.3.4. Quality of Life
2.4. Statistical Analysis
2.4.1. Study Population
2.4.2. Longitudinal Course of Sleep, Distress, and QoL
2.4.3. Predictive Determinants of Sleep and Distress
2.4.4. Relationships between Sleep, Distress, and QoL
3. Results
3.1. Study Population
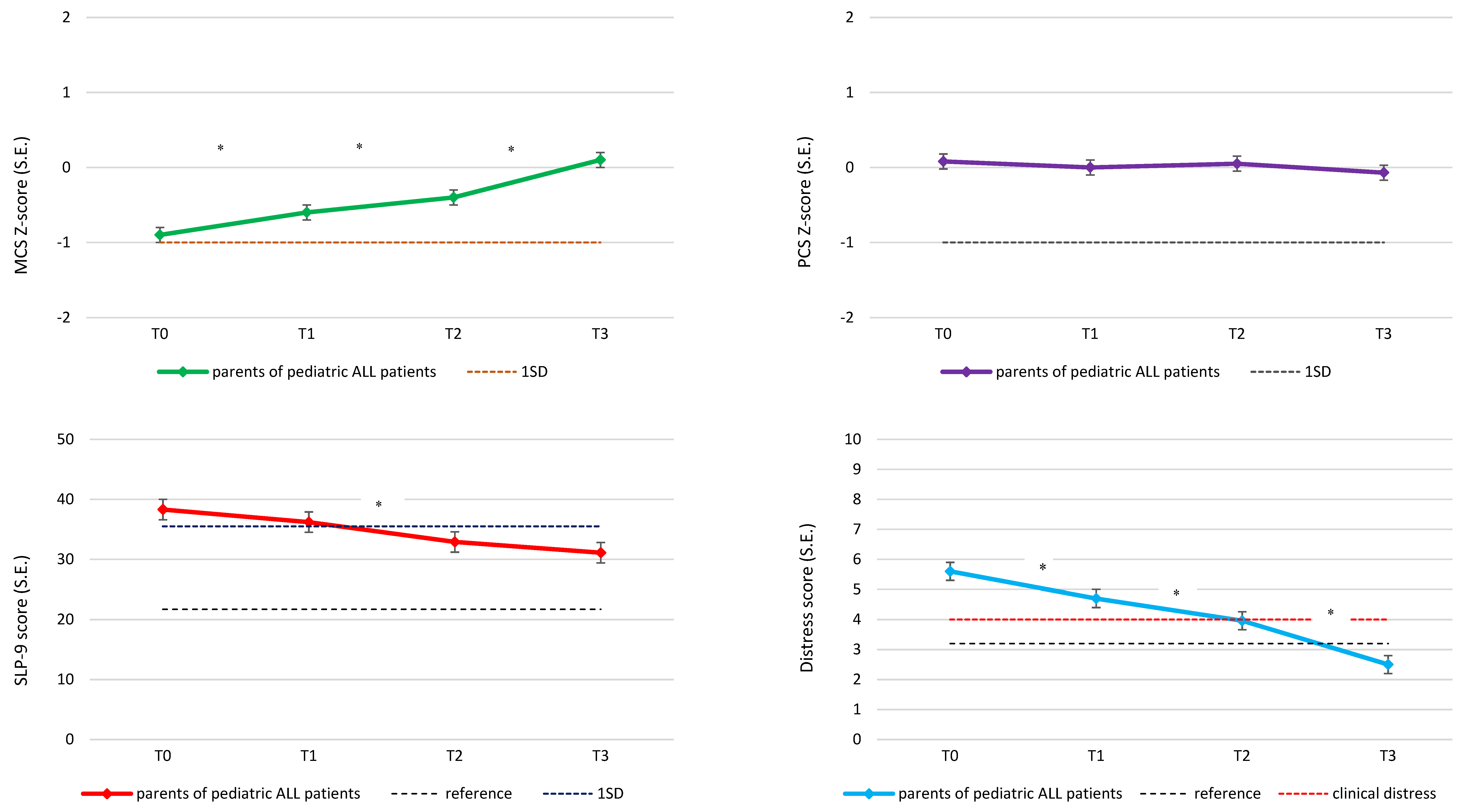
3.2. Longitudinal Course of Sleep, Distress and QoL
3.3. Predictive Determinants of Sleep and Distress
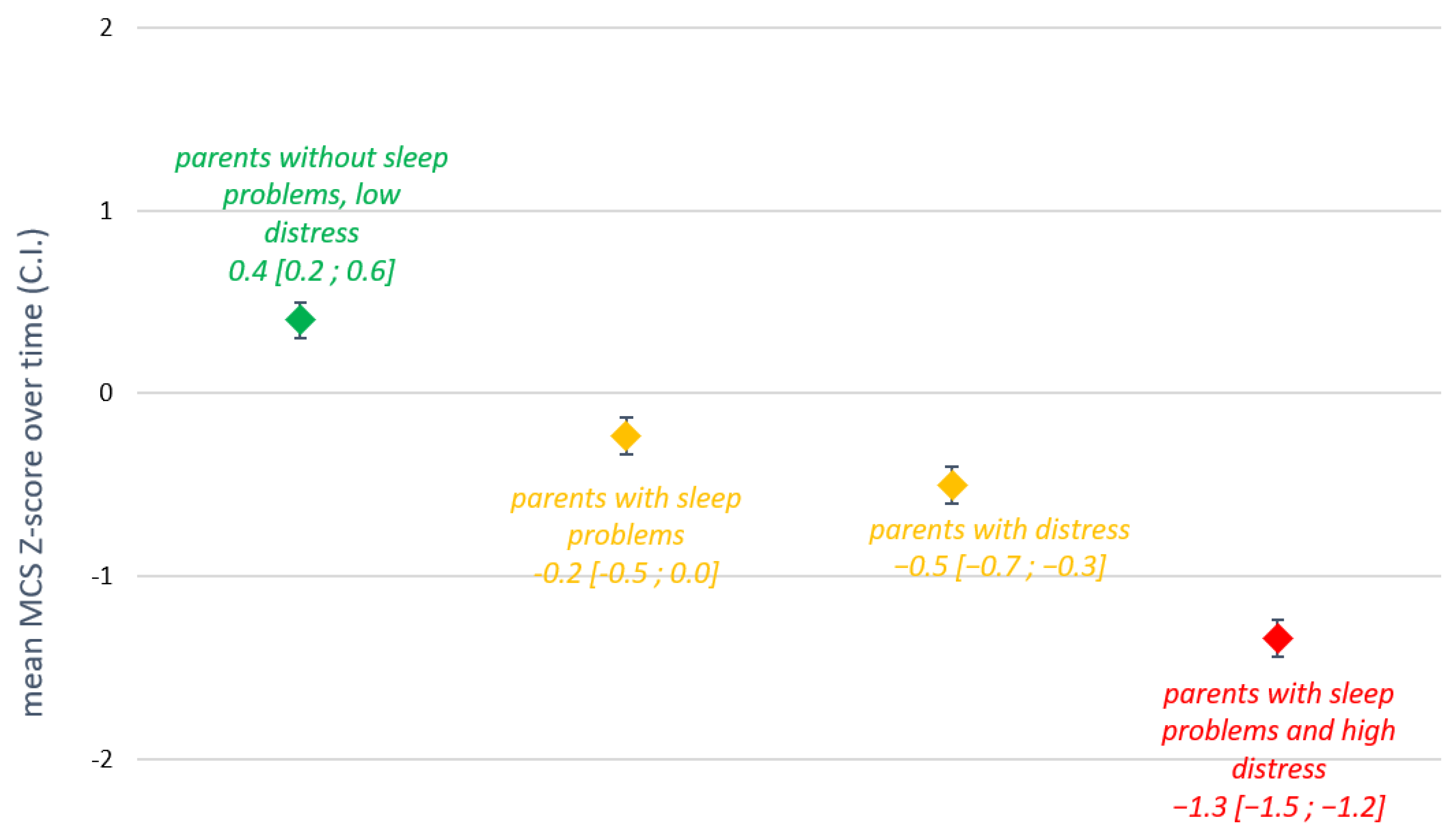
3.4. Relationships between Sleep, Distress, and QoL
4. Discussion
4.1. Main Findings
4.2. Clinical Implications
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fardell, J.E.; Vetsch, J.; Trahair, T.; Mateos, M.K.; Grootenhuis, M.A.; Touyz, L.M.; Marshall, G.M.; Wakefield, C.E. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: A systematic review. Pediatr. Blood Cancer 2017, 64, e26489. [Google Scholar] [CrossRef] [PubMed]
- Kearney, J.A.; Salley, C.G.; Muriel, A.C. Standards of Psychosocial Care for Parents of Children with Cancer. Pediatr. Blood Cancer 2015, 62 (Suppl. 5), S632–S683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klassen, A.F.; Klaassen, R.; Dix, D.; Pritchard, S.; Yanofsky, R.; O’Donnell, M.; Scott, A.; Sung, L. Impact of caring for a child with cancer on parents’ health-related quality of life. J. Clin. Oncol. 2008, 26, 5884–5889. [Google Scholar] [CrossRef] [PubMed]
- Rensen, N.; Steur, L.M.; Schepers, S.A.; Merks, J.H.; Moll, A.C.; Kaspers, G.J.; Grootenhuis, M.A.; Van Litsenburg, R.R. Gender-specific differences in parental health-related quality of life in childhood cancer. Pediatr. Blood Cancer 2019, 66, e27728. [Google Scholar] [CrossRef] [Green Version]
- Vrijmoet-Wiersma, C.M.; van Klink, J.M.; Kolk, A.M.; Koopman, H.M.; Ball, L.M.; Maarten Egeler, R. Assessment of parental psychological stress in pediatric cancer: A review. J. Pediatr. Psychol. 2008, 33, 694–706. [Google Scholar] [CrossRef] [Green Version]
- Price, J.; Kassam-Adams, N.; Alderfer, M.A.; Christofferson, J.; Kazak, A.E. Systematic Review: A Reevaluation and Update of the Integrative (Trajectory) Model of Pediatric Medical Traumatic Stress. J. Pediatr. Psychol. 2016, 41, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Zupanec, S.; Jones, H.; Stremler, R. Sleep habits and fatigue of children receiving maintenance chemotherapy for ALL and their parents. J. Pediatr. Oncol. Nurs. 2010, 27, 217–228. [Google Scholar] [CrossRef]
