Post-Irradiation Sinus Mucosa Disease in Nasopharyngeal Carcinoma Patients Treated with Intensity-Modulated Proton Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Radiotherapy Protocol
2.3. Protocol of MRI
2.4. Post-Treatment Follow-Up
2.5. Evaluation of Paranasal Sinus
2.6. Evaluation of Nasopharynx
2.7. Statistical Methods
3. Results
3.1. Patients and Tumor Characteristics
3.2. Post-Irradiation Sinus Mucosa Abnormality and Localized Nasopharyngeal Inflammation
3.3. Abnormal Rates of SMD before and after Radiation Therapy
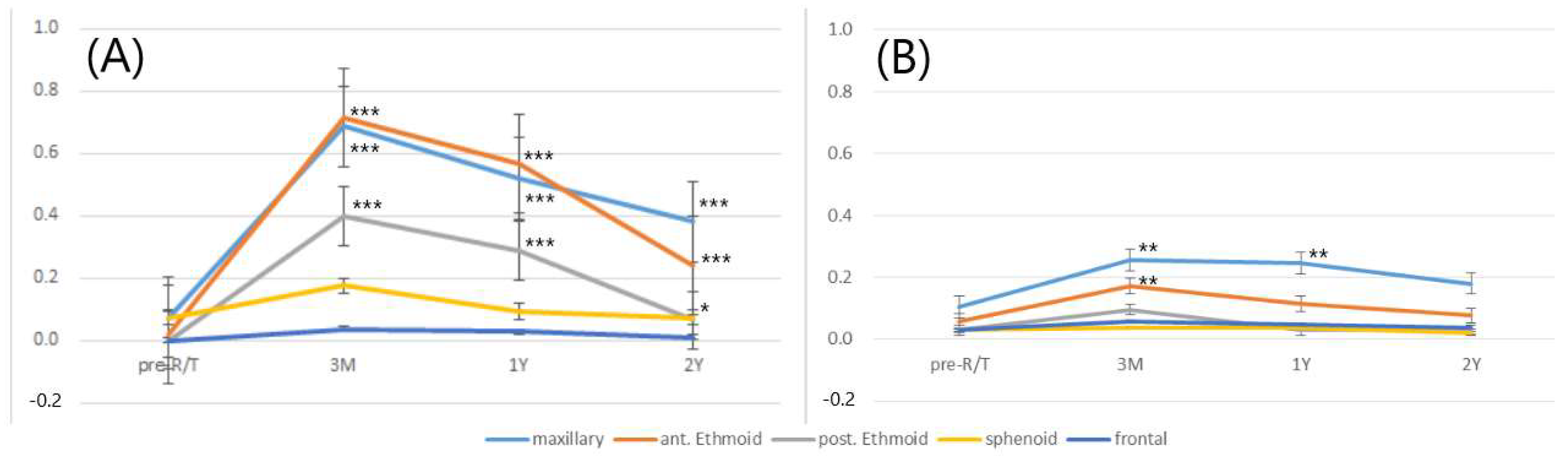
3.4. Chronological Changes in Severity of SMD during Different Follow-Up Periods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Huang, S.F.; Hsiao, J.H.; Young, C.K.; Chien, H.T.; Kuo, C.F.; See, L.C.; Luo, S.F.; Huang, L.H.; Liao, C.T.; Chang, T.J. Familial aggregation of nasopharyngeal carcinoma in Taiwan. Oral Oncol. 2017, 73, 10–15. [Google Scholar] [CrossRef]
- Chiang, C.J.; Lo, W.C.; Yang, Y.W.; You, S.L.; Chen, C.J.; Lai, M.S. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J. Formos. Med. Assoc. 2016, 115, 1076–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, P.M.; Ma, B.B.; Chan, A.T. Radiotherapy for nasopharyngeal carcinoma-transition from two-dimensional to three-dimensional methods. Radiother. Oncol. 2004, 73, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Chien, C.Y.; Tsai, W.L.; Liao, K.C.; Chou, S.Y.; Lin, H.C.; Luo, S.D.; Lee, T.F.; Lee, C.H.; Fang, F.M. Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head Neck 2016, 38, E1026–E1032. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Huang, S.F.; Lee, T.J.; Ng, S.H.; Chang, J.T. Postirradiation sinus mucosa disease in nasopharyngeal carcinoma patients. Laryngoscope 2007, 117, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.X.; Liu, L.P.; Li, L.; Li, X.; Cao, X.J.; Dong, W.; Yang, X.H.; Xu, J.; Yu, S.; Hao, J.F. Factors influencing the incidence of sinusitis in nasopharyngeal carcinoma patients after intensity-modulated radiation therapy. Eur. Arch. Otorhinolaryngol. 2014, 271, 3195–3201. [Google Scholar] [CrossRef]
- Hsin, C.H.; Tseng, H.C.; Lin, H.P.; Chen, T.H. Sinus mucosa status in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: A 5-year follow-up. Head Neck 2016, 38, 29–35. [Google Scholar] [CrossRef]
- Teo, P.M.; Kwan, W.H.; Leung, S.F.; Leung, W.T.; Chan, A.; Choi, P.; Yu, P.; Lee, W.Y.; Johnson, P. Early tumour response and treatment toxicity after hyperfractionated radiotherapy in nasopharyngeal carcinoma. Br. J. Radiol. 1996, 69, 241–248. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Kamel, R.; Al-Badawy, S.; Khairy, A.; Kandil, T.; Sabry, A. Nasal and paranasal sinus changes after radiotherapy for nasopharyngeal carcinoma. Acta Otolaryngol. 2004, 124, 532–535. [Google Scholar] [CrossRef]
- Lou, P.J.; Chen, W.P.; Tai, C.C. Delayed irradiation effects on nasal epithelium in patients with nasopharyngeal carcinoma. An ultrastructural study. Ann Otol. Rhinol. Laryngol. 1999, 108, 474–480. [Google Scholar] [CrossRef]
- Riva, G.; Franco, P.; Provenzano, E.; Arcadipane, F.; Bartoli, C.; Lava, P.; Ricardi, U.; Pecorari, G. Radiation-Induced Rhinitis: Cytological and Olfactory Changes. Am. J. Rhinol. Allergy 2019, 33, 153–161. [Google Scholar]
