Genetic Mutations of Pancreatic Cancer and Genetically Engineered Mouse Models
Abstract
Simple Summary
Abstract
1. Introduction
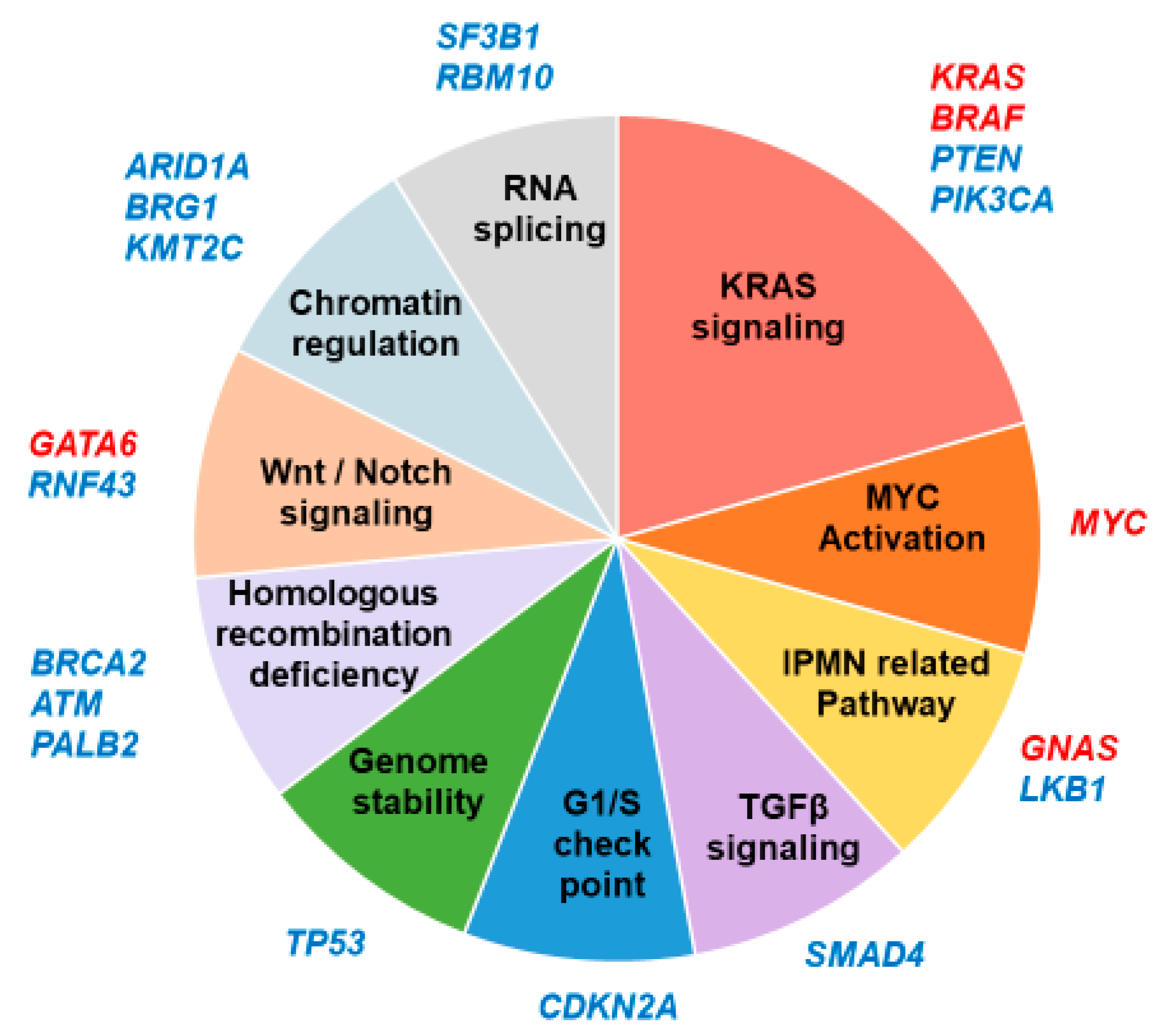
2. Altered Pathways of Pancreatic Cancer and GEMM
2.1. KRAS Pathway
2.2. MYC Activation
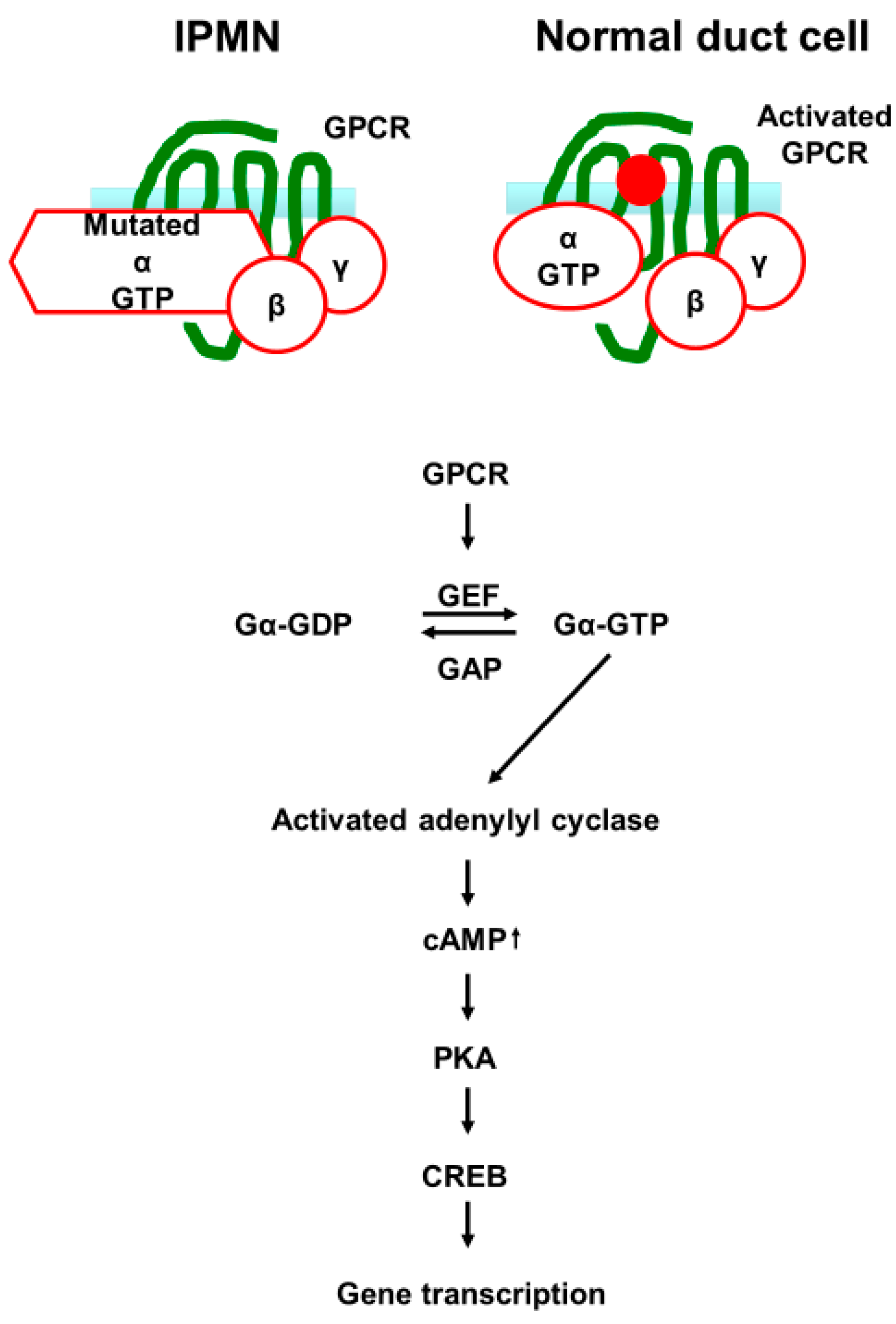
2.3. IPMN Related Pathways
2.4. SMAD4
2.5. CDKN2A (p16INK4 and p14ARF)
2.6. TP53(p53)
2.7. Homologous Recombination Deficiency
2.8. WNT Signaling
2.9. Chromatin Regulation Related Genes; ARID1A, BRG1 and KMT2C
3. Discussion and Future Directions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cancer Information Service, N.C.C., Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare). Cancer Statistics. Available online: https://www.mhlw.go.jp/english/database/db-hw/index.html (accessed on 7 December 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. Stock. Swed. 2016, 55, 1158–1160. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Hansel, D.E.; Kern, S.E.; Hruban, R.H. Molecular Pathogenesis of Pancreatic Cancer. Annu. Rev. Genom. Hum. Genet. 2003, 4, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Hong, J.; Iacobuzio-Donahue, C.A. The pancreatic cancer genome revisited. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 469–481. [Google Scholar] [CrossRef]
- Chan-Seng-Yue, M.; Kim, J.C.; Wilson, G.W.; Ng, K.; Flores-Figueroa, E.; O’Kane, G.M.; Connor, A.A.; Denroche, R.E.; Grant, R.C.; McLeod, J.; et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 2020, 52, 231–240. [Google Scholar] [CrossRef]
- Hruban, R.H.; Adsay, N.V.; Albores-Saavedra, J.; Compton, C.; Garrett, E.S.; Goodman, S.N.; Kern, S.E.; Klimstra, D.S.; Klöppel, G.; Longnecker, D.S.; et al. Pancreatic Intraepithelial Neoplasia. Am. J. Surg. Pathol. 2001, 25, 579–586. [Google Scholar] [CrossRef]
- Hruban, R.H.; Takaori, K.; Klimstra, D.S.; Adsay, V.; Albores-Saavedra, J.; Biankin, A.; A Biankin, S.; Compton, C.; Fukushima, N.; Furukawa, T.; et al. An Illustrated Consensus on the Classification of Pancreatic Intraepithelial Neoplasia and Intraductal Papillary Mucinous Neoplasms. Am. J. Surg. Pathol. 2004, 28, 977–987. [Google Scholar] [CrossRef]
- Adsay, N.V.; Adair, C.F.; Heffess, C.S.; Klimstra, D.S. Intraductal Oncocytic Papillary Neoplasms of the Pancreas. Am. J. Surg. Pathol. 1996, 20, 980–994. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Shimizu, M.; Ban, S.; Koyama, I.; Hatori, T.; Fujita, I.; Yamamoto, M.; Kawamura, S.; Kobayashi, M.; Ishida, K.; et al. Intraductal Tubulopapillary Neoplasms of the Pancreas Distinct from Pancreatic Intraepithelial Neoplasia and Intraductal Papillary Mucinous Neoplasms. Am. J. Surg. Pathol. 2009, 33, 1164–1172. [Google Scholar] [CrossRef]
- Fukushima, N.; Fukayama, M. Mucinous cystic neoplasms of the pancreas: Pathology and molecular genetics. J. Hepato-Biliary-Pancreat. Surg. 2007, 14, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Goggins, M.; Parsons, J.; E Kern, S. Progression model for pancreatic cancer. Clin. Cancer Res. 2000, 6, 2969–2972. [Google Scholar]
- Xiao, H.D.; Yamaguchi, H.; Dias-Santagata, D.; Kuboki, Y.; Akhavanfard, S.; Hatori, T.; Yamamoto, M.; Shiratori, K.; Kobayashi, M.; Shimizu, M.; et al. Molecular characteristics and biological behaviours of the oncocytic and pancreatobiliary subtypes of intraductal papillary mucinous neoplasms. J. Pathol. 2011, 224, 508–516. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kuboki, Y.; Hatori, T.; Yamamoto, M.; Shimizu, K.; Shiratori, K.; Shibata, N.; Shimizu, M.; Furukawa, T. The discrete nature and distinguishing molecular features of pancreatic intraductal tubulopapillary neoplasms and intraductal papillary mucinous neoplasms of the gastric type, pyloric gland variant. J. Pathol. 2013, 231, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.L.; Dubois, C.L.; Sarai, K.; Zarei, S.; Schaeffer, D.F.; Sander, M.; Kopp, J.L. Cell of origin affects tumour development and phenotype in pancreatic ductal adenocarcinoma. Gut 2019, 68, 487–498. [Google Scholar] [CrossRef]