- Daniel, L.C.; Walsh, C.M.; Meltzer, L.J.; Barakat, L.P.; Kloss, J.D. The relationship between child and caregiver sleep in acute lymphoblastic leukemia maintenance. Support Care Cancer 2018, 26, 1123–1132. [Google Scholar] [CrossRef]
- Matthews, E.E.; Neu, M.; Cook, P.F.; King, N. Sleep in mother and child dyads during treatment for pediatric acute lymphoblastic leukemia. Oncol. Nurs. Forum. 2014, 41, 599–610. [Google Scholar] [CrossRef]
- Rensen, N.; Steur, L.M.H.; Schepers, S.A.; Merks, J.H.M.; Moll, A.C.; Grootenhuis, M.A.; Kaspers, G.J.L.; van Litsenburg, R.R.L. Concurrence of sleep problems and distress: Prevalence and determinants in parents of children with cancer. Eur. J. Psychotraumatol. 2019, 10, 1639312. [Google Scholar] [CrossRef] [Green Version]
- Steur, L.M.H.; Rensen, N.; Grootenhuis, M.A.; van Eijkelenburg, N.K.A.; van der Sluis, I.M.; Dors, N.; Bos, C.V.D.; Tissing, W.J.; Kaspers, G.J.; Van Litsenburg, R.R. Parental sleep after induction therapy for childhood acute lymphoblastic leukemia. J. Psychosoc. Oncol. Res. Pract. 2021, 3, e045. [Google Scholar] [CrossRef]
- Spielman, A.J.; Yang, C.-M.; Glovinsky, P.B. Assessment Techniques for Insomnia. In Principles and Practice of Sleep Medicine; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 1632–1645. [Google Scholar]
- Lichstein, K.L.; Taylor, D.J.; McCrae, C.S.; Ruiter, M.E. Insomnia: Epidemiology and risk factors. In Principles and Practice of Sleep Medicine; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 827–837. [Google Scholar]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Precipitating factors of insomnia. Behav. Sleep Med. 2004, 2, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M. Psychological and Behavioral Treatments for Insomnia. In Principles and Practice of Sleep Medicine; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 866–883. [Google Scholar]
- Kalmbach, D.A.; Cuamatzi-Castelan, A.S.; Tonnu, C.V.; Tran, K.M.; Anderson, J.R.; Roth, T.; Drake, C.L. Hyperarousal and sleep reactivity in insomnia: Current insights. Nat. Sci. Sleep. 2018, 10, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Martire, V.; Caruso, D.; Palagini, L.; Zoccoli, G.; Bastianini, S. Stress & Sleep: A Relationship Lasting a Lifetime. Neurosci. Biobehav. Rev. 2019, 117, 65–77. [Google Scholar] [PubMed]
