Expansion of Liver Transplantation Criteria for Hepatocellular Carcinoma from Milan to UCSF in Australia and New Zealand and Justification for Metroticket 2.0
Abstract
Simple Summary
Abstract
1. Introduction
- (a)
- to compare the long-term outcomes of patients with HCC transplanted before 2007 and after 2007, when the UCSF was the sole criterion used for listing patients for LT for HCC;
- (b)
- to compare the long-term outcomes for LT within the Milan versus the UCSF criteria in order to determine the true benefit of the expansion of the criteria;
- (c)
- to retrospectively validate the MT2 criteria [12]. This was done to explore the possibility for further expansion of the criteria for LT for HCC in ANZ.
2. Materials and Methods
2.1. Metroticket 2.0 Framework
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival in the Milan and UCSF Eras
3.3. Was the Survival Advantage in the UCSF Era due to Patients Transplanted within the Milan Criteria?
3.4. Validating the MT2 Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Are, C.; Meyer, B.; Stack, A.; Ahmad, H.; Smith, L.; Qian, B.; Song, T.; Chowdhury, S. Global Trends in the Burden of Liver Cancer. J. Surg. Oncol. 2017, 115, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Burroughs, A.; Graziadei, I.; Pirenne, J.; Valdecasas, J.C.; Muiesan, P.; Samuel, D.; Forns, X. Easl Clinical Practice Guidelines: Liver Transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.C.; Fuster, J. Expansion of the Hepatocellular Carcinoma Milan Criteria in Liver Transplantation: Future Directions. World J. Gastroenterol. 2018, 24, 3626. [Google Scholar] [CrossRef]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Expansion of the Tumor Size Limits Does Not Adversely Impact Survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Sugawara, Y.; Tamura, S.; Makuuchi, M. Living Donor Liver Transplantation for Hepatocellular Carcinoma: Tokyo University Series. Dig. Dis. 2007, 25, 310–312. [Google Scholar] [CrossRef]
- Zheng, S.S.; Xu, X.; Wu, J.; Chen, J.; Wang, W.L.; Zhang, M.; Liang, T.B.; Li, M. Liver Transplantation for Hepatocellular Carcinoma: Hangzhou Experiences. Transplantation 2008, 85, 1726–1732. [Google Scholar] [CrossRef]
- Ito, T.; Takada, Y.; Ueda, M.; Haga, H.; Maetani, Y.; Oike, F.; Ogawa, K.; Sakamoto, S.; Ogura, Y.; Egawa, H.; et al. Expansion of Selection Criteria for Patients with Hepatocellular Carcinoma in Living Donor Liver Transplantation. Liver Transpl 2007, 13, 1637–1644. [Google Scholar] [CrossRef]
- Fan, J.; Yang, G.S.; Fu, Z.R.; Peng, Z.H.; Xia, Q.; Peng, C.H.; Qian, J.M.; Zhou, J.; Xu, Y.; Qiu, S.J.; et al. Liver Transplantation Outcomes in 1078 Hepatocellular Carcinoma Patients: A Multi-Center Experience in Shanghai, China. J. Cancer Res. Clin. Onco.l 2009, 135, 1403–1412. [Google Scholar] [CrossRef]
- Chen, J.W.; Kow, L.; Verran, D.J.; McCall, J.L.; Munn, S.; Balderson, G.A.; Fawcett, J.W.; Gow, P.J.; Jones, R.M.; Jeffrey, G.P.; et al. Poorer Survival in Patients Whose Explanted Hepatocellular Carcinoma (Hcc) Exceeds Milan or Ucsf Criteria. An Analysis of Liver Transplantation in Hcc in Australia and New Zealand. HPB 2009, 11, 81–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting Survival after Liver Transplantation in Patients with Hepatocellular Carcinoma Beyond the Milan Criteria: A Retrospective, Exploratory Analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death after Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Costentin, C.E.; Bababekov, Y.J.; Zhu, A.X.; Yeh, H. Is It Time to Reconsider the Milan Criteria for