- Grippo, P.J.; Nowlin, P.S.; Demeure, M.J.; Longnecker, D.S.; Sandgren, E.P. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 2003, 63, 2016–2019. [Google Scholar]
- Hingorani, S.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef]
- Guerra, C.; Schuhmacher, A.J.; Cañamero, M.; Grippo, P.J.; Verdaguer, L.; Pérez-Gallego, L.; Dubus, P.; Sandgren, E.P.; Barbacid, M. Chronic Pancreatitis Is Essential for Induction of Pancreatic Ductal Adenocarcinoma by K-Ras Oncogenes in Adult Mice. Cancer Cell 2007, 11, 291–302. [Google Scholar] [CrossRef]
- Singh, K.; Pruski, M.; Bland, R.; Younes, M.; Guha, S.; Thosani, N.; Maitra, A.; Cash, B.D.; McAllister, F.; Logsdon, C.D.; et al. Kras mutation rate precisely orchestrates ductal derived pancreatic intraepithelial neoplasia and pancreatic cancer. Lab. Investig. 2021, 101, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Schönhuber, N.; Seidler, B.; Schuck, K.; Veltkamp, C.; Schachtler, C.; Zukowska, M.; Eser, S.; Feyerabend, T.B.; Paul, M.C.; Eser, P.; et al. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat. Med. 2014, 20, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, X.; Nayak, S.G.; Pitarresi, J.R.; Cuitino, M.C.; Yu, L.; Hildreth, B.E.; Thies, K.A.; Schilling, D.J.; Fernandez, S.A.; et al. Generation of a pancreatic cancer model using a Pdx1-Flp recombinase knock-in allele. PLoS ONE 2017, 12, e0184984. [Google Scholar] [CrossRef]
- Collisson, E.A.; Trejo, C.L.; Silva, J.M.; Gu, S.; Korkola, J.E.; Heiser, L.M.; Charles, R.-P.; Rabinovich, B.A.; Hann, B.; Dankort, D.; et al. A Central Role for RAF→MEK→ERK Signaling in the Genesis of Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2012, 2, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.N.; Maher, M.E.; Tran, N.H.; Van De Hey, D.R.; Foley, T.M.; Yueh, A.E.; Leystra, A.; Pasch, C.A.; Jeffrey, J.J.; Clipson, L.; et al. PIK3CA mutations can initiate pancreatic tumorigenesis and are targetable with PI3K inhibitors. Oncogenesis 2015, 4, e169. [Google Scholar] [CrossRef]
- Kopp, J.L.; Dubois, C.L.; Schaeffer, D.F.; Samani, A.; Taghizadeh, F.; Cowan, R.W.; Rhim, A.D.; Stiles, B.L.; Valasek, M.; Sander, M. Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia from Pancreatic Ductal Cells in Mice. Gastroenterology 2018, 154, 1509–1523.e5. [Google Scholar] [CrossRef] [PubMed]
- Sandgren, E.P.; Quaife, C.J.; Paulovich, A.G.; Palmiter, R.D.; Brinster, R.L. Pancreatic tumor pathogenesis reflects the causative genetic lesion. Proc. Natl. Acad. Sci. USA 1991, 88, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Rajbhandari, N.; Liu, C.; Sakamoto, K.; Zhang, Q.; Triplett, A.A.; Batra, S.K.; Opavsky, R.; Felsher, D.W.; DiMaio, D.J.; et al. Dormant Cancer Cells Contribute to Residual Disease in a Model of Reversible Pancreatic Cancer. Cancer Res. 2013, 73, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, N.; Lin, W.-C.; Wehde, B.L.; Triplett, A.A.; Wagner, K.-U. Autocrine IGF1 Signaling Mediates Pancreatic Tumor Cell Dormancy in the Absence of Oncogenic Drivers. Cell Rep. 2017, 18, 2243–2255. [Google Scholar] [CrossRef]
- Maddipati, R.; Norgard, R.J.; Baslan, T.; Rathi, K.S.; Zhang, A.; Saeid, A.; Higashihara, T.; Wu, F.; Kumar, A.; Annamalai, V.; et al. MYC levels regulate metastatic heterogeneity in pancreatic adenocarcinoma. Cancer Discov. 2021, 1–58. [Google Scholar] [CrossRef]
- Taki, K.; Ohmuraya, M.; Tanji, E.; Komatsu, H.; Hashimoto, D.; Semba, K.; Araki, K.; Kawaguchi, Y.; Baba, H.; Furukawa, T. GNASR201H and KrasG12D cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene 2016, 35, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Collet, L.; Ghurburrun, E.; Meyers, N.; Assi, M.; Pirlot, B.; Leclercq, I.A.; Couvelard, A.; Komuta, M.; Cros, J.; Demetter, P.; et al. Kras and Lkb1 mutations synergistically induce intraductal papillary mucinous neoplasm derived from pancreatic duct cells. Gut 2020, 69, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Bardeesy, N.; Cheng, K.-H.; Berger, J.H.; Chu, G.C.; Pahler, J.; Olson, P.; Hezel, A.F.; Horner, J.; Lauwers, G.Y.; Hanahan, D.; et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006, 20, 3130–3146. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.J.; Bardeesy, N.; Sinha, M.; Lopez, L.; Tuveson, D.A.; Horner, J.; Redston, M.S.; DePinho, R.A. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003, 17, 3112–3126. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Wang, L.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Kato, Y.; Mizukami, Y.; Widholz, S.; Boukhali, M.; Revenco, I.; Grossman, E.A.; Ji, F.; Sadreyev, R.I.; Liss, A.S.; et al. Mutant GNAS drives pancreatic tumourigenesis by inducing PKA-mediated SIK suppression and reprogramming lipid metabolism. Nat. Cell Biol. 2018, 20, 811–822. [Google Scholar] [CrossRef]
- Drosos, Y.; Escobar, D.; Chiang, M.-Y.; Roys, K.; Valentine, V.; Valentine, M.B.; Rehg, J.E.; Sahai, V.; Begley, L.A.; Ye, J.; et al. ATM-deficiency increases genomic instability and metastatic potential in a mouse model of pancreatic cancer. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Mishra, A.; Emamgholi, F.; Erlangga, Z.; Hartleben, B.; Unger, K.; Wolff, K.; Teichmann, U.; Kessel, M.; Woller, N.; Kühnel, F.; et al. Generation of focal mutations and large genomic deletions in the pancreas using inducible in vivo genome editing. Carcinogenesis 2020, 41, 334–344. [Google Scholar] [CrossRef]
- Kimura, Y.; Fukuda, A.; Ogawa, S.; Maruno, T.; Takada, Y.; Tsuda, M.; Hiramatsu, Y.; Araki, O.; Nagao, M.; Yoshikawa, T.; et al. ARID1A Maintains Differentiation of Pancreatic Ductal Cells and Inhibits Development of Pancreatic Ductal Adenocarcinoma in Mice. Gastroenterology 2018, 155, 194–209.e2. [Google Scholar] [CrossRef]
- Wang, W.; Friedland, S.C.; Guo, B.; O’Dell, M.R.; Alexander, W.; Whitney-Miller, C.L.; Agostini-Vulaj, D.; Huber, A.R.; Myers, J.R.; Ashton, J.; et al. ARID1A, a SWI/SNF subunit, is critical to acinar cell homeostasis and regeneration and is a barrier to transformation and epithelial-mesenchymal transition in the pancreas. Gut 2019, 68, 1245–1258. [Google Scholar] [CrossRef]
- Von Figura, G.; Fukuda, A.; Roy, N.; Liku, M.E.; Iv, J.P.M.; Kim, G.E.; Russ, H.A.; Firpo, M.A.; Mulvihill, S.J.; Dawson, D.W.; et al. The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat. Cell Biol. 2014, 16, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.L.; Willis, N.; Mercer, K.; Bronson, R.T.; Crowley, D.; Montoya, R.; Jacks, T.; Tuveson, D.A. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001, 15, 3243–3248. [Google Scholar] [CrossRef]
- Kopp, J.L.; Dubois, C.L.; Schaffer, A.E.; Hao, E.; Shih, H.P.; Seymour, P.A.; Ma, J.; Sander, M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 2011, 138, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Colicelli, J. Human RAS Superfamily Proteins and Related GTPases. Sci. Stke 2004, 2004, re13. [Google Scholar] [CrossRef] [PubMed]
- Taparowsky, E.; Suard, Y.; Fasano, O.; Shimizu, K.; Goldfarb, M.; Wigler, M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature 1982, 300, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; McMillan, E.A.; Balaji, U.; Baek, G.; Lin, W.-C.; Mansour, J.C.; Mollaee, M.; Wagner, K.-U.; Koduru, P.; Yopp, A.C.; et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015, 6, 6744. [Google Scholar] [CrossRef]
- Yanagisawa, A.; Ohtake, K.; Ohashi, K.; Hori, M.; Kitagawa, T.; Sugano, H.; Kato, Y. Frequent c-Ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res. 1993, 53, 953–956. [Google Scholar] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Zebisch, A.; Troppmair, J. Back to the roots: The remarkable RAF oncogene story. Cell. Mol. Life Sci. 2006, 63, 1314–1330. [Google Scholar] [CrossRef]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef]
- Zhao, L.; Vogt, P.K. Hot-spot mutations in p110α of phosphatidylinositol 3-kinase (PI3K): Differential interactions with the regulatory subunit p85 and with RAS. Cell Cycle 2010, 9, 596–600. [Google Scholar] [CrossRef]
- Basturk, O.; Berger, M.F.; Yamaguchi, H.; Adsay, V.; Askan, G.; Bhanot, U.K.; Zehir, A.; Carneiro, F.; Hong, S.-M.; Zamboni, G.; et al. Pancreatic intraductal tubulopapillary neoplasm is genetically distinct from intraductal papillary mucinous neoplasm and ductal adenocarcinoma. Mod. Pathol. 2017, 30, 1760–1772. [Google Scholar] [CrossRef]
- Chow, J.T.-S.; Salmena, L. Recent advances in PTEN signalling axes in cancer. Fac. Rev. 2020, 9, 31. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Hodakoski, C.; Barrows, D.; Mense, S.M.; Parsons, R.E. PTEN function: The long and the short of it. Trends Biochem. Sci. 2014, 39, 183–190. [Google Scholar] [CrossRef]
- Ross, J.; Miron, C.E.; Plescia, J.; Laplante, P.; McBride, K.; Moitessier, N.; Möröy, T. Targeting MYC: From understanding its biology to drug discovery. Eur. J. Med. Chem. 2021, 213, 113137. [Google Scholar] [CrossRef]
- Bergmann, F.; Aulmann, S.; Sipos, B.; Kloor, M.; Von Heydebreck, A.; Schweipert, J.; Harjung, A.; Mayer, P.; Hartwig, W.; Moldenhauer, G.; et al. Acinar cell carcinomas of the pancreas: A molecular analysis in a series of 57 cases. Virchows Arch. 2014, 465, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Schleger, C.; Verbeke, C.; Hildenbrand, R.; Zentgraf, H.; Bleyl, U. c-MYC Activation in Primary and Metastatic Ductal Adenocarcinoma of the Pancreas: Incidence, Mechanisms, and Clinical Significance. Mod. Pathol. 2002, 15, 462–469. [Google Scholar] [CrossRef]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Shi, S.; Liu, M.; Qin, Y.; Meng, Q.; Hua, J.; Ji, S.; Zhang, Y.; Yang, J.; Xu, J.; et al. PIN1 Maintains Redox Balance via the c-Myc/NRF2 Axis to Counteract Kras-Induced Mitochondrial Respiratory Injury in Pancreatic Cancer Cells. Cancer Res. 2019, 79, 133–145. [Google Scholar] [CrossRef]
- O’Hayre, M.; Vazquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef]
- Turan, S.; Bastepe, M. GNAS Spectrum of Disorders. Curr. Osteoporos. Rep. 2015, 13, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Larribère, L.; Utikal, J. Update on GNA Alterations in Cancer: Implications for Uveal Melanoma Treatment. Cancers 2020, 12, 1524. [Google Scholar] [CrossRef]
- Furukawa, T.; Kuboki, Y.; Tanji, E.; Yoshida, S.; Hatori, T.; Yamamoto, M.; Shibata, N.; Shimizu, K.; Kamatani, N.; Shiratori, K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci. Rep. 2011, 1, 161. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Matthaei, H.; Maitra, A.; Dal Molin, M.; Wood, L.D.; Eshleman, J.R.; Goggins, M.; Canto, M.I.; Schulick, R.D.; Edil, B.H.; et al. Recurrent GNAS Mutations Define an Unexpected Pathway for Pancreatic Cyst Development. Sci. Transl. Med. 2011, 3, 92ra66. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Shimizu, K.; Hatori, T.; Yamamoto, M.; Shibata, N.; Shiratori, K.; Furukawa, T. Molecular Biomarkers for Progression of Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas 2015, 44, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Klöppel, G.; Volkan Adsay, N.; Albores-Saavedra, J.; Fukushima, N.; Horii, A.; Hruban, R.H.; Kato, Y.; Klimstra, D.S.; Longnecker, D.S.; et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch. Int. J. Pathol. 2005, 447, 794–799. [Google Scholar] [CrossRef]
- Nishikawa, G.; Sekine, S.; Ogawa, R.; Matsubara, A.; Mori, T.; Taniguchi, H.; Kushima, R.; Hiraoka, N.; Tsuta, K.; Tsuda, H.; et al. Frequent GNAS mutations in low-grade appendiceal mucinous neoplasms. Br. J. Cancer 2013, 108, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Ma, R.; Li, Y. The biological basis and function of GNAS mutation in pseudomyxoma peritonei: A review. J. Cancer Res. Clin. Oncol. 2020, 146, 2179–2188. [Google Scholar] [CrossRef]
- Williams, T.; Brenman, J.E. LKB1 and AMPK in cell polarity and division. Trends Cell Biol. 2008, 18, 193–198. [Google Scholar] [CrossRef]
- Hearle, N.; Schumacher, V.; Menko, F.H.; Olschwang, S.; Boardman, L.A.; Gille, J.; Keller, J.J.; Westerman, A.M.; Scott, R.J.; Lim, W.; et al. Frequency and Spectrum of Cancers in the Peutz-Jeghers Syndrome. Clin. Cancer Res. 2006, 12, 3209–3215. [Google Scholar] [CrossRef]
- van Lier, M.G.F.; Wagner, A.; Mathus-Vliegen, E.M.H.; Kuipers, E.J.; Steyerberg, E.; van Leerdam, M.E. High Cancer Risk in Peutz–Jeghers Syndrome: A Systematic Review and Surveillance Recommendations. Am. J. Gastroenterol. 2010, 105, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Basturk, O.; Brannon, A.R.; Bhanot, U.; Scott, S.N.; Bouvier, N.; LaFemina, J.; Jarnagin, W.R.; Berger, M.F.; Klimstra, D.; et al. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J. Am. Coll. Surg. 2015, 220, 845–854.e1. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Molin, M.D.; Mafficini, A.; Yu, J.; Malleo, G.; Rusev, B.; Fassan, M.; Antonello, D.; Sadakari, Y.; Castelli, P.; et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J. Pathol. 2014, 233, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Ono, Y.; Morikawa, T.; Motoi, F.; Higuchi, R.; Yamamoto, M.; Hayakawa, Y.; Karasaki, H.; Mizukami, Y.; Unno, M.; et al. Serine/Threonine Kinase 11 Plays a Canonical Role in Malignant Progression of KRAS-mutant and GNAS-wild-type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2021. Publish Ah. [Google Scholar] [CrossRef]
- Sato, N.; Rosty, C.; Jansen, M.; Fukushima, N.; Ueki, T.; Yeo, C.J.; Cameron, J.L.; Iacobuzio-Donahue, C.A.; Hruban, R.H.; Goggins, M. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am. J. Pathol. 2001, 159, 2017–2022. [Google Scholar] [CrossRef]
- Su, G.H.; Hruban, R.H.; Bansal, R.K.; Bova, G.S.; Tang, D.J.; Shekher, M.C.; Westerman, A.M.; Entius, M.M.; Goggins, M.; Yeo, C.J.; et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am. J. Pathol. 1999, 154, 1835–1840. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Bierie, B.; Moses, H.L. TGFβ: The molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 2006, 6, 506–520. [Google Scholar] [CrossRef]
- Zavadil, J.; Böttinger, E.P. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 2005, 24, 5764–5774. [Google Scholar] [CrossRef]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.-M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Inoue, H.; Furukawa, T.; Sunamura, M.; Takeda, K.; Matsuno, S.; Horii, A. Exclusion ofSMAD4 mutation as an early genetic change in human pancreatic ductal tumorigenesis. GenesChromosom. Cancer 2001, 31, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Wilentz, R.E.; Iacobuzio-Donahue, C.A.; Argani, P.; McCarthy, D.M.; Parsons, J.L.; Yeo, C.J.; Kern, S.E.; Hruban, R.H. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: Evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000, 60, 2002–2006. [Google Scholar]
- Iacobuzio-Donahue, C.A.; Wilentz, R.E.; Argani, P.; Yeo, C.J.; Cameron, J.L.; Kern, S.E.; Hruban, R.H. Dpc4 protein in mucinous cystic neoplasms of the pancreas: Frequent loss of expression in invasive carcinomas suggests a role in genetic progression. Am. J. Surg. Pathol. 2000, 24, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Liggett, W.H.; Sidransky, D. Role of the p16 tumor suppressor gene in cancer. J. Clin. Oncol. 1998, 16, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Stott, F.J.; Bates, S.; James, M.C.; McConnell, B.B.; Starborg, M.; Brookes, S.M.; Palmero, I.; Ryan, K.M.; Hara, E.; Vousden, K.H.; et al. The alternative product from the human CDKN2A locus, p14ARF, participates in a regulatory feedback loop with p53 and Mdm. EMBO J. 1998, 17, 5001–5014. [Google Scholar] [CrossRef]