- Riva, G.; Boita, M.; Ravera, M.; Moretto, F.; Badellino, S.; Rampino, M.; Ricardi, U.; Pecorari, G.; Garzaro, M. Nasal cytological changes as late effects of radiotherapy for nasopharyngeal cancer. Am. J. Rhinol. Allergy 2015, 29, e41–e45. [Google Scholar] [CrossRef]
- Kuhar, H.N.; Tajudeen, B.A.; Heilingoetter, A.; Mahdavinia, M.; Gattuso, P.; Ghai, R.; Gunawan, F.; Diaz, A.Z.; Tolekidis, G.; Batra, P.S. Distinct histopathologic features of radiation-induced chronic sinusitis. Int. Forum Allergy Rhinol. 2017, 7, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Nakai, Y.; Ikeoka, H.; Koshimo, H.; Esaki, Y.; Nakata, J.; Onoyama, Y. Functional and morphological pathology of the nasal mucosa after X-ray irradiation. Clin. Otolaryngol. Allied Sci. 1988, 13, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Himi, T.; Kukuminato, Y.; Kita, H.; Yoshioka, I.; Kataura, A. Effect of radiotherapy on the levels of secretory immunoglobulin A against indigenous and virulent streptococci. Otolaryngol. Head Neck. Surg. 1997, 117, 433–437. [Google Scholar] [PubMed]
- Zubizarreta, P.A.; D’Antonio, G.; Raslawski, E.; Gallo, G.; Preciado, M.V.; Casak, S.J.; Scopinaro, M.; Morales, G.; Sackmann-Muriel, F. Nasopharyngeal carcinoma in childhood and adolescence: A single-institution experience with combined therapy. Cancer 2000, 89, 690–695. [Google Scholar] [CrossRef]
- Leeman, J.E.; Romesser, P.B.; Zhou, Y.; McBride, S.; Riaz, N.; Sherman, E.; Cohen, M.A.; Cahlon, O.; Lee, N. Proton therapy for head and neck cancer: Expanding the therapeutic window. Lancet Oncol. 2017, 18, 254–265. [Google Scholar] [CrossRef]
- Holliday, E.B.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Gunn, G.B.; Phan, J.; Beadle, B.M.; Zhu, X.R.; Zhang, X.; et al. Proton therapy reduces treatment-related toxicities for patients with nasopharyngeal cancer: A case-match control study of intensity-modulated proton therapy and intensity-modulated photon therapy. Int. J. Part. Ther. 2015, 2, 19–28. [Google Scholar] [CrossRef]
- McDonald, M.W.; Liu, Y.; Moore, M.G.; Johnstone, P.A. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: Cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat. Oncol. 2016, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.C.; Fan, K.H.; Lin, C.Y.; Hung, T.M.; Huang, B.S.; Chang, K.P.; Kang, C.J.; Huang, S.F.; Chang, P.H.; Hsu, C.L.; et al. Intensity Modulated Proton Beam Therapy versus Volumetric Modulated Arc Therapy for Patients with Nasopharyngeal Cancer: A Propensity Score-Matched Study. Cancers 2021, 13, 3555. [Google Scholar] [CrossRef]
- Huang, P.W.; Lin, C.Y.; Hsieh, C.H.; Hsu, C.L.; Fan, K.H.; Huang, S.F.; Liao, C.T.; Ng, S.K.; Yen, T.C.; Chang, J.T.; et al. A phase II randomized trial comparing neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in advanced squamous cell carcinoma of the pharynx or larynx. Biomed. J. 2018, 41, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar] [PubMed]
- Sievers, K.W.; Greess, H.; Baum, U.; Dobritz, M.; Lenz, M. Paranasal sinuses and nasopharynx CT and MRI. Eur. J. Radiol. 2000, 33, 185–202. [Google Scholar] [CrossRef]
- Lund, V.J.; Kennedy, D.W. Staