BRCA-Mutated Pancreatic Cancer: From Discovery to Novel Treatment Paradigms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Discovery of BRCA1 and BRCA2
3. Predisposition to Pancreatic Cancer with BRCA and Associated Gene Mutations
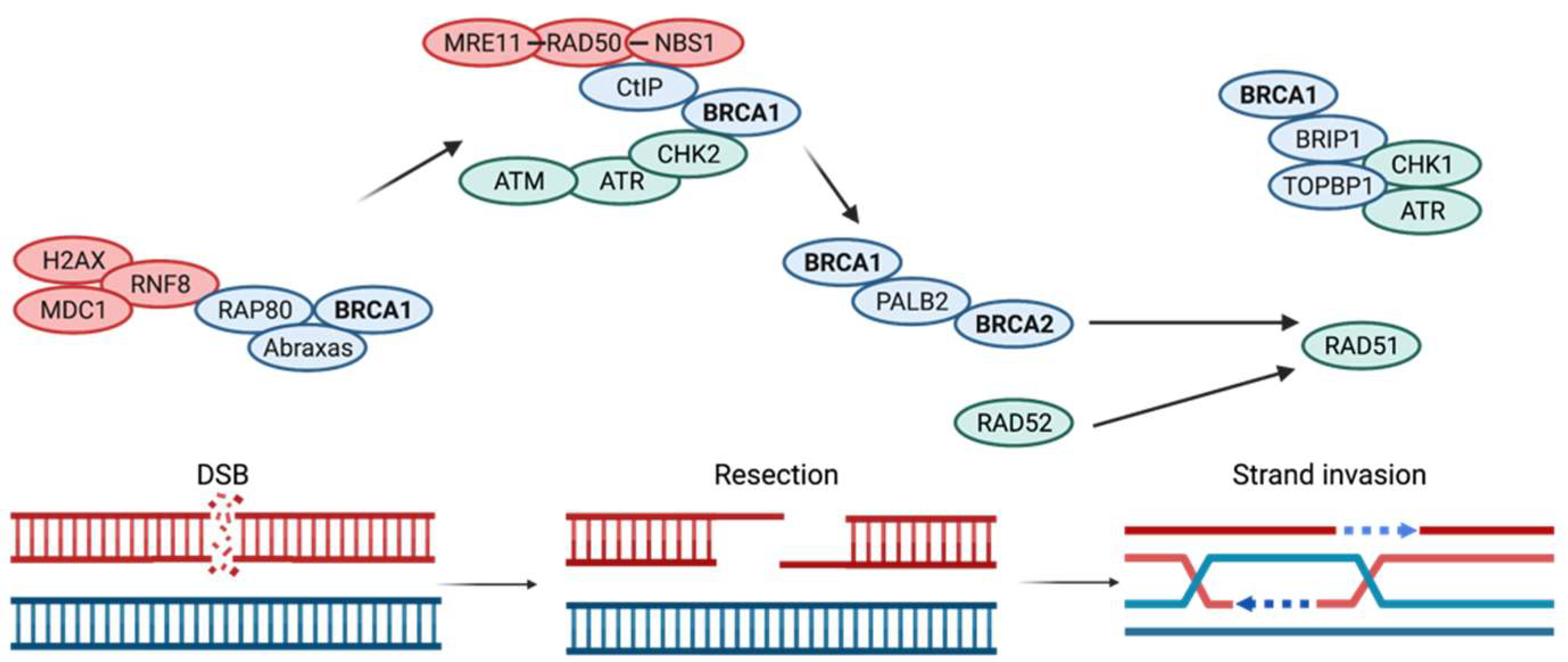
4. DNA Repair Mechanisms Compromised by BRCA1 and BRCA2 Mutations
5. BRCA1 Has Also Been Associated with the Regulation of the G2-M Checkpoint
6. BRCA1 Is a Fundamental Protein in Multiple Cellular Processes
7. Additional Functions of BRCA2
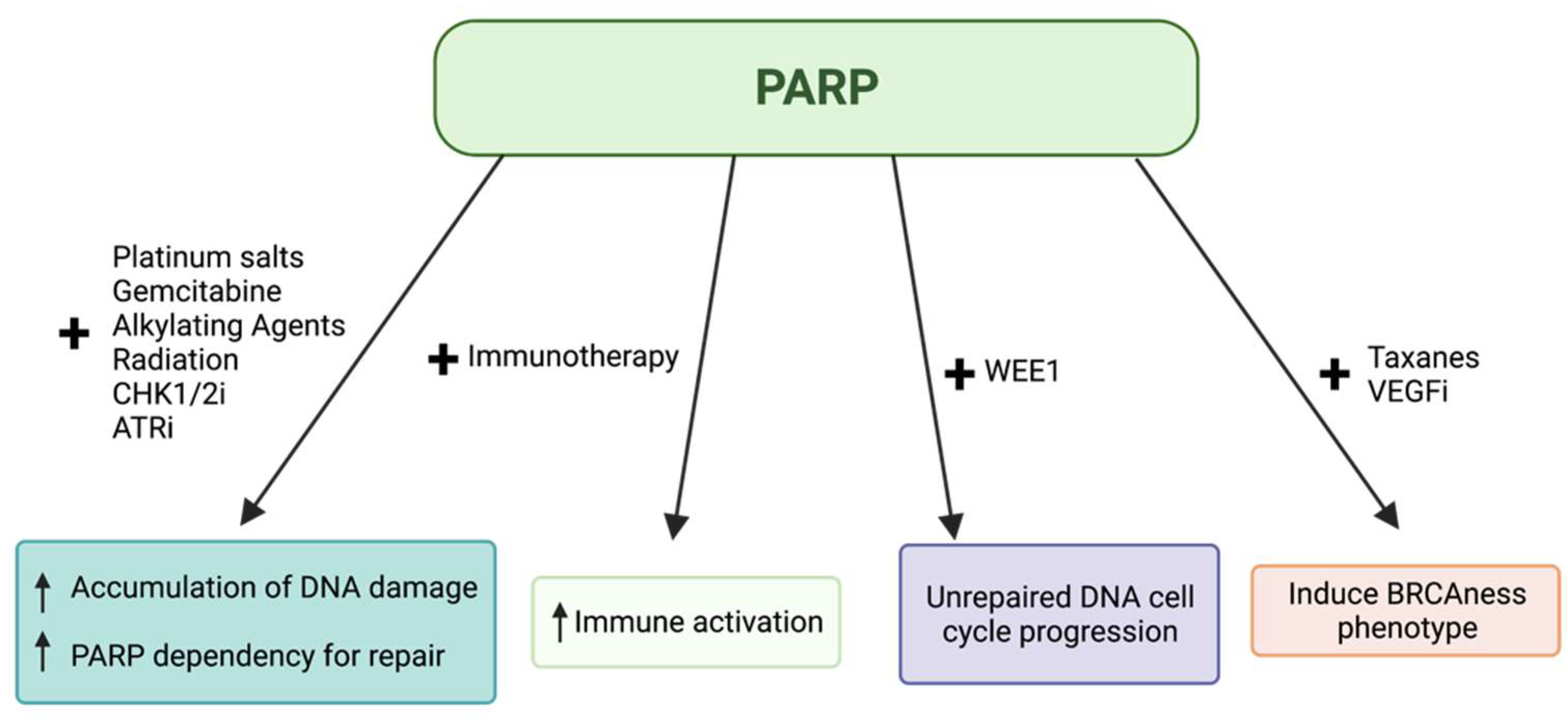
8. Current Available Treatments and Areas of Research
- A.
- Chemotherapy in BRCA-Mutated Cancers
- B.
- PARP Inhibitors in BRCA-Mutated Cancers
| Investigators | Phase | Patient Population | Number of PDAC Patients | Intervention | Outcome | Ref |
|---|---|---|---|---|---|---|
| Kauffman et al. | II | PDAC with gBRCA1/2 mutation following progression on gemcitabine | 23 | Olaparib 400mg PO BID | ORR 22% PFS 4.6 months OS 9.8 months | [91] |
| Shroff et al. | II | PDAC with any BRCA mutation, previously treated with 1-2 lines | 19 | Rucaparib 600mg PO BID | ORR 16% | [83] |
| Lowery et al. | II | PDAC with gBRCA mutation or PALB2 mutation, 1-2 prior lines of treatment | 16 | Veliparib PO BID PO | PFS 1.7 months OS 3.1 months | [85] |
| Golan et al. | II | PDAC with BRCA-appearing phenotype, first or second line | 32 | Olaparib PO BID | PFS 14 weeks in Israel 25 weeks in the US | [81] |
| Golan et al. | III | PDAC with gBRCA mutation that has not progressed on firstline platinum-based treatment | 92 olaparib 62 placebo | 3:2 olaparib versus placebo | ORR 37% | [80] |
| Reiss et al. | II | PDAC with g or s BRCA or PALB2 mutations that has not progressed on firstline platinum-based treatment | 24 | Rucaparib 600mg PO BID | ORR 37% | [82] |
| Chiorean et al. | II | PDAC including g or s BRCA or PALB2 mutations | 108 | 1:1 veliparib + FOLFIRI versus FOLFIRI alone | OS 5.1 vs 5.9 months PFS 2 months vs 3 months | [92] |
| Pishvaian et al | I/II | PDAC with g or S BRCA or PALB2 mutations or relevant breast or ovarian family history | 22 | Veliparib + mFOLFOX6 | OS 8.5 months PFS 3.7 months | [93] |
- C.
- PARP Inhibitors in Combination with Other Agents
- D.
- Other Novel Treatments
- E.
- Future Research Endeavors
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A Strong Candidate for the Breast and Ovarian Cancer Susceptibility Gene BRCA1. Source Sci. New Ser. 1994, 266, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; Collins, N.; Gregory, S.; Gumbs, C.; Micklem, G.; et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.B.; Whitcomb, D.C. Role of BRCA1 and BRCA2 mutations in pancreatic cancer. Gut 2007, 56, 601–605. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2012, 12, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Lowery, M.A.; Wong, W.; Jordan, E.J.; Lee, J.W.; Kemel, Y.; Vijai, J.; Mandelker, D.; Zehir, A.; Capanu, M.; Salo-Mullen, E.; et al. Prospective Evaluation of Germline Alterations in Patients with Exocrine Pancreatic Neoplasms. J. Natl. Cancer Inst. 2018, 110, 1067–1074. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Guo, M. Synthetic lethality strategies: Beyond BRCA1/2 mutations in pancreatic cancer. Cancer Sci. 2021, 111, 3111–3121. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific Killing of BRCA2-Deficient Tumours with Inhibitors of Poly(ADP-Ribose). Polymerase Nat. 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Rosen, M.N.; Goodwin, R.A.; Vickers, M.M. BRCA mutated pancreatic cancer: A change is coming. World J. Gastroenterol. 2021, 27, 1943–1958. [Google Scholar] [CrossRef]
- Goldstein, J.B.; Zhao, L.; Wang, X.; Ghelman, Y.; Overman, M.J.; Javle, M.M.; Shroff, R.T.; Varadhachary, G.R.; Wolff, R.A.; McAllister, F.; et al. Germline DNA sequencing reveals novel mutations predictive of overall survival in a cohort of patients with pancreatic cancer. Clin. Cancer Res. 2020, 26, 1385–1394. [Google Scholar] [CrossRef]
- Moynahan, M.E.; Pierce, A.J.; Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 2001, 7, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Shahid, T.; Soroka, J.; Kong, E.H.; Malivert, L.; McIlwraith, M.J.; Pape, T.; West, S.C.; Zhang, X. Structure and mechanism of action of the BRCA2 breast cancer tumor suppressor. Nat. Struct. Mol. Biol. 2014, 21, 962–968. [Google Scholar] [CrossRef] [Green Version]
- Newman, B.; Austin, M.A.; King, M.C. Inheritance of human breast cancer: Evidence for autosomal dominant transmission in high-risk families. Proc. Natl. Acad. Sci. USA 1988, 85, 3044–3048. [Google Scholar] [CrossRef] [Green Version]
- Goldgar, D.E.; Fields, P.; Lewis, C.M.; Tran, T.D.; Cannon-Albright, L.A.; Ward, J.H.; Swensen, J.; Skolnick, M.H. A large kindred with 17q-linked breast and ovarian cancer: Genetic, phenotypic, and genealogical analysis. J. Natl. Cancer Inst. 1994, 86, 200–209. [Google Scholar] [CrossRef]
- Cartwright-Smith, L. Patenting Genes: What Does Association for Molecular Pathology v. Myriad Genetics Mean for Genetic Testing and Research? Public Health Rep. 2014, 129, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Grover, S.; Syngal, S. Hereditary Pancreatic Cancer. Gastroenterology 2010, 139, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Silverman, D.T. Risk factors for pancreatic cancer: A case-control study based on direct interviews. Teratog Carcinog Mutagen 2001, 21, 7–25. [Google Scholar] [CrossRef]
- Easton, D. Cancer risks in BRCA2 mutation carriers. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar]
- Couch, F.J.; Johnson, M.R.; Rabe, K.G.; Rabe, K.G.; Brune, K.; deAndrade, M.; Goggins, M.; Rothenmund, H.; Gallinger, S.; Klein, A.; et al. The Prevalence of BRCA2 Mutations in Familial Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Hahn, S.A.; Greenhalf, B.; Ellis, I.; Sina-Frey, M.; Rieder, H.; Korte, B.; Gerdes, B.; Kress, R.; Ziegler, A.; Raeburn, J.A.; et al. BRCA2 germline mutations in familial pancreatic carcinoma. J. Natl. Cancer Inst. 2003, 95, 214–221. [Google Scholar] [CrossRef]
- Goggins, M.; Schutte, M.; Lu, J.; Moskaluk, C.A.; Weinstein, C.L.; Petersen, G.M.; Yeo, C.J.; Jackson, C.E.; Lynch, H.T.; Hruban, R.H.; et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996, 56, 5360–5364. [Google Scholar]
- Chen, Y.; Farmer, A.A.; Chen, C.; Jones, D.C.; Chen, P.; Lee, W. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 1996, 56, 3168–3172. [Google Scholar]
- Bork, P.; Hofmann, K.; Bucher, P.; Neuwald, A.F.; Altschul, S.F.; Koonin, E.V. A Superfamily of Conserved Domains in DNA Damage-Responsive Cell Cycle Checkpoint Proteins. FASEB J. 1997, 11, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Koonin, E.V.; Altschul, S.F.; Bork, P. BRCA1 protein products...Functional motifs. Nat. Genet. 1996, 13, 266–268. [Google Scholar] [CrossRef]
- Callebaut, I.; Mornon, J.P. From BRCA1 to RAP1: A widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997, 400, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Jensen, D.E.; Proctor, M.; Marquis, S.T.; Gardner, H.P.; Ha, S.I.; Chodosh, L.A.; Ishov, A.M.; Tommerup, N.; Vissing, H. BAP1: A novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998, 16, 1097–1112. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Li, S.; Boyer, T.G.; Lee, W. Lessons learned from BRCA1 and BRCA2. Oncogene 2000, 19, 6159–6175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallery, D.L.; Vandenberg, C.J.; Hiom, K. Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains. EMBO J. 2002, 21, 6755–6762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Feng, W.; Lim, P.X.; Kass, E.M.; Jasin, M. Homology-Directed Repair and the Role of BRCA1, BRCA2, and Related Proteins in Genome Integrity and Cancer. Annu. Rev. Cancer Biol. 2018, 2, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Chial, H. Tumor suppressor (TS) genes and the two-hit hypothesis. Nat. Educ. 2008, 1, 177. [Google Scholar]
- Review of Cancer Genetics. Available online: https://www.cooperhealth.org/sites/default/files/pdfs/Review_of_Cancer_Genetics.pdf (accessed on 27 February 2022).
- The Genetics of Cancer—National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/causes-prevention/genetics (accessed on 27 February 2022).
- Macklin-Mantia, S.K.; Hines, S.L.; Kasi, P.M. Retrospective review of outcomes in patients with DNA-damage repair related pancreatic cancer. Hered. Cancer Clin. Pract. 2020, 18, 1–5. [Google Scholar] [CrossRef]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018, 319, 2401–2409. [Google Scholar] [CrossRef]
- Iqbal, J.; Ragone, A.; Lubinski, J.; Lynch, H.T.; Moller, P.; Ghadirian, P.; Foulkes, W.D.; Armel, S.; Eisen, A.; Neuhausen, S.L.; et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 2012, 107, 2005–2009. [Google Scholar] [CrossRef]
- Golan, T.; Kanji, Z.S.; Epelbaum, R.; Devaud, N.; Dagan, E.; Holter, S.; Aderka, D.; Paluch-Shimon, S.; Kaufman, B.; Gershoni-Baruch, R.; et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br. J. Cancer 2014, 111, 1132. [Google Scholar] [CrossRef]
- Su, G.H.; Hruban, R.H.; Bansal, R.K.; Bova, G.S.; Tang, D.J.; Shekher, M.C.; Westerman, A.M.; Entius, M.M.; Goggins, M.; Yeo, C.J.; et al. Germline and Somatic Mutations of the STK11/LKB1 Peutz-Jeghers Gene in Pancreatic and Biliary Cancers. Am. J. Pathol. 1999, 154, 1835–1840. [Google Scholar] [CrossRef] [Green Version]
- Genetics of Pancreatic Cancer|Columbia Surgery. Available online: https://columbiasurgery.org/pancreas/genetics-pancreatic-cancer (accessed on 27 February 2022).
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Kastrinos, F.; Mukherjee, B.; Tayob, N.; Wang, F.; Sparr, J.; Raymond, V.M.; Bandipalliam, P.; Stoffel, E.M.; Gruber, S.B.; Syngal, S. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009, 302, 1790–1795. [Google Scholar] [CrossRef]
- Hsu, F.; Roberts, N.J.; Childs, E.; Porter, N.; Rabe, K.G.; Borgida, A.; Ukaegbu, C.; Goggins, M.G.; Hruban, R.H.; Zogopoulos, G.; et al. Risk of Pancreatic Cancer Among Individuals with Pathogenic Variants in the ATM Gene. JAMA Oncol. 2021, 7, 1664–1668. [Google Scholar] [CrossRef]
- Rupnik, A.; Grenon, M.; Lownedes, N. The MRN complex. Curr. Biol. 2008, 18, R455–R457. [Google Scholar] [CrossRef] [Green Version]
- Jasin, M.; Rothstein, R. Repair of Strand Breaks by Homologous Recombination. Cold Spring Harb. Perspect. Biol. 2013, 5, a012740. [Google Scholar] [CrossRef]
- Powell, S.N.; Kachnic, L.A. Roles of BRCA1 and BRCA2 in homologous recombination, DNA replication fidelity and the cellular response to ionizing radiation. Oncogene 2003, 22, 5784–5791. [Google Scholar] [CrossRef] [Green Version]
- Venkitaraman, A.R. Cancer Susceptibility and the Functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Yarden, R.I.; Pardo-Reoyo, S.; Sgagias, M.; Cowan, K.H.; Brody, L.C. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat. Genet. 2002, 30, 285–289. [Google Scholar] [CrossRef]
- Fabbro, M.; Savage, K.; Hobson, K.; Deans, A.J.; Powell, S.N.; McArthur, G.A.; Khanna, K.K. BRCA1-BARD1 Complexes Are Required for p53Ser-15 Phosphorylation and a G1/S Arrest following Ionizing Radiation-induced DNA Damage. J. Biol. Chem. 2004, 279, 31251–31258. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Zheng, M.; Lu, R.; Du, J.; Zhao, Q.; Li, Z.; Li, Y.; Zhang, S. The role of CDC25C in cell cycle regulation and clinical cancer therapy: A systematic review. Cancer Cell Int. 2020, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rufini, A.; Tucci, P.; Melino, G. Senescence and aging: The critical roles of p53. Oncogene 2013, 32, 5129–5143. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef] [Green Version]
- Parvin, J.D. Overview of History and Progress in BRCA1 Research: The First BRCA1 Decade. Cancer Bio. Ther. 2004, 3, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Mullan, P.B.; Quinn, J.E.; Harkin, D.P. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene 2006, 25, 5854–5863. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Pao, G.M.; Chen, H.; Verma, I.M.; Hunter, T. Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J. Biol. Chem. 2003, 278, 5255–5263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fradet-Turcotte, A.; Sitz, J.; Grapton, D.; Orthwein, A. BRCA2 functions: From DNA repair to replication fork stabilization. Endocr. Relat. Cancer 2016, 23, T1–T17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorodetska, I.; Kozeretska, I.; Dubrovska, A. BRCA Genes: The Role in Genome Stability, Cancer Stemness and Therapy Resistance. J. Cancer 2019, 10, 2109–2127. [Google Scholar] [CrossRef] [Green Version]
- Preobrazhenska, O.; Yakymovych, M.; Kanamoto, T.; Yakymovych, I.; Stoika, R.; Heldin, C.; Souchelnytskyi, S. BRCA2 and Smad3 synergize in regulation of gene transcription. Oncogene 2002, 21, 5660–5664. [Google Scholar] [CrossRef] [Green Version]
- Morand, S.; Stanbery, L.; Walter, A.; Rocconi, R.P.; Nemunaitis, J. BRCA1/2 Mutation Status Impact on Autophagy and Immune Response: Unheralded Target. JNCI Cancer Spectrum 2020, 4, pkaa077. [Google Scholar] [CrossRef]
- Bhatia, V.; Barroso, S.I.; Garcia-Rubio, M.L.; Tumini, E.; Herrera-Moyano, E.; Aguilera, A. BRCA2 prevents R-loop accumulation and associates with TREX-2 mRNA export factor PCID2. Nature 2014, 511, 362–365. [Google Scholar] [CrossRef]
- Lemasters, J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005, 8, 3–5. [Google Scholar] [CrossRef]
- Andres, F.; Yufeng, L.; Chen, D.; Grizzle, W.E.; Updike, K.L.; Merz, N.D.; Downs-Kelly, E.; Burwell, T.C.; Vaklavas, C.; Buchsbaum, D.J.; et al. Expression of the MHC Class II Pathway in Triple-Negative Breast Cancer Tumor Cells Is Associated with a Good Prognosis and Infiltrating Lymphocytes. Cancer Immunol. Res. 2016, 4, 390–399. [Google Scholar]
- Arun, B.; Akar, U.; Gutierrez-Barrera, A.M.; Hortobagyi, G.N.; Ozpolat, B. The PARP inhibitor AZD2281 (Olaparib) induces autophagy/mitophagy in BRCA1 and BRCA2 mutant breast cancer cells. Int. J. Oncol. 2015, 47, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Jo, A.; Park, P.; Lee, H.; Lee, H.-O. Brca2 Deficiency Leads to T Cell Loss and Immune Dysfunction. Mol. Cells 2015, 38, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 5 March 2022).
- Rebelatto, T.F.; Falavigna, M.; Pozzari, M.; Spada, F.; Cella, C.A.; Laffi, A.; Pellicori, S.; Fazio, N. Should platinum-based chemotherapy be preferred for germline BReast CAncer genes (BRCA) 1 and 2-mutated pancreatic ductal adenocarcinoma (PDAC) patients? A systematic review and meta-analysis. Cancer Treat. Rev. 2019, 80, 101895. [Google Scholar] [CrossRef] [PubMed]
- Waddell, N.; Pajic, M.; Patch, A.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emelyanova, M.; Pudova, E.; Khomich, D.; Krasnov, G.; Popova, A.; Abramov, I.; Mikhailovich, V.; Filipenko, M.; Menshikova, S.; Tjulandin, S.; et al. Platinum-based chemotherapy for pancreatic cancer: Impact of mutations in the homologous recombination repair and Fanconi anemia genes. Ther. Adv. Med. Oncol. 2022, 14, 17588359221083050. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma, Version 2.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Golan, T.; Barenboim, A.; Lahat, G.; Nachmany, I.; Goykhman, Y.; Shmueli, S.; Halpern, N.; Brazowski, E.; Geva, R.; Wolf, I.; et al. Increased Rate of Complete Pathologic Response After Neoadjuvant FOLFIRINOX for BRCA Mutation Carriers with Borderline Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2020, 27, 3963–3970. [Google Scholar] [CrossRef]
- Orsi, G.; DiMarco, M.; Cavaliere, A.; Niger, M.; Bozzarelli, S.; Giordano, G.; Noventa, S.; Rapposelli, I.G.; Garajova, I.; Tortora, G.; et al. Chemotherapy toxicity and activity in patients with pancreatic ductal adenocarcinoma and germline BRCA1-2 pathogenic variants (gBRCA1-2pv): A multicenter survey. ESMO Open 2021, 6, 100238. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Lee, J.W.; Zalupski, M.; Capanu, M.; Park, J.; Golan, T.; Tahover, E.; Lower, M.A.; Chou, J.F.; Sahai, V.; et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin with or Without Veliparib in Patients with Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020, 38, 1378. [Google Scholar] [CrossRef]
- Osher, D.J.; Kushner, Y.B.; Arseneau, J.; Foulkes, W.D. Melphalan as a treatment for BRCA-related ovarian carcinoma: Can you teach an old drug new tricks? J. Clin. Path. 2011, 64, 924–926. [Google Scholar] [CrossRef]
- Evers, B.; Schut, E.; VanDerBurg, E.; Braumuller, T.M.; Egan, D.A.; Holstege, H.; Edser, P.; Adams, D.J.; Wade-Martins, R.; Bouwman, P.; et al. A High-Throughput Pharmaceutical Screen Identifies Compounds with Specific Toxicity against BRCA2-Deficient Tumors. Clin. Cancer Res. 2010, 16, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Tacconi, E.M.C.; Badie, S.; DeGregoriis, G.; Reislander, T.; Lai, X.; Porru, M.; Folio, C.; Moore, J.; Kopp, A.; Torres, J.B.; et al. Chlorambucil targets BRCA1/2-deficient tumours and counteracts PARP inhibitor resistance. EMBO Mol. Med. 2019, 11, e9982. [Google Scholar] [CrossRef]
- NCT04150042. A Study of Melphalan, BCNU, Vitamin B12b, Vitamin C, and Stem Cell Infusion in People with Advanced Pancreatic Cancer and BRCA Mutations. Available online: https://clinicaltrials.gov/ct2/show/NCT04150042 (accessed on 3 March 2022).
- NCT04692740. Chlorambucil in Metastatic PDAC Patients Bearing a Germ Line DNA Defects Repair Mutations (SALE Trial) (SALE). Available online: https://clinicaltrials.gov/ct2/show/NCT04692740 (accessed on 3 March 2022).
- Chi, J.; Chung, S.Y.; Parakrama, R.; Fayyaz, F.; Jose, J.; Saif, M.W. The role of PARP inhibitors in BRCA mutated pancreatic cancer. Therap. Adv. Gastroenterol. 2021, 14, 17562848211014818. [Google Scholar] [CrossRef]
- Chen, A. PARP Inhibitors: Its role in treatment of cancer. Chin. J. Cancer 2011, 30, 463–471. [Google Scholar] [CrossRef]
- Prescribing Information. Available online: www.accessdata.fda.gov (accessed on 8 March 2022).
- Golan, T.; Hammel, P.; Reni, M.; VanCutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.; et al. Overall survival from the phase 3 POLO trial: Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. J. Clin. Oncol. 2021, 39, 378. [Google Scholar] [CrossRef]
- Golan, T.; Varadhachary, G.R.; Sela, T.; Fogelman, D.R.; Halperin, N.; Shroff, R.T.; Halperin, S.; Xiao, L.; Aderka, D.; Maitra, A.; et al. Phase II study of olaparib for BRCAness phenotype in pancreatic cancer. J. Clin. Oncol. 2018, 36, 297. [Google Scholar] [CrossRef]
- Reiss, K.A.; Mick, R.; O-Hara, M.H.; Teitelbaum, U.; Karasic, T.B.; Schneider, C.; Cowden, S.; Southwell, T.; Romeo, J.; Izgur, N.; et al. Phase II Study of Maintenance Rucaparib in Patients with Platinum-Sensitive Advanced Pancreatic Cancer and a Pathogenic Germline or Somatic Variant in BRCA1, BRCA2, or PALB2. J. Clin. Oncol. 2021, 39, 2497–2505. [Google Scholar] [CrossRef]
- Shroff, R.T.; Hendifar, A.; McWilliams, R.R.; Geva, R.; Epelbaum, R.; Rolfe, L.; Goble, S.; Lin, K.K.; Biankin, A.V.; Giordano, H.; et al. Rucaparib Monotherapy in Patients with Pancreatic Cancer and a Known Deleterious BRCA Mutation. JCO Precis. Oncol. 2018, 2, 1–15. [Google Scholar] [CrossRef]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Ben-Baruch, N.E.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef]
- Lowery, M.A.; Kelsen, D.P.; Capanu, M.; Smith, S.C.; Lee, J.W.; Stadler, Z.K.; Moore, M.J.; Kindler, H.L.; Golan, T.; Segal, A.; et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur. J. Cancer 2018, 89, 19–26. [Google Scholar] [CrossRef]
- Murai, J.; Huang, S.Y.N.; Renaud, A.; Zhang, Y.; Ji, J.; Takeda, S.; Morris, J.; Teicher, B.; Doroshow, J.H.; Pommier, Y. Stereospecific PARP Trapping by BMN 673 and Comparison with Olaparib and Rucaparib. Mol. Cancer Ther. 2014, 13, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Rehman, F.L.; Feng, Y.; Boshuizen, J.; Bajrami, I.; Elliot, R.; Wang, B.; Lord, C.J.; Post, L.E.; Ashworth, A. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin. Cancer Res. 2013, 19, 5003–5015. [Google Scholar] [CrossRef] [Green Version]
- De Bono, J.; Ramanathan, R.K.; Mina, L.; Chugh, R.; Glaspy, J.; Rafii, S.; Kaye, S.; Sachdev, J.; Heymach, J.; Smith, D.C.; et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017, 7, 620–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.; Iyer, R.; Fountzilas, C. Poly(ADP-Ribose) Polymerase Inhibitors in Pancreatic Cancer: A New Treatment Paradigms and Future Implications. Cancers 2019, 11, 1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germ-line BRCA1/2mutation: An open-label phase II study. J. Clin. Oncol. 2013, 31, 244–250. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Guthrie, K.A.; Philip, P.A.; Swisher, E.M.; Jalikis, F.; Pishvaian, M.J.; Berlin, J.; Noel, M.S.; Suga, J.M.; Garrido-Laguna, I.; et al. Randomized phase II study of second-line modified FOLFIRI with PARP inhibitor ABT-888 (Veliparib) (NSC-737664) versus FOLFIRI in metastatic pancreatic cancer (mPC): SWOG S1513. J. Clin. Oncol. 2021, 27, 6314–6322. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Wang, H.; Zhuang, T.; He, A.R.; Hwang, J.J.; Hankin, A.; Ley, L.; White, K.; Littman, S.J.; Weiner, L.M.; et al. A phase I/II study of ABT-888 in combination with 5-fluorouracil (5-FU) and oxaliplatin (Ox) in patients with metastatic pancreatic cancer (MPC). J. Clin. Oncol. 2013, 31, 147. [Google Scholar] [CrossRef]
- Yap, T.A.; Tan, D.S.; Stathis, A.; Shapiro, G.I.; Iwasa, S.; Joerger, M.; Zhang, J.; Plummer, R.; Sawyer, M.; Tan, A.C.; et al. PETRA: First in class, first in human trial of the next generation PARP1-selective inhibitor AZD5305 in patients (pts) with BRCA1/2, PALB2 or RAD51C/D mutations. In Proceedings of the American Association for Cancer Research Annual Meeting, New Orleans, LA, USA, 8–13 April 2022. [Google Scholar]
- Yao, W.; Maitra, A.; Ying, H. Recent insights into the biology of pancreatic cancer. EBioMed 2020, 53, 102655. [Google Scholar] [CrossRef]
- Gros, A.; Tran, E.; Parkhurst, M.R.; Ilyas, S.; Pasetto, A.; Groh, E.M.; Robbins, P.F.; Yossef, R.; Garcia-Garijo, A.; Fajarardo, C.A.; et al. Recognition of human gastrointestinal cancer neoantigens by circulating PD-1+ lymphocytes. J. Clin. Invest. 2019, 129, 4992–5004. [Google Scholar] [CrossRef] [Green Version]
- Chung, V.; Guthrie, K.A.; Pishvaian, M.J.; Lowy, A.M.; Chiorean, E.G.; Duong, M.T.; O’Reilly, E.M.; Philip, P.A. Randomized phase II trial of olaparib + pembrolizumab versus olaparib alone as maintenance therapy in metastatic pancreatic cancer patients with germline BRCA1 or BRCA2 (gBRCA1/2+) mutations: SWOG S2001. J. Clin. Oncol. 2021, 39, TPS447. [Google Scholar] [CrossRef]
- NCT04548752. Testing the Addition of Pembrolizumab, an Immunotherapy Cancer Drug to Olaparib Alone as Therapy for Patients with Pancreatic Cancer That Has Spread with Inherited BRCA Mutations. Available online: https://clinicaltrials.gov/ct2/show/NCT04548752?term=Nct04548752&draw=2&rank=1 (accessed on 25 April 2022).
- NCT04493060. Niraparib and Dostarlimab for the Treatment of Germline or Somatic BRCA1/2 and PALB2 Mutated Metastatic Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04493060 (accessed on 3 March 2022).
- Terrero, G.; Datta, J.; Dennison, J.; Sussman, D.A.; Lohse, I.; Merchant, N.B.; Hosein, P.J. Ipilimumab/Nivolumab Therapy in Patients with Metastatic Pancreatic or Biliary Cancer with Homologous Recombination Deficiency Pathogenic Germline Variants. JAMA Oncol. 2022. [Google Scholar] [CrossRef]
- NCT03205176. AZD5153 in Patients with Relapsed or Refractory Solid Tumors, Including Lymphomas. Available online: https://clinicaltrials.gov/ct2/show/NCT03205176?term=Nct03205176&draw=2&rank=1 (accessed on 25 April 2022).
- Wang, J.S.Z.; DeVita, S.; Karlix, J.L.; Cook, C.; Littlewood, G.M.; Hattersley, M.M.; Moorthy, G.; Edlung, H.; Fabbri, G.; Sachsenmeier, K.F.; et al. First-in-human study of AZD5153, a small molecule inhibitor of bromodomain protein 4 (BRD4), in patients (pts) with relapsed/refractory (RR) malignant solid tumor and lymphoma: Preliminary data. Dev. Ther. Tumor Biol. 2019, 37, 3085. [Google Scholar] [CrossRef]
- Liu, J.F.; Barry, W.T.; Birrer, M.; Lee, J.; Buckanovich, R.J.; Fleming, G.F.; Rimel, B.J.; Buss, M.K.; Nattam, S.; Hurteau, J.; et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: A randomised phase 2 study. Lancet 2014, 15, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- NCT02498613. A Phase 2 Study of Cediranib in Combination With Olaparib in Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT02498613?term=NCT02498613&draw=2&rank=1 (accessed on 25 April 2022).
- Golan, T.; O’Kane, G.M.; Denroche, R.E.; Raitses-Gurevich, M.; Grant, R.C.; Holter, S.; Wang, Y.; Zhang, A.; Jang, G.H.; Stossel, C.; et al. Genomic Features and Classification of Homologous Recombination Deficient Pancreatic Ductal Adenocarcinoma. Gastroenterology 2021, 160, 2119–2132. [Google Scholar] [CrossRef]
- Murai, J.; Feng, Y.; Yu, G.K.; Ru, Y.; Tang, S.; Shen, Y.; Pommier, Y. Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget 2016, 7, 76534–76550. [Google Scholar] [CrossRef] [Green Version]
- Jaspers, J.E.; Kersbergen, A.; Boon, U.; vanDeemter, L.; Zander, S.A.; Drost, R.; Wientjens, E.; Ji, J.; Aly, A.; Doroshow, J.H.; et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013, 3, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; George, E.; Ragland, R.; Rafail, S.; Zhang, R.; Krepler, C.; Morgan, M.; Herlyn, M.; Brown, E.; Simpkins, F. Targeting the ATR/CHK1 Axis with PARP Inhibition Results in Tumor Regression in BRCA-Mutant Ovarian Cancer Models. Clin. Cancer Res. 2017, 23, 3097–3108. [Google Scholar] [CrossRef] [Green Version]
- NCT03462342. Combination ATR and PARP Inhibitor (CAPRI) Trial with AZD6738 and Olaparib in Recurrent Ovarian Cancer (CAPRI). Available online: https://clinicaltrials.gov/ct2/show/NCT03462342 (accessed on 3 March 2022).
- Takebe, N.; Naqash, A.R.; O’Sullivan Coyne, G.; Kummar, S.; Do, K.; Bruns, A.; Juwara, L.; Zlott, J.; Rubinstein, L.; Piekarz, R.; et al. Safety, Antitumor Activity, and Biomarker Analysis in a Phase I Trial of the Once-daily Wee1 Inhibitor Adavosertib (AZD1775) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3834–3844. [Google Scholar] [CrossRef]
- Hartman, S.J.; Bagby, S.M.; Yacob, B.W.; Simmons, D.M.; MacBeth, M.; Lieu, C.H.; Davis, S.L.; Leal, A.D.; Tentler, J.J.; Diamond, J.R.; et al. WEE1 Inhibition in Combination with Targeted Agents and Standard Chemotherapy in Preclinical Models of Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2021, 11, 957. [Google Scholar] [CrossRef]
- Cuneo, K.C.; Morgan, M.A.; Sahai, V.; Schipper, M.J.; Parsels, L.A.; Parsels, J.D.; Devasia, T.; Al-Hawaray, M.; Cho, C.S.; Nathan, H.; et al. Dose Escalation Trial of the Wee1 Inhibitor Adavosertib (AZD1775) in Combination with Gemcitabine and Radiation for Patients with Locally Advanced Pancreatic Cancer. J. Clin. Oncol. 2019, 27, 2643–2650. [Google Scholar] [CrossRef]
- Uson, P.S.L.; Samadder, N.J.; Riegert-Johnson, D.; Boardman, L.; Borad, M.J.; Ahn, D.; Sonbol, M.B.; Faigel, D.O.; Fukami, N.; Pannala, R.; et al. Clinical Impact of Pathogenic Germline Variants in Pancreatic Cancer: Results from a Multicenter, Prospective, Universal Genetic Testing Study. Clin. Transl. Gastroenterol. 2021, 12, e00414. [Google Scholar] [CrossRef]
- Mersch, J.; Brown, N.; Pirzadeh-Miller, S.; Mundt, E.; Cox, H.C.; Brown, K.; Aston, M.; Esterling, L.; Manley, S.; Ross, T. Prevalence of Variant Reclassification Following Hereditary Cancer Genetic Testing. JAMA 2018, 320, 1266–1274. [Google Scholar] [CrossRef]
- Kweon, J.; Jang, A.H.; Shin, H.R.; See, J.E.; Lee, W.; Lee, J.W.; Chang, S.; Kim, K.; & Kim, Y. A CRISPR-based base-editing screen for the functional assessment of BRCA1 variants. Oncogene 2020, 39, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Momtaz, P.; O’Connor, C.A.; Chou, J.F.; Capanu, M.; Park, W.; Bandlamudi, C.; Berger, M.F.; Kelsen, D.P.; Suehnholz, S.P.; Chakravarty, D.; et al. Pancreas cancer and BRCA: A critical subset of patients with improving therapeutic outcomes. Cancer 2021, 127, 4393–4402. [Google Scholar] [CrossRef]
- NCT03601923. Niraparib in Patients with Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03601923 (accessed on 25 April 2022).
- NCT04171700. A Study to Evaluate Rucaparib in Patients with Solid Tumors and With Deleterious Mutations in HRR Genes (LODESTAR). Available online: https://clinicaltrials.gov/ct2/show/NCT04171700 (accessed on 25 April 2022).
- NCT04550494. Measuring the Effects of Talazoparib in Patients with Advanced Cancer and DNA Repair Variations. Available online: https://clinicaltrials.gov/ct2/show/NCT04550494 (accessed on 25 April 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devico Marciano, N.; Kroening, G.; Dayyani, F.; Zell, J.A.; Lee, F.-C.; Cho, M.; Valerin, J.G. BRCA-Mutated Pancreatic Cancer: From Discovery to Novel Treatment Paradigms. Cancers 2022, 14, 2453. https://doi.org/10.3390/cancers14102453
Devico Marciano N, Kroening G, Dayyani F, Zell JA, Lee F-C, Cho M, Valerin JG. BRCA-Mutated Pancreatic Cancer: From Discovery to Novel Treatment Paradigms. Cancers. 2022; 14(10):2453. https://doi.org/10.3390/cancers14102453
Chicago/Turabian StyleDevico Marciano, Naomie, Gianna Kroening, Farshid Dayyani, Jason A. Zell, Fa-Chyi Lee, May Cho, and Jennifer Goldstein Valerin. 2022. "BRCA-Mutated Pancreatic Cancer: From Discovery to Novel Treatment Paradigms" Cancers 14, no. 10: 2453. https://doi.org/10.3390/cancers14102453
APA StyleDevico Marciano, N., Kroening, G., Dayyani, F., Zell, J. A., Lee, F.-C., Cho, M., & Valerin, J. G. (2022). BRCA-Mutated Pancreatic Cancer: From Discovery to Novel Treatment Paradigms. Cancers, 14(10), 2453. https://doi.org/10.3390/cancers14102453