- Schutte, M.; Hruban, R.H.; Geradts, J.; Maynard, R.; Hilgers, W.; Rabindran, S.K.; Moskaluk, C.A.; Hahn, S.; Schwarte-Waldhoff, I.; Schmiegel, W.; et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997, 57, 3126–3130. [Google Scholar]
- Fukushima, N.; Sato, N.; Ueki, T.; Rosty, C.; Walter, K.M.; Wilentz, R.E.; Yeo, C.J.; Hruban, R.H.; Goggins, M. Aberrant Methylation of Preproenkephalin and p16 Genes in Pancreatic Intraepithelial Neoplasia and Pancreatic Ductal Adenocarcinoma. Am. J. Pathol. 2002, 160, 1573–1581. [Google Scholar] [CrossRef]
- Wilentz, R.E.; Geradts, J.; Maynard, R.; Offerhaus, G.J.; Kang, M.; Goggins, M.; Yeo, C.J.; Kern, S.E.; Hruban, R.H. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: Loss of intranuclear expression. Cancer Res. 1998, 58, 4740–4744. [Google Scholar]
- Sharpless, N.; Bardeesy, N.; Lee, K.-H.; Carrasco, D.; Castrillon, D.H.; Aguirre, A.J.; Wu, E.A.; Horner, J.W.; DePinho, R. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nat. Cell Biol. 2001, 413, 86–91. [Google Scholar] [CrossRef]
- Takeuchi, S.; Doi, M.; Ikari, N.; Yamamoto, M.; Furukawa, T. Mutations in BRCA1, BRCA2, and PALB2, and a panel of 50 cancer-associated genes in pancreatic ductal adenocarcinoma. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Shindo, K.; Yu, J.; Suenaga, M.; Fesharakizadeh, S.; Cho, C.; Macgregor-Das, A.; Siddiqui, A.; Witmer, P.D.; Tamura, K.; Song, T.J.; et al. Deleterious Germline Mutations in Patients with Apparently Sporadic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2017, 35, 3382–3390. [Google Scholar] [CrossRef]
- Murphy, K.M.; Brune, K.A.; Griffin, C.; Sollenberger, J.E.; Petersen, G.M.; Bansal, R.; Hruban, R.H.; Kern, S.E. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: Deleterious BRCA2 mutations in 17%. Cancer Res. 2002, 62, 3789–3793. [Google Scholar] [PubMed]
- Takai, E.; Nakamura, H.; Chiku, S.; Kubo, E.; Ohmoto, A.; Totoki, Y.; Shibata, T.; Higuchi, R.; Yamamoto, M.; Furuse, J.; et al. Whole-exome Sequencing Reveals New Potential Susceptibility Genes for Japanese Familial Pancreatic Cancer. Ann. Surg. 2020. Publish Ah. [Google Scholar] [CrossRef]
- Takai, E.; Yachida, S.; Shimizu, K.; Furuse, J.; Kubo, E.; Ohmoto, A.; Suzuki, M.; Hruban, R.H.; Okusaka, T.; Morizane, C.; et al. Germline mutations in Japanese familial pancreatic cancer patients. Oncotarget 2016, 7, 74227–74235. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1. J. Natl. Compr. Cancer Netw. 2020, 18, 380–391. [Google Scholar] [CrossRef]
- Sugiura, T.; Yamaguchi, A.; Miyamoto, K. A cancer-associated RING finger protein, RNF43, is a ubiquitin ligase that interacts with a nuclear protein, Hapexp. Cell Res. 2008, 314, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-K.; Spit, M.; Jordens, I.; Low, T.Y.; Stange, D.; Van De Wetering, M.; Van Es, J.H.; Mohammed, S.; Heck, A.; Maurice, M.; et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nat. Cell Biol. 2012, 488, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, M.; Hodis, E.; Mu, X.J.; Yamauchi, M.; Rosenbluh, J.; Cibulskis, K.; Saksena, G.; Lawrence, M.S.; Qian, Z.R.; Nishihara, R.; et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 2014, 46, 1264–1266. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kuboki, Y.; Hatori, T.; Yamamoto, M.; Sugiyama, M.; Shibata, N.; Shimizu, K.; Shiratori, K.; Furukawa, T. Clinicopathological significance of somatic RNF43 mutation and aberrant expression of ring finger protein 43 in intraductal papillary mucinous neoplasms of the pancreas. Mod. Pathol. 2015, 28, 261–267. [Google Scholar] [CrossRef]
- Wu, J.; Jiao, Y.; Dal Molin, M.; Maitra, A.; de Wilde, R.F.; Wood, L.D.; Eshleman, J.R.; Goggins, M.G.; Wolfgang, C.L.; Canto, M.I.; et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 21188–21193. [Google Scholar] [CrossRef]
- Centore, R.C.; Sandoval, G.J.; Soares, L.M.M.; Kadoch, C.; Chan, H.M. Mammalian SWI/SNF Chromatin Remodeling Complexes: Emerging Mechanisms and Therapeutic Strategies. Trends Genet. 2020, 36, 936–950. [Google Scholar] [CrossRef]
- Wang, L.; Shilatifard, A. UTX Mutations in Human Cancer. Cancer Cell 2019, 35, 168–176. [Google Scholar] [CrossRef]
- Wang, X.; Nagl, J.N.G.; Wilsker, D.; Van Scoy, M.; Pacchione, S.; Yaciuk, P.; Dallas, P.; Moran, E. Two related ARID family proteins are alternative subunits of human SWI/SNF complexes. Biochem. J. 2004, 383, 319–325. [Google Scholar] [CrossRef]
- Jones, S.; Li, M.; Parsons, D.W.; Zhang, X.; Wesseling, J.; Kristel, P.; Schmidt, M.; Markowitz, S.; Yan, H.; Bigner, D.; et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum. Mutat. 2012, 33, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Giacomini, C.P.; Matsukuma, K.; Karikari, C.A.; Bashyam, M.D.; Hidalgo, M.; Maitra, A.; Pollack, J.R. Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2012, 109, E252–E259. [Google Scholar] [CrossRef] [PubMed]
- Dal Molin, M.; Hong, S.M.; Hebbar, S.; Sharma, R.; Scrimieri, F.; de Wilde, R.F.; Mayo, S.C.; Goggins, M.; Wolfgang, C.L.; Schulick, R.D.; et al. Loss of expression of the SWI/SNF chromatin remodeling subunit BRG1/SMARCA4 is frequently observed in intraductal papillary mucinous neoplasms of the pancreas. Hum. Pathol. 2012, 43, 585–591. [Google Scholar] [CrossRef][Green Version]
- Mann, K.M.; Ward, J.M.; Yew, C.C.K.; Kovochich, A.; Dawson, D.W.; Black, M.; Brett, B.T.; Sheetz, T.E.; Dupuy, A.; Chang, D.; et al. Sleeping Beauty mutagenesis reveals cooperating mutations and pathways in pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 2012, 109, 5934–5941. [Google Scholar] [CrossRef]
- Leach, S.D. Mouse models of pancreatic cancer: The fur is finally flying! Cancer Cell 2004, 5, 7–11. [Google Scholar] [CrossRef]
- Viger, R.S.; Guittot, S.M.; Anttonen, M.; Wilson, D.B.; Heikinheimo, M. Role of the GATA Family of Transcription Factors in Endocrine Development, Function, and Disease. Mol. Endocrinol. 2008, 22, 781–798. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Fu, B.; Pan, F.; Yachida, S.; Dhara, M.; Albesiano, E.; Li, L.; Naito, Y.; Vilardell, F.; et al. GATA6 Activates Wnt Signaling in Pancreatic Cancer by Negatively Regulating the Wnt Antagonist Dickkopf-1. PLoS ONE 2011, 6, e22129. [Google Scholar] [CrossRef]
- O’Kane, G.M.; Grünwald, B.T.; Jang, G.-H.; Masoomian, M.; Picardo, S.; Grant, R.C.; Denroche, R.E.; Zhang, A.; Wang, Y.; Lam, B.; et al. GATA6 Expression Distinguishes Classical and Basal-like Subtypes in Advanced Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 4901–4910. [Google Scholar] [CrossRef]
- Martinelli, P.; Madriles, F.; Cañamero, M.; Pau, E.C.-D.S.; del Pozo, N.; Guerra, C.; Real, F.X. The acinar regulator Gata6 suppressesKrasG12V-driven pancreatic tumorigenesis in mice. Gut 2016, 65, 476–486. [Google Scholar] [CrossRef]
- Sakamoto, H.; Attiyeh, M.A.; Gerold, J.M.; Makohon-Moore, A.P.; Hayashi, A.; Hong, J.; Kappagantula, R.; Zhang, L.; Melchor, J.P.; Reiter, J.G.; et al. The Evolutionary Origins of Recurrent Pancreatic Cancer. Cancer Discov. 2020, 10, 792–805. [Google Scholar] [CrossRef]
- Escobar-Hoyos, L.; Knorr, K.; Abdel-Wahab, O. Aberrant RNA Splicing in Cancer. Annu. Rev. Cancer Biol. 2019, 3, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Obeng, E.A.; Chappell, R.J.; Seiler, M.; Chen, M.C.; Campagna, D.R.; Schmidt, P.J.; Schneider, R.K.; Lord, A.M.; Wang, L.; Gambe, R.G.; et al. Physiologic Expression of Sf3b1 K700E Causes Impaired Erythropoiesis, Aberrant Splicing, and Sensitivity to Therapeutic Spliceosome Modulation. Cancer Cell 2016, 30, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Saborowski, M.; Saborowski, A.; Morris, J.P.; Bosbach, B.; Dow, L.E.; Pelletier, J.; Klimstra, D.S.; Lowe, S.W. A modular and flexible ESC-based mouse model of pancreatic cancer. Genes Dev. 2014, 28, 85–97. [Google Scholar] [CrossRef]
- Ocal, O.; Pashkov, V.; Kollipara, R.K.; Zolghadri, Y.; Cruz, V.H.; Hale, M.A.; Heath, B.R.; Artyukhin, A.B.; Christie, A.L.; Tsoulfas, P.; et al. A rapid in vivo screen for pancreatic ductal adenocarcinoma therapeutics. Dis. Model. Mech. 2015, 8, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and Dissemination Precede Pancreatic Tumor Formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Maddipati, R.; Stanger, B.Z. Pancreatic Cancer Metastases Harbor Evidence of Polyclonality. Cancer Discov. 2015, 5, 1086–1097. [Google Scholar] [CrossRef]
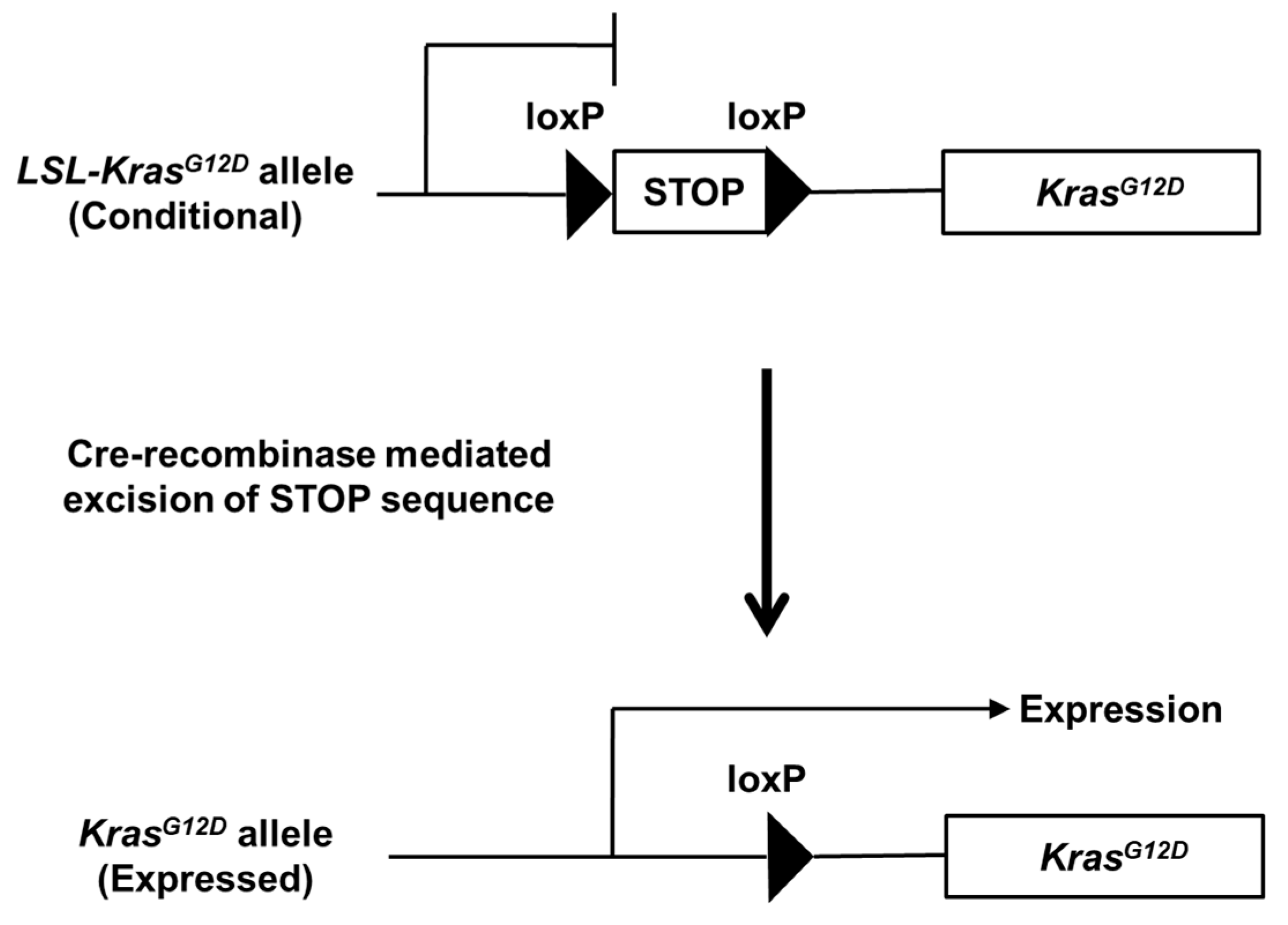
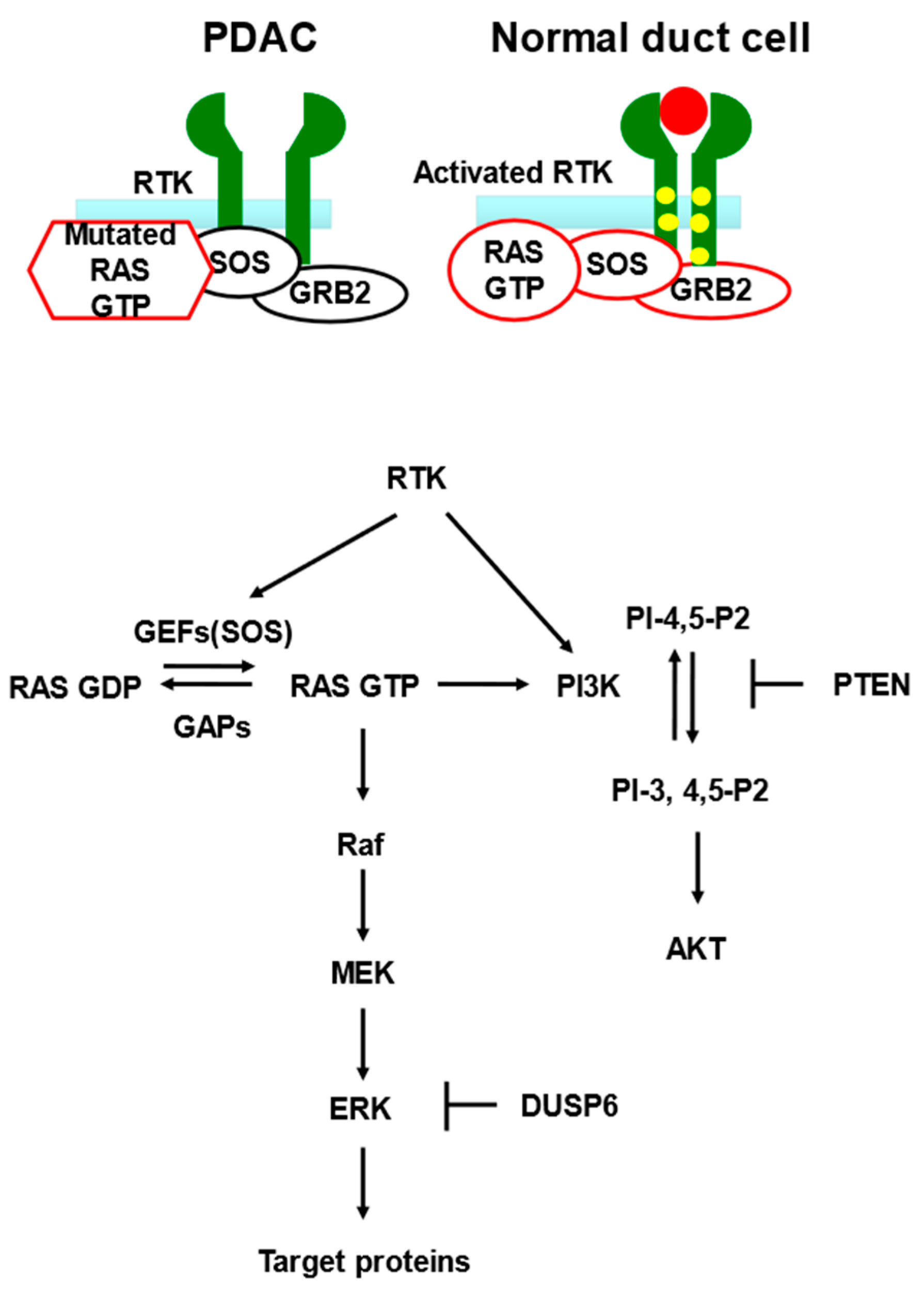
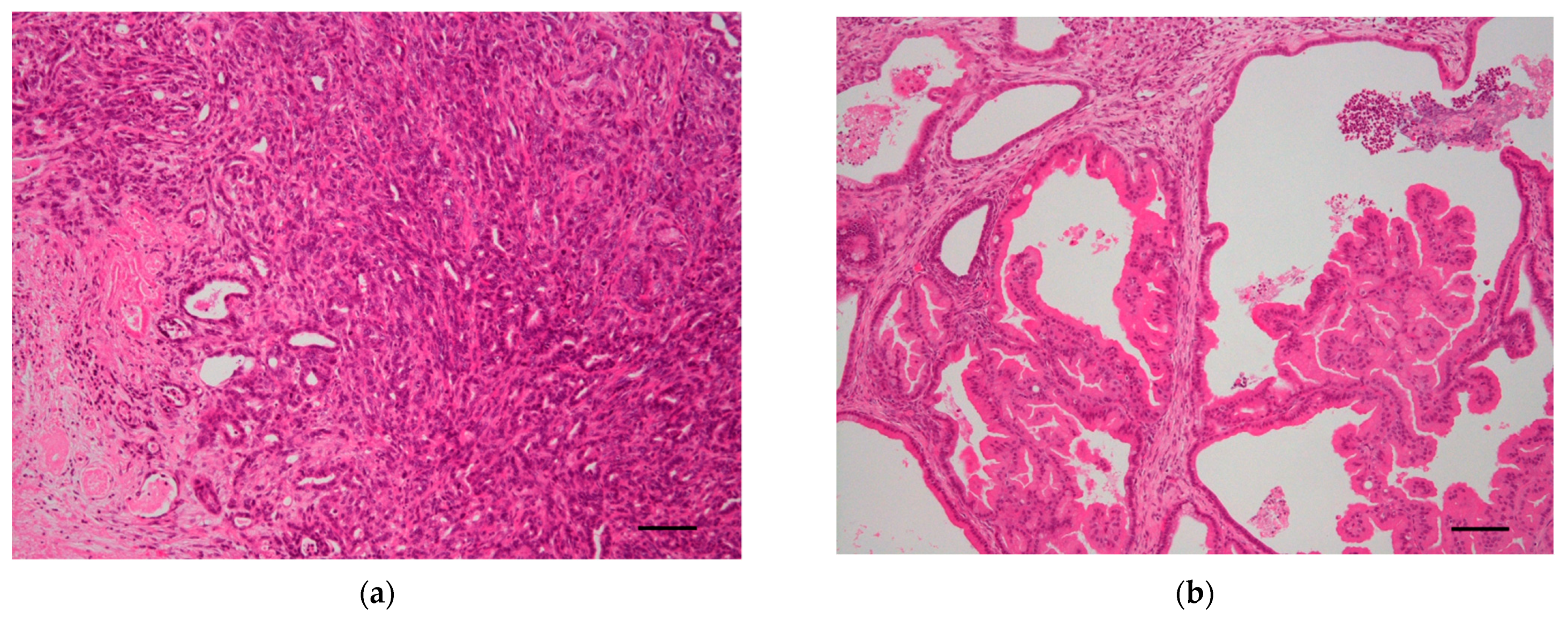
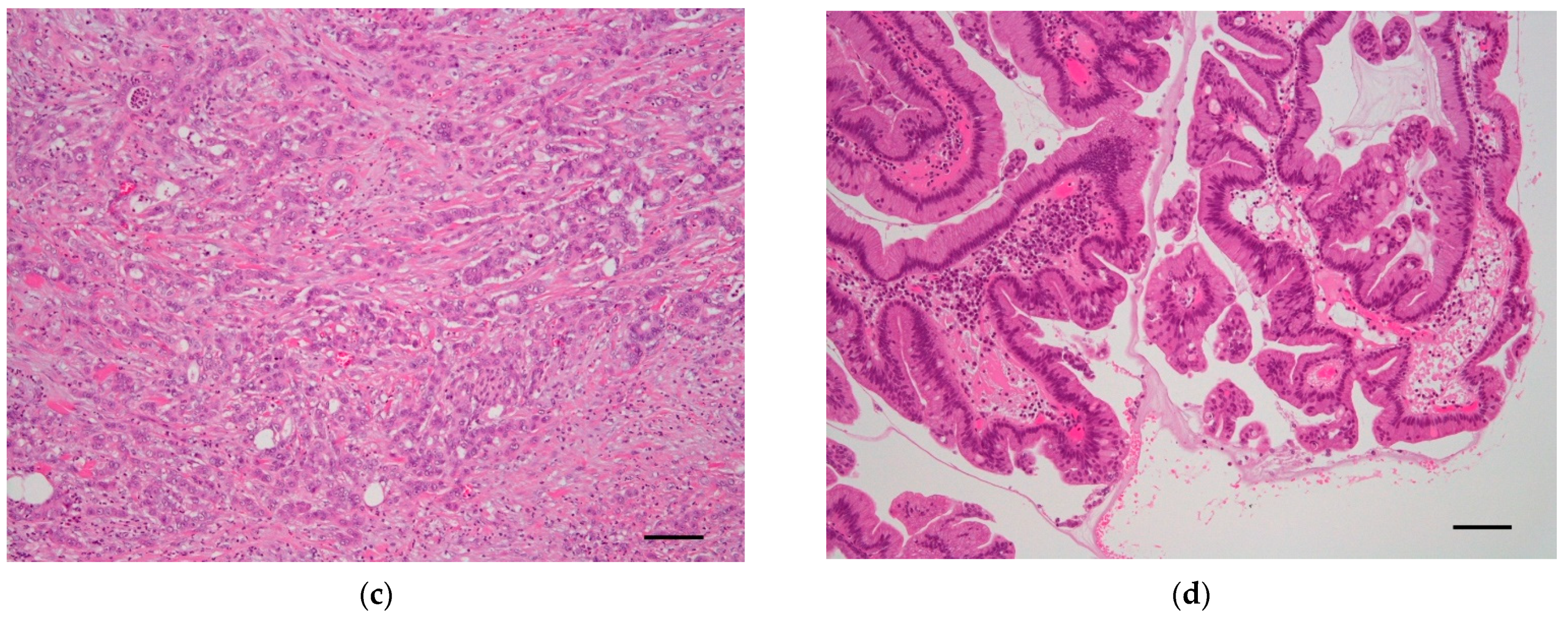
| Genotype | Time of Expression | Phenotype | Reference |
|---|---|---|---|
| Sox9CreER;KasLSL-G12D;Trp53flox/flox Ptf1aCreER;KrasLSL-G12D;Trp53flox/flox | Inducible | PanIN, PDAC | Lee, 2019 [18] |
| Tg(Ela5-KrasG12D) | ~P30 | Preinvasive ductal neoplasia, acinar cell dysplasia | Grippo, 2003 [19] |
| Pdx1-Cre;LSL-KrasG12D | E8.5 | PanIN, PDAC | Hingorani, 2003 [20] |
| Ptf1Cre/+;LSL-KrasG12D | E9.5 | PanIN, PDAC | Hingorani, 2003 [20] |
| KrasG12Vgeo;Elas-tTA/tetO-Cre | Inducible | PanIn, PDAC | Guerra, 2007 [21] |
| Tg(CAG-lox-GFP-stop-lox-KrasG12V);Hinf1b/CreERT2 | Inducible | PanIN, PDAC | Singh, 2021 [22] |
| Pdx1-Flp;FSF-KrasG12D/+;FSF-R26CAG-CreERT2 | E9.5 | PanIN, PDAC | Schönhuber, 2014 [23] |
| Pdx1-FlpO;Frt-STOP-Frt krasG12D | E9.5 | PanIN, PDAC | Wu, 2017 [24] |
| Pdx1-CreERT2;BrafCA/+ | Inducible | PanIN | Collisson, 2012 [25] |
| Tg(Pdx1-Cre)Pik3cap110* | E8.5 | PanIN, PDAC | Payne, 2015 [26] |
| Tg(Pdx1-Cre);Pik3caH1047R | E8.5 | PanIN, PDAC | Payne, 2015 [26] |
| Sox9-CreERT2;Ptenflox/flox;LSL-KrasG12D | Inducible | IPMN, PDAC | Kopp, 2018, [27] |
| Tg(Ela-1-myc) | ~P30 | Mixed acinar/ductal adenocarcinoma | Sandgren, 1991 [28] |
| Pdx1-Cre;CAG-tTA;TetO-Myc | Inducible | PanIN, PDAC | Lin, 2013 [29] |
| Pdx1-Cre;CAG-tTA;TetO-KrasG12D | Inducible | PanIN, PDAC | Rajbhandari, 2017 [30] |
| Pdx1CreER;KrasG12D;Trp53fl/+; Rosaconfetti/YFP | Inducible | PanIN, PDAC | Maddipati, 2021 [31] |
| Tg(CAG-LSL-GNASR201H); LSL-KrasG12D;Ptf1Cre/+ | E9.5 | IPMN | Taki, 2016 [32] |
| Sox9-CreERT2;LSL-KrasG12D;Lkb1flox/flox | Inducible | IPMN | Collet, 2020 [33] |
| Pdx1-Cre; LSL-KrasG12D; Smad4flox/flox | E8.5 | IPMN, PanIN | Bardeesy, 2006 [34] |
| Pdx1-Cre;LSL-KrasG12D;Ink4a/Arfflox/flox;Smad4flox/flox | E8.5 | IPMN, differentiated PDAC | Bardeesy, 2006 [34] |
| Pdx1-Cre; LSL-KrasG12D; Ink4a/Arfflox/flox | E8.5 | PanIN, pooly differentiated PDAC | Aguirre AJ, 2003 [35] |
| Ptf1Cre/+;LSL-KrasG12D;LSL-Trp53R172H/+ | E9.5 | PanIN, PDAC | Hingorani, 2005 [36] |
| Ptf1a-CreER;LSL-KrasG12D;Tp53loxP/+;LSL-GnasR201C | Inducible | IPMN, PDAC | Patra, 2018 [37] |
| LSL-KasG12D;ptf1a+/cre;ATMloxp/loxP | E8.5 | PDAC, metastaic | Drosos, 2016 [38] |
| Ptf1Cre/+;LSL-KrasG12D;rtTA3lox/lox;sgRnf43;Tre3g-Cas9 | Inducible gene editing | PanIN, PDAC | Misha, 2020 [39] |
| Ptf1Cre/+;LSL-KrasG12D;Arid1aflox/flox | E9.5 | IPMN, PDAC | Kimura, 2018 [40] Wang, 2019 [41] |
| Ptf1Cre/+,LSL-KrasG12D;Brg1flox/flox | E9.5 | IPMN, PDAC | Von Figura, 2014 [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiki, Y.; Jiang, C.; Ohmuraya, M.; Furukawa, T. Genetic Mutations of Pancreatic Cancer and Genetically Engineered Mouse Models. Cancers 2022, 14, 71. https://doi.org/10.3390/cancers14010071
Saiki Y, Jiang C, Ohmuraya M, Furukawa T. Genetic Mutations of Pancreatic Cancer and Genetically Engineered Mouse Models. Cancers. 2022; 14(1):71. https://doi.org/10.3390/cancers14010071
Chicago/Turabian StyleSaiki, Yuriko, Can Jiang, Masaki Ohmuraya, and Toru Furukawa. 2022. "Genetic Mutations of Pancreatic Cancer and Genetically Engineered Mouse Models" Cancers 14, no. 1: 71. https://doi.org/10.3390/cancers14010071
APA StyleSaiki, Y., Jiang, C., Ohmuraya, M., & Furukawa, T. (2022). Genetic Mutations of Pancreatic Cancer and Genetically Engineered Mouse Models. Cancers, 14(1), 71. https://doi.org/10.3390/cancers14010071