- Steur, L.M.H.; Grootenhuis, M.A.; Van Someren, E.J.W.; Van Eijkelenburg, N.K.A.; Van der Sluis, I.M.; Dors, N.; Bos, C.V.D.; Tissing, W.J.E.; Kaspers, G.J.L.; Van Litsenburg, R.R.L. High prevalence of parent-reported sleep problems in pediatric patients with acute lymphoblastic leukemia after induction therapy. Pediatr. Blood Cancer 2020, 67, e28165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutch Childhood Oncology Group. PROTOCOL ALL-11 Treatment Study Protocol of the Dutch Childhood Oncology Group for Children and Adolescents (1–19 Year) with Newly Diagnosed Acute Lymphoblastic Leukemia. 2012. Available online: https://www.skion.nl/voor-professionals/behandelrichtlijnen/protocollen/100/all-11/?pid=ac-p (accessed on 1 December 2019).
- Centraal Bureau Voor de Statistiek. Standaard Onderwijsindeling 2016 [Standard Educational Classification] Den Haag/Heerlen: Centraal Bureau voor de Statistiek [Statistics Netherlands]. 2016. Available online: https://www.cbs.nl/nl-nl/onze-diensten/methoden/classificaties/onderwijs-en-beroepen/standaard-onderwijsindeling--soi--/standaard-onderwijsindeling-2016 (accessed on 22 August 2018).
- Hays, R.D.; Martin, S.A.; Sesti, A.M.; Spritzer, K.L. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005, 6, 41–44. [Google Scholar] [CrossRef]
- Spritzer, K.L.; Hays, R.D. MOS Sleep Scale: A Manual for Use and Scoring; Version 1.0; RAND: Los Angeles, CA, USA, 2003. [Google Scholar]
- de Weerd, A.; de Haas, S.; Otte, A.; Trenite, D.K.; van Erp, G.; Cohen, A.; de Kam, M.; van Gerven, J. Subjective sleep disturbance in patients with partial epilepsy: A questionnaire-based study on prevalence and impact on quality of life. Epilepsia 2004, 45, 1397–1404. [Google Scholar] [CrossRef]
- Haverman, L.; van Oers, H.A.; Limperg, P.F.; Houtzager, B.A.; Huisman, J.; Darlington, A.S.; Maurice-Stam, H.; Grootenhuis, M.A. Development and validation of the distress thermometer for parents of a chronically ill child. J. Pediatr. 2013, 163, 1140–1146.e2. [Google Scholar] [CrossRef]
- van Oers, H.A.; Schepers, S.A.; Grootenhuis, M.A.; Haverman, L. Dutch normative data and psychometric properties for the Distress Thermometer for Parents. Qual. Life Res. 2017, 26, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Ware, J.E.; Kosinski, M.; Turner-Bowker, D.M.; Gandek, B. User’s Manual for the SF-12v2 Health Survey with a Supplement Documenting SF-12 Health Survey; QualityMetric Incorporated: Lincoln, RI, USA, 2002. [Google Scholar]
- Mols, F.; Pelle, A.J.; Kupper, N. Normative data of the SF-12 health survey with validation using postmyocardial infarction patients in the Dutch population. Qual. Life Res. 2009, 18, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Schoors, M.; Caes, L.; Knoble, N.B.; Goubert, L.; Verhofstadt, L.L.; Alderfer, M.A. Systematic Review: Associations between Family Functioning and Child Adjustment after Pediatric Cancer Diagnosis: A Meta-Analysis. J. Pediatr. Psychol. 2017, 42, 6–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Essen, L.; Sjödén, P.O.; Mattsson, E. Swedish mothers and fathers of a child diagnosed with cancer A Look at Their Quality of Life. Acta Oncol. 2004, 43, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Litzelman, K.; Catrine, K.; Gangnon, R.; Witt, W.P. Quality of life among parents of children with cancer or brain tumors: The impact of child characteristics and parental psychosocial factors. Qual. Life Res. 2011, 20, 1261–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, W.P.; Litzelman, K.; Wisk, L.E.; Spear, H.A.; Catrine, K.; Levin, N.; Gottlieb, C.A. Stress-mediated quality of life outcomes in parents of childhood cancer and brain tumor survivors: A case-control study. Qual. Life Res. 2010, 19, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Coleman, K.; Flesch, L.; Petiniot, L.; Pate, A.; Lin, L.; Crosby, L.; Beebe, D.W.; Nelson, A.; Alonso, P.B.; Davies, S.M.; et al. Sleep disruption in caregivers of pediatric stem cell recipients. Pediatr. Blood Cancer 2018, 65, e26965. [Google Scholar] [CrossRef] [Green Version]
- McLoone, J.K.; Wakefield, C.E.; Yoong, S.L.; Cohn, R.J. Parental sleep experiences on the pediatric oncology ward. Support. Care Cancer 2013, 21, 557–564. [Google Scholar] [CrossRef]
- Wright, M. Children receiving treatment for cancer and their caregivers: A mixed methods study of their sleep characteristics. Pediatr. Blood Cancer 2011, 56, 638–645. [Google Scholar] [CrossRef]
- Ljungman, L.; Cernvall, M.; Gronqvist, H.; Ljotsson, B.; Ljungman, G.; von Essen, L. Long-term positive and negative psychological late effects for parents of childhood cancer survivors: A systematic review. PLoS ONE 2014, 9, e103340. [Google Scholar] [CrossRef] [Green Version]
- Seixas, A.A.; Nunes, J.V.; Airhihenbuwa, C.O.; Williams, N.J.; Pandi-Perumal, S.R.; James, C.C.; Jean-Louis, G. Linking emotional distress to unhealthy sleep duration: Analysis of the 2009 National Health Interview Survey. Neuropsychiatr. Dis. Treat. 2015, 11, 2425–2530. [Google Scholar] [CrossRef] [Green Version]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, K.M.; Elmore-Staton, L.; Buckhalt, J.A.; El-Sheikh, M. The link between maternal sleep and permissive parenting during late adolescence. J. Sleep Res. 2018, 27, e12676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuillan, M.E.; Bates, J.E.; Staples, A.D.; Deater-Deckard, K. Maternal stress, sleep, and parenting. J. Fam. Psychol. 2019, 33, 349–359. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.C.; Bastiani, J.; Williams, L.K. Are parenting behaviors associated with child sleep problems during treatment for acute lymphoblastic leukemia? Cancer Med. 2016, 5, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, W.; Bona, K. Assessment of Financial Burden as a Standard of Care in Pediatric Oncology. Pediatr. Blood Cancer 2015, 62 (Suppl. 5), S619–S631. [Google Scholar] [CrossRef] [Green Version]
- Kelada, L.; Wakefield, C.E.; Vetsch, J.; Schofield, D.; Sansom-Daly, U.M.; Hetherington, K.; O’Brien, T.; Cohn, R.J.; Anazodo, A.; Viney, R.; et al. Financial toxicity of childhood cancer and changes to parents’ employment after treatment completion. Pediatr. Blood Cancer 2020, 67, e28345. [Google Scholar] [CrossRef]
- Santacroce, S.J.; Kneipp, S.M. Influence of pediatric cancer-related financial burden on parent distress and other stress-related symptoms. Pediatr. Blood Cancer 2020, 67, e28093. [Google Scholar] [CrossRef]
- Sultan, S.; Leclair, T.; Rondeau, E.; Burns, W.; Abate, C. A systematic review on factors and consequences of parental distress as related to childhood cancer. Eur. J. Cancer Care 2016, 25, 616–637. [Google Scholar] [CrossRef] [Green Version]
- Rensen, N.; Steur, L.M.H.; Grootenhuis, M.A.; van Eijkelenburg, N.K.A.; van der Sluis, I.M.; Dors, N.; Bos, C.V.D.; Tissing, W.J.; Kaspers, G.J.; Van Litsenburg, R.R. Parental functioning during maintenance treatment for childhood acute lymphoblastic leukemia: Effects of treatment intensity and dexamethasone pulses. Pediatr. Blood Cancer 2020, 67, e28697. [Google Scholar] [CrossRef]
- McGrath, P.; Pitcher, L. ‘Enough is enough’: Qualitative findings on the impact of dexamethasone during reinduction/consolidation for paediatric acute lymphoblastic leukaemia. Support. Care Cancer 2002, 10, 146–155. [Google Scholar] [CrossRef]