Selecting Patients with Hepatocellular Carcinoma for Deceased-Donor Liver Transplantation? Hepatology 2019, 69, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Seppala, M. Studies of Carcino-Fetal Proteins: Physical and Chemical Properties of Human Alpha-Fetoprotein. Int. J. Cancer 1971, 7, 218–225. [Google Scholar] [CrossRef]
- Jain, A.; Reyes, J.; Kashyap, R.; Dodson, S.F.; Demetris, A.J.; Ruppert, K.; Abu-Elmagd, K.; Marsh, W.; Madariaga, J.; Mazariegos, G.; et al. Long-Term Survival after Liver Transplantation in 4000 Consecutive Patients at a Single Center. Ann. Surg 2000, 232, 490–500. [Google Scholar] [CrossRef]
- Onaca, N.; Davis, G.L.; Jennings, L.W.; Goldstein, R.M.; Klintmalm, G.B. Improved Results of Transplantation for Hepatocellular Carcinoma: A Report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2009, 15, 574–580. [Google Scholar] [CrossRef]
- Crocetti, L.; Bozzi, E.; Scalise, P.; Bargellini, I.; Lorenzoni, G.; Ghinolfi, D.; Campani, D.; Balzano, E.; De Simone, P.; Cioni, R. Locoregional Treatments for Bridging and Downstaging HCC to Liver Transplantation. Cancers 2021, 13, 5558. [Google Scholar] [CrossRef]
- El-Fattah, M.A.; Aboelmagd, M.; Elhamouly, M. Prognostic Factors of Hepatocellular Carcinoma Survival after Radiofrequency Ablation: A Us Population-Based Study. United Eur. Gastroenterol. J. 2017, 5, 227–235. [Google Scholar] [CrossRef]
- Bai, D.S.; Zhang, C.; Chen, P.; Jin, S.J.; Jiang, G.Q. The Prognostic Correlation of Afp Level at Diagnosis with Pathological Grade, Progression, and Survival of Patients with Hepatocellular Carcinoma. Sci. Rep. 2017, 7, 12870. [Google Scholar] [CrossRef] [PubMed]
- Cancer Australia. Available online: Liver cancer in Australia statistics|Cancer Australia (accessed on 13 April 2022).
- Chamberlain, J.; Sarfati, D.; Cunningham, R.; Koea, J.; Gurney, J.; Blakely, T. Incidence and Management of Hepatocellular Carcinoma among Maori and Non-Maori New Zealanders. Aust. N. Z. J. Public Health 2013, 37, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Koczwara, B.; Deckx, L.; Ullah, S.; Van den Akker, M. Impact of Comorbidities on Physical Function and Survival of Middle-Aged as Compared to Older, Individuals with Cancer. Support. Care Cancer 2022, 30, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, I.; Colle, I.; Geerts, A.; Smeets, P.; Berardi, G.; Praet, M.; Rogiers, X.; de Hemptinne, B.; Van Vlierberghe, H.; Troisi, R.I. Liver Transplantation for Hepatocellular Carcinoma Comparing the Milan, Ucsf, and Asan Criteria: Long-Term Follow-up of a Western Single Institutional Experience. Clin. Transplant. 2015, 29, 425–433. [Google Scholar] [CrossRef]
- Duffy, J.P.; Vardanian, A.; Benjamin, E.; Watson, M.; Farmer, D.G.; Ghobrial, R.M.; Lipshutz, G.; Yersiz, H.; Lu, D.S.K.; Lassman, C.; et al. Liver Transplantation Criteria for Hepatocellular Carcinoma Should Be Expanded: A 22-Year Experience with 467 Patients at Ucla. Ann. Surg. 2007, 246, 502–511. [Google Scholar] [CrossRef]
- Piñero, F.; Costa, P.; Boteon, Y.L.; Duque, S.H.; Marciano, S.; Anders, M.; Varon, A.; Zerega, A.; Poniachik, J.; Soza, A.; et al. Results of Liver Transplantation for Hepatocellular Carcinoma in a Multicenter Latin American Cohort Study. Ann. Hepatol. 2018, 17, 256–267. [Google Scholar] [CrossRef]
- Unek, T.; Karademir, S.; Arslan, N.C.; Egeli, T.; Atasoy, G.; Sagol, O.; Obuz, F.; Akarsu, M.; Astarcioglu, I. Comparison of Milan and Ucsf Criteria for Liver Transplantation to Treat Hepatocellular Carcinoma. World J. Gastroenterol. 2011, 17, 4206–4212. [Google Scholar] [CrossRef]
- Lee, S.G.; Hwang, S.; Moon, D.B.; Ahn, C.S.; Kim, K.H.; Sung, K.B.; Ko, G.Y.; Park, K.M.; Ha, T.Y.; Song, G.W. Expanded Indication Criteria of Living Donor Liver Transplantation for Hepatocellular Carcinoma at One Large-Volume Center. Liver Transpl. 2008, 14, 935–945. [Google Scholar] [CrossRef]
- Degroote, H.; Callebout, E.; Iesari, S.; Dekervel, J.; Schreiber, J.; Pirenne, J.; Verslype, C.; Ysebaert, D.; Michielsen, P.; Lucidi, V.; et al. Extended Criteria for Liver Transplantation in Hepatocellular Carcinoma. A Retrospective, Multicentric Validation Study in Belgium. Surg. Oncol. 2020, 33, 231–238. [Google Scholar] [CrossRef]
- Raj, A.; McCall, J.; Gane, E. Validation of the “Metroticket” Predictor in a Cohort of Patients Transplanted for Predominantly Hbv-Related Hepatocellular Carcinoma. J. Hepatol. 2011, 55, 1063–1068. [Google Scholar] [CrossRef]
- Menon, K.V.; Hakeem, A.R.; Heaton, N.D. Review Article: Liver Transplantation for Hepatocellular Carcinoma—A Critical Appraisal of the Current Worldwide Listing Criteria. Aliment. Pharmacol. Ther. 2014, 40, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Centonze, L.; Di Sandro, S.; Lauterio, A.; De Carlis, R.; Sgrazzutti, C.; Ciulli, C.; Vella, I.; Vicentin, I.; Incarbone, N.; Bagnardi, V.; et al. A Retrospective Single-Centre Analysis of the Oncological Impact of Li-Rads Classification Applied to Metroticket 2.0 Calculator in Liver Transplantation: Every Nodule Matters. Transpl. Int. 2021, 34, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. European Association for the Study of the Liver. Electronic address, easloffice easloffice eu, Chair Clinical Practice Guidelines Panel, Easl Governing Board representative, and members Panel. Easl Recommendations on Treatment of Hepatitis C: Final Update of the Series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar]
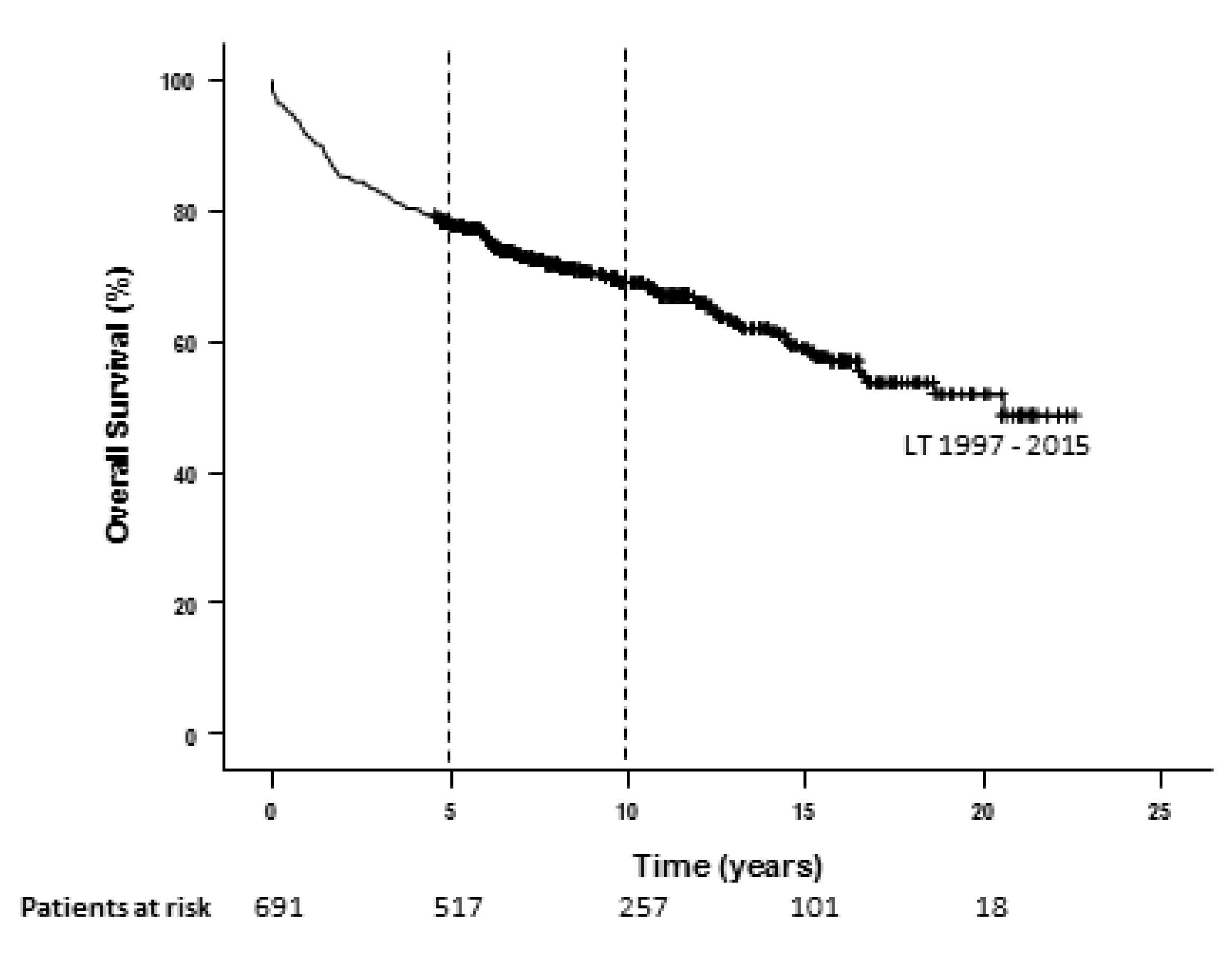
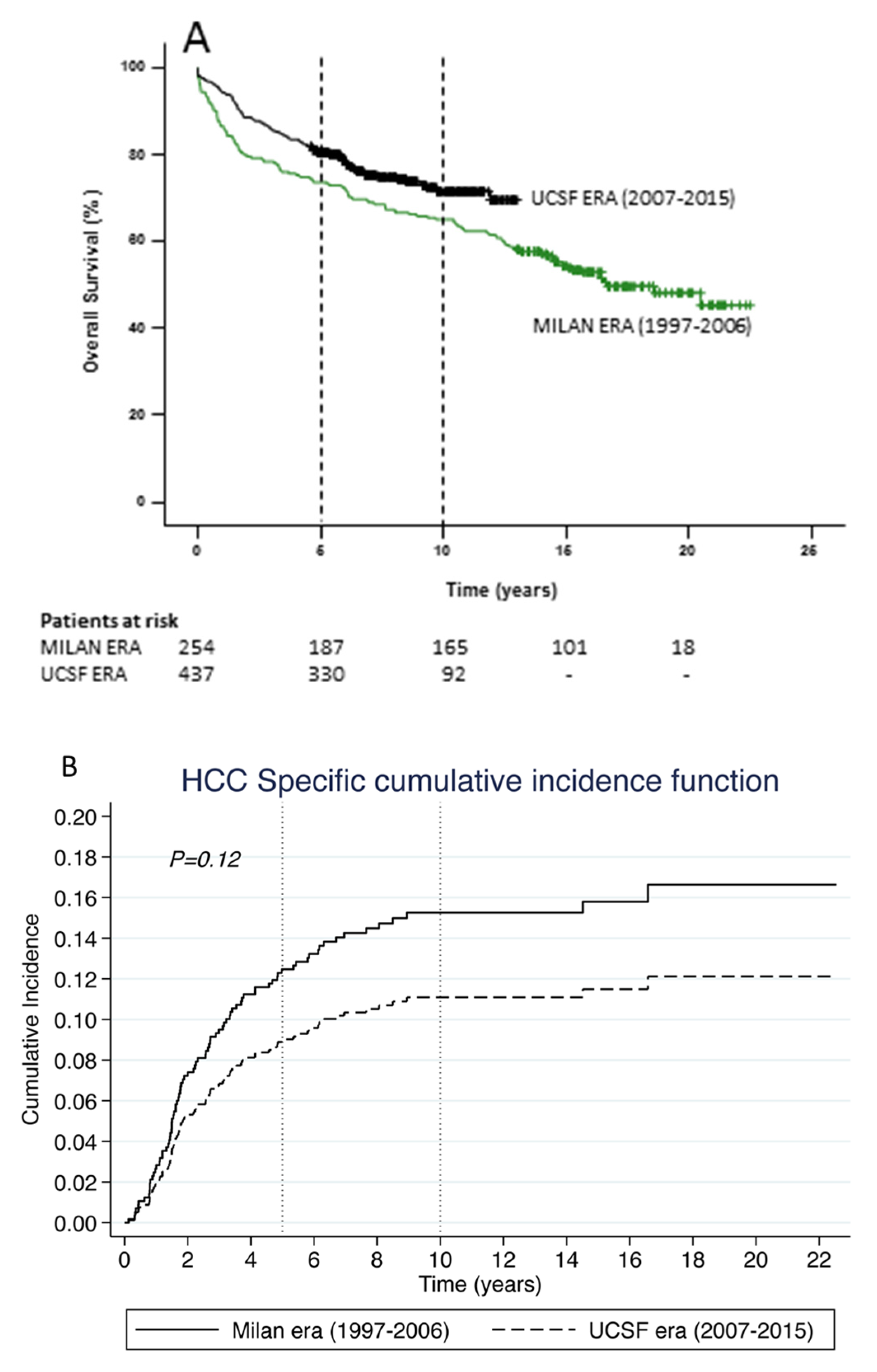

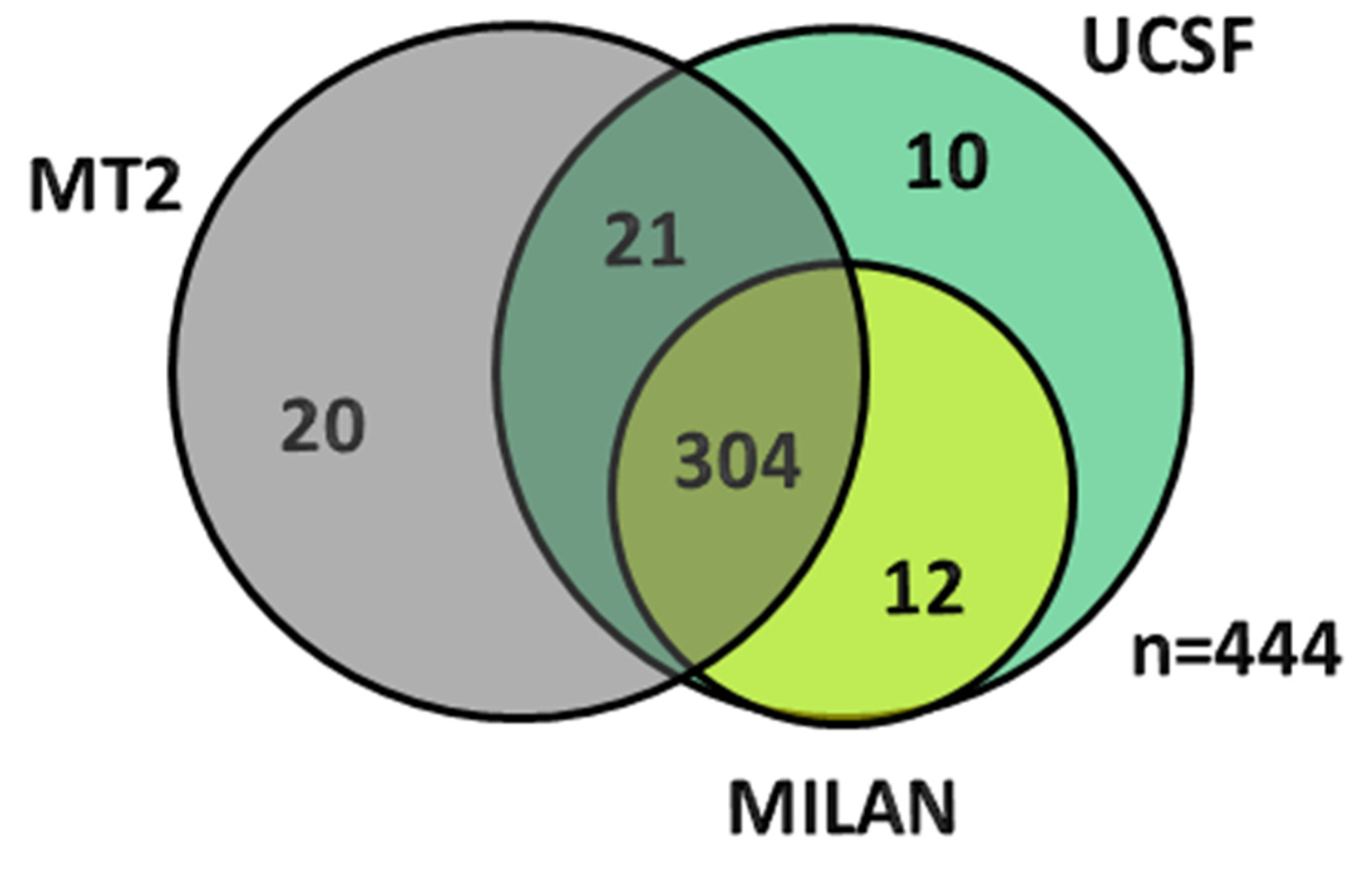
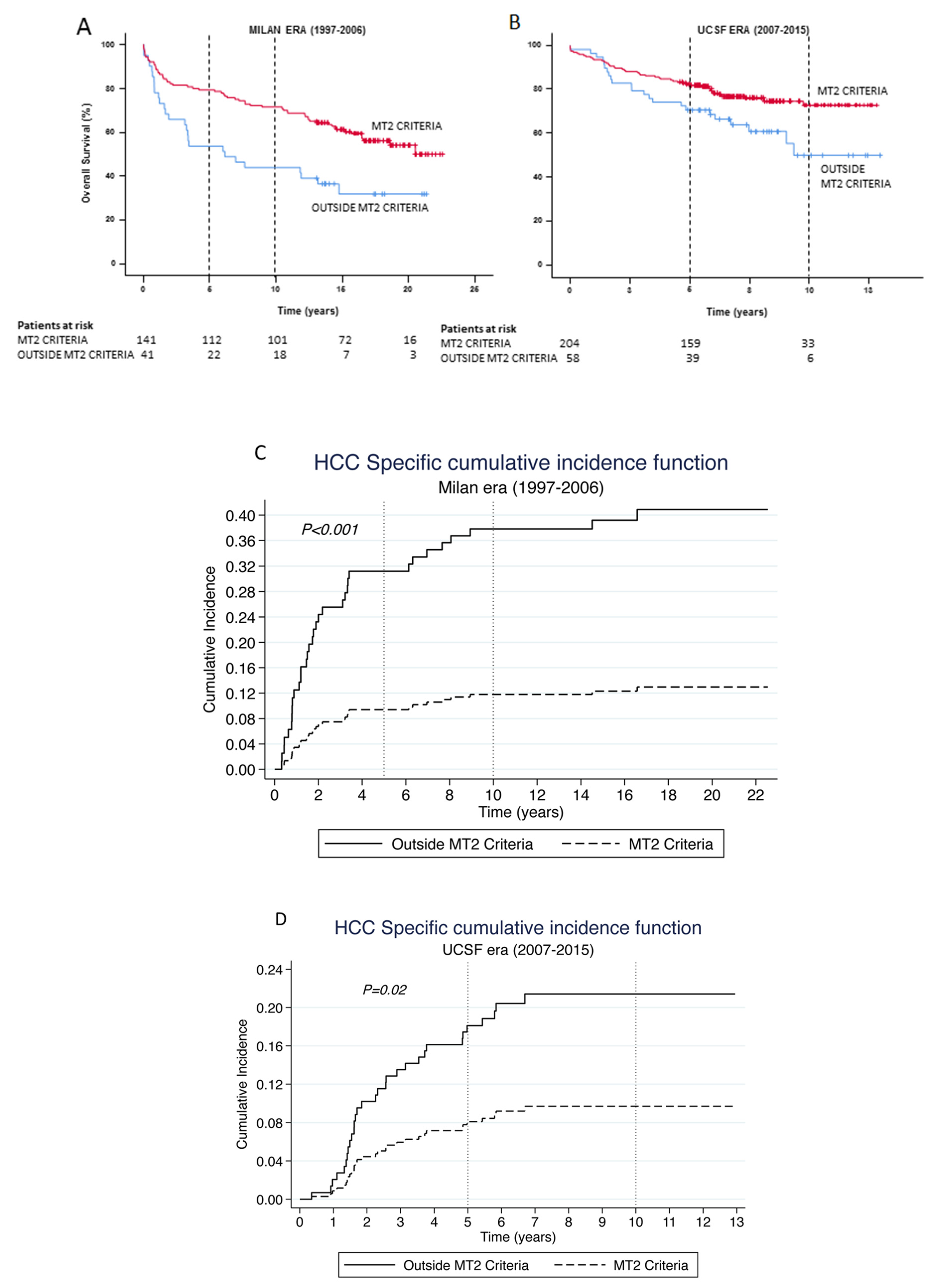
| Demographic Characteristics | Overall Cohort (n = 691) | Milan era (n = 254) 1997–2006 | UCSF era (n = 437) 2007–2015 | P Value (Era Comparison) |
|---|---|---|---|---|
| Gender male/female n (%) | 595 (86)/96 (14) | 219 (86)/35 (14) | 377 (86)/60 (14) | 0.871 # |
| Median Age in years (IQR) | 55.7 (51.5–60.1) | 54.7 (49.1–58.8) | 56.4 (52.2–60.4) | 0.001 * |
| Underlying disease n (%) Viral hepatitis Alcohol-related disease Immune/Inflammatory /metabolic NAFLD | 543 (79) 65 (9) 45 (7) 37 (5) | 200 (79) 28 (11) 13 (5) 13 (5) | 343 (79) 37 (9) 32 (7) 24 (5) | 0.502 # |
| Median serum α-FP at listing in ng/ml (IQR) | 12 (5.0–44.0) | 17 (6.0–80.7) | 9.8 (4.0–28.4) | <0.001 * |
| Hepatitis status n (%) Hepatitis C Hepatitis B Hepatitis B & C No hepatitis | 362 (53) 165 (24) 16 (2) 148 (21) | 112 (44) 78 (31) 10 (4) 54 (21) | 250 (57) 87 (20) 6 (1) 94 (22) | 0.001 # |
| Pathological characteristics at explant | ||||
| Mean tumor number ( ± SD) | 2 (1.7) | 2 (1.9) | 2 (1.5) | 0.468 * |
| Mean size of largest tumor in cm (±SD) | 2.7 (1.8) | 2.6 (1.6) | 2.8 (1.9) | 0.659 * |
| Mean total tumor diameter in cm (±SD) | 3.9 (2.9) | 3.7 (2.8) | 4.1 (3.0) | 0.054 * |
| Capsular invasion n (%) | 33 (5) | 16 (6) | 17 (4) | 0.151 # |
| Vascular invasion n (%) Macrovascular invasion Microvascular invasion No invasion Not available | 22 (3) 73 (11) 436 (63) 160 (23) | 13 (5) 27 (11) 178 (70) 36 (14) | 9 (2) 46 (11) 258 (59) 124 (28) | 0.177 # |
| Lymph node status n (%) Involved Uninvolved Not available | 21 (3) 339 (49) 331 (48) | 6 (2) 172 (68) 76 (30) | 15 (3) 167 (38) 255 (59) | 0.049 # |
| Variables | 5y Survival (n at Risk) | 10y Survival (n at Risk) | 95% CI | χ2 | P Log-Rank Comparison (Mantel-Cox) | |
|---|---|---|---|---|---|---|
| Age (years) | <55 (n = 312) | 81% (244) | 72% (136) | 15.04–17.18 | 5.72 | 0.017 |
| ≥55 (n = 379) | 75% (272) | 67% (121) | 13.02–15.15 | |||
| Underlying disease | Viral hepatitis (n = 543) | 78% (411) | 71% (208) | 14.63–16.32 | 6.08 | 0.014 |
| Alcohol-related cirrhosis (n = 65) | 72% (46) | 57% (22) | 10.22–10.74 | |||
| Hepatitis status | Hep B (n = 165) | 82% (132) | 79% (85) | 15.57–18.31 | 7.25 | 0.007 |
| Hep C (n = 362) | 76% (266) | 67% (115) | 13.31–15.57 | |||
| α-FP | <10 ng/mL (n = 216) | 79% (152) | 73% (180) | 14.99–17.72 | 4.31 | 0.038 |
| ≥10 ng/mL (n = 228) | 75% (64) | 63% (94) | 12.70–15.11 | |||
| Capsular invasion | no (n = 656) | 79% (496) | 70% (250) | 14.47–16.03 | 4.21 | 0.04 |
| yes (n = 33) | 61% (20) | 48% (6) | 8.42–15.46 | |||
| Vascular invasion | no (n = 436) | 80% (343) | 72% (185) | 14.64–16.47 | 5.712 | 0.017 |
| yes microvascular (n = 73) | 67% (46) | 52% (12) | 9.93–14.70 | |||
| no (n = 436) | 80% (343) | 72% (185) | 14.64–16.47 | 29.64 | <0.001 | |
| yes macrovascular (n = 22) | 32% (7) | 26% (4) | 2.92–8.78 | |||
| Step | Variable | HR | 95% CI | P |
|---|---|---|---|---|
| 1 | age | 1.027 | 1.003–1.052 | 0.027 |
| 2 | age | 1.029 | 1.005–1.054 | 0.018 |
| α-FP | 1.000 | 1.000–1.000 | 0.057 | |
| 3 | age | 1.027 | 1.003–1.052 | 0.027 |
| Author/Year [Ref] | Region | Within UCSF Criteria | |
|---|---|---|---|
| 5-Year Survival | 10-Year Survival | ||
| Duffy et al. 2007 [26] | USA | 64% | n.a. |
| Lee et al. 2008 [29] | Korea | 76% | n.a. |
| Unek et al. 2011 [28] | Turkey | 54% | n.a. |
| Bonadio et al. 2015 [25] | Belgium | 74% | n.a. |
| Pinero et al. 2018 [27] | Latin America | 66% | n.a. |
| Current study 2022 | ANZ | 80% | 72% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, S.G.; Strasser, S.I.; McCaughan, G.W.; Fink, M.A.; Jones, R.; McCall, J.; Munn, S.; Macdonald, G.A.; Hodgkinson, P.; Jeffrey, G.P.; et al. Expansion of Liver Transplantation Criteria for Hepatocellular Carcinoma from Milan to UCSF in Australia and New Zealand and Justification for Metroticket 2.0. Cancers 2022, 14, 2777. https://doi.org/10.3390/cancers14112777
Barreto SG, Strasser SI, McCaughan GW, Fink MA, Jones R, McCall J, Munn S, Macdonald GA, Hodgkinson P, Jeffrey GP, et al. Expansion of Liver Transplantation Criteria for Hepatocellular Carcinoma from Milan to UCSF in Australia and New Zealand and Justification for Metroticket 2.0. Cancers. 2022; 14(11):2777. https://doi.org/10.3390/cancers14112777
Chicago/Turabian StyleBarreto, Savio G., Simone I. Strasser, Geoffrey W. McCaughan, Michael A. Fink, Robert Jones, John McCall, Stephen Munn, Graeme A. Macdonald, Peter Hodgkinson, Gary P. Jeffrey, and et al. 2022. "Expansion of Liver Transplantation Criteria for Hepatocellular Carcinoma from Milan to UCSF in Australia and New Zealand and Justification for Metroticket 2.0" Cancers 14, no. 11: 2777. https://doi.org/10.3390/cancers14112777
APA StyleBarreto, S. G., Strasser, S. I., McCaughan, G. W., Fink, M. A., Jones, R., McCall, J., Munn, S., Macdonald, G. A., Hodgkinson, P., Jeffrey, G. P., Jaques, B., Crawford, M., Brooke-Smith, M. E., & Chen, J. W. (2022). Expansion of Liver Transplantation Criteria for Hepatocellular Carcinoma from Milan to UCSF in Australia and New Zealand and Justification for Metroticket 2.0. Cancers, 14(11), 2777. https://doi.org/10.3390/cancers14112777