- McGrath, P.; Rawson-Huff, N. Corticosteroids during continuation therapy for acute lymphoblastic leukemia: The psycho-social impact. Issues Compr. Pediatr. Nurs. 2010, 33, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J.; Buysse, D.J. Treatment Guidelines for Insomnia. In Principles and Practice of Sleep Medicine; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 931–937. [Google Scholar]
- Salem, H.; Johansen, C.; Schmiegelow, K.; Winther, J.F.; Wehner, P.S.; Hasle, H.; Rosthøj, S.; Kazak, A.E.; Bidstrup, P.E. FAMily-Oriented Support (FAMOS): Development and feasibility of a psychosocial intervention for families of childhood cancer survivors. Acta Oncol. 2017, 56, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kazak, A.E.; Alderfer, M.A.; Streisand, R.; Simms, S.; Rourke, M.T.; Barakat, L.P.; Gallagher, P.; Cnaan, A. Treatment of posttraumatic stress symptoms in adolescent survivors of childhood cancer and their families: A randomized clinical trial. J. Fam. Psychol. 2004, 18, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, C.E.; Sansom-Daly, U.M.; McGill, B.C.; Ellis, S.J.; Doolan, E.L.; Robertson, E.G.; Mathur, S.; Cohn, R.J. Acceptability and feasibility of an e-mental health intervention for parents of childhood cancer survivors: “Cascade”. Support. Care Cancer 2016, 24, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.L.; Patino-Fernandez, A.M.; McSherry, M.; Beele, D.; Alderfer, M.A.; Reilly, A.T.; Hwang, W.-T.; Kazak, A.E. The Psychosocial Assessment Tool (PAT2.0): Psychometric properties of a screener for psychosocial distress in families of children newly diagnosed with cancer. J. Pediatr. Psychol. 2008, 33, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Schepers, S.A.; Sint Nicolaas, S.M.; Maurice-Stam, H.; Haverman, L.; Verhaak, C.M.; Grootenhuis, M.A. Parental distress 6 months after a pediatric cancer diagnosis in relation to family psychosocial risk at diagnosis. Cancer 2018, 124, 381–390. [Google Scholar] [CrossRef]
- Le Blanc, M.; Mérette, C.; Savard, J.; Ivers, H.; Baillargeon, L.; Morin, C.M. Incidence and Risk Factors of Insomnia in a Population-Based Sample. Sleep 2009, 32, 1027–1037. [Google Scholar] [CrossRef]
| Sex | N (%) |
| Male | 83 (59.7) |
| Female | 56 (40.3) |
| Risk group stratification | N (%) |
| Standard risk | 32 (23.2) |
| Medium risk | 105 (76.1) |
| of which with confirmed IKZF1 deletion and third year of treatment | 8 (7.6) |
| High risk | 1 (0.7) |
| Unknown (deceased before risk group stratification) | 1 (0.7) |
| Age at diagnosis | Median (interquartile range) |
| in years | 4.8 (3.1–8.7) |
| Chronic illness (other than ALL) | N (%) |
| Yes | 8 (5.8) |
| No | 124 (89.2) |
| Unknown | 7 (5.0) |
| Family’s educational level | N (%) |
| Low | 5 (3.6) |
| Middle | 39 (28.1) |
| High | 88 (63.3) |
| Unknown | 7 (5.0) |
| T0 N = 120 | T1 N = 112 | T2 N = 101 | T3 N = 92 | |
|---|---|---|---|---|
| Parent’s sex | N (%) | |||
| male | 26 (21.7) | 19 (17.0) | 18 (17.8) | 16 (17.4) |
| female | 94 (78.3) | 92 (82.1) | 82 (81.2) | 75 (81.5) |
| unknown | 0 (0.0) | 1 (0.9) | 1 (1.0) | 1 (1.1) |
| Parent’s age | Mean (SD) | |||
| in years | 38.6 (6.4) | 39.0 (6.1) | 39.9 (6.4) | 40.9 (6.0) |
| Chronic illness | N (%) | |||
| yes | 14 (11.7) | 12 (10.7) | 7 (6.9) | 5 (5.4) |
| no | 106 (88.3) | 100 (89.3) | 93 (92.1) | 87 (94.6) |
| Time since child’s ALL diagnosis | Mean (SD) | |||
| in months | 4.7 (1.3) | 13.5 (1.3) | 24.2 (1.7) | 36.7 (1.8) |
| Parent-reported pain of the child | N (%) | |||
| clinically relevant pain score (≥4) | 54 (45.0) | 36 (32.1) | 33 (32.7) | 20 (21.7) |
| no clinically relevant pain score (<4) | 65 (54.2) | 72 (64.3) | 68 (67.3) | 68 (73.9) |
| unknown | 1 (0.8) | 4 (3.6) | 0 (0.0) | 4 (4.3) |
| SLP-9 | ||||
| mean score (SD) | 36.4 (16.7) | 34.3 (18.3) | 30.9 (17.1) | 28.4 (16.4) |
| % clinically relevant sleep problems | 51.7 | 40.2 | 40.0 | 32.6 |
| Distress | ||||
| mean thermometer score (SD) | 5.4 (2.8) | 4.5 (2.4) | 3.9 (2.8) | 2.4 (2.5) |
| % clinical distress | 72.0 | 66.7 | 51.1 | 26.8 |
| Quality of life | ||||
| mean MCS z-score (SD) | −0.9 (1.2) | −0.5 (1.1) | −0.4 (1.0) | 0.1 (1.0) |
| % clinically impaired | 48.3 | 36.1 | 29.3 | 11.5 |
| mean PCS z-score (SD) | 0.1 (1.2) | 0.0 (1.0) | 0.2 (0.9) | 0.1 (0.8) |
| % clinically impaired | 14.7 | 13.0 | 10.1 | 9.2 |
| Parenting problems | N (%) | |||
| yes | 56 (46.7) | 49 (43.8) | 42 (41.6) | 23 (25.0) |
| no | 63 (52.5) | 63 (56.3) | 58 (57.4) | 67 (72.8) |
| unknown | 1 (0.8) | 0 (0.0) | 1 (1.0) | 2 (2.2) |
| Social support | N (%) | |||
| sufficient | 106 (88.3) | 94 (83.9) | 82 (81.2) | 82 (89.1) |
| insufficient | 14 (11.7) | 17 (15.2) | 18 (17.8) | 10 (10.9) |
| unknown | 0 (0.0) | 1 (0.9) | 1 (1.0) | 0 (0.0) |
| Wish for referral | N (%) | |||
| yes/maybe | 51 (42.5) | 43 (38.4) | 27 (26.7) | 20 (21.7) |
| no | 67 (55.8) | 69 (61.6) | 73 (72.3) | 70 (76.1) |
| unknown | 2 (1.7) | 0 (0.0) | 1 (1.0) | 2 (2.2) |
| Sleep Problems, Low Distress a | High Distress, No Sleep Problems a | Sleep Problems and High Distress a | |
|---|---|---|---|
| Parent variables | OR [95% C.I.] | OR [95% C.I.] | OR [95% C.I.] |
| Chronic illness | 2.2 [0.4; 12.4] | 1.2 [0.3; 5.5] | 3.7 [0.9; 15.9] * |
| Child variables | |||
| TSD (per one year increase) | 0.9 [0.6; 1.4] | 0.5 [0.4; 0.7] **** | 0.5 [0.3; 0.7] **** |
| Medium or high risk group | 1.0 [0.4; 2.6] | 2.1 [0.9; 4.7] * | 3.0 [1.2; 7.7] ** |
| Clinically relevant pain (parent-rated) | 0.5 [0.2; 1.6] | 2.7 [1.3; 5.5] *** | 4.3 [2.0; 9.5] **** |
| Psychosocial variables | |||
| Parenting problems | 2.3 [0.9; 5.7] * | 3.8 [1.9; 7.7] **** | 4.5 [2.1; 9.5] **** |
| Insufficient social support | 1.8 [0.3; 11.0] | 4.9 [1.2; 19.8] ** | 15.2 [3.8; 61.2 ] **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rensen, N.; Steur, L.; Grootenhuis, M.; Twisk, J.; van Eijkelenburg, N.; van der Sluis, I.; Dors, N.; van den Bos, C.; Tissing, W.; Kaspers, G.; et al. Parental Sleep, Distress, and Quality of Life in Childhood Acute Lymphoblastic Leukemia: A Longitudinal Report from Diagnosis up to Three Years Later. Cancers 2022, 14, 2779. https://doi.org/10.3390/cancers14112779
Rensen N, Steur L, Grootenhuis M, Twisk J, van Eijkelenburg N, van der Sluis I, Dors N, van den Bos C, Tissing W, Kaspers G, et al. Parental Sleep, Distress, and Quality of Life in Childhood Acute Lymphoblastic Leukemia: A Longitudinal Report from Diagnosis up to Three Years Later. Cancers. 2022; 14(11):2779. https://doi.org/10.3390/cancers14112779
Chicago/Turabian StyleRensen, Niki, Lindsay Steur, Martha Grootenhuis, Jos Twisk, Natasha van Eijkelenburg, Inge van der Sluis, Natasja Dors, Cor van den Bos, Wim Tissing, Gertjan Kaspers, and et al. 2022. "Parental Sleep, Distress, and Quality of Life in Childhood Acute Lymphoblastic Leukemia: A Longitudinal Report from Diagnosis up to Three Years Later" Cancers 14, no. 11: 2779. https://doi.org/10.3390/cancers14112779
APA StyleRensen, N., Steur, L., Grootenhuis, M., Twisk, J., van Eijkelenburg, N., van der Sluis, I., Dors, N., van den Bos, C., Tissing, W., Kaspers, G., & van Litsenburg, R. (2022). Parental Sleep, Distress, and Quality of Life in Childhood Acute Lymphoblastic Leukemia: A Longitudinal Report from Diagnosis up to Three Years Later. Cancers, 14(11), 2779. https://doi.org/10.3390/cancers14112779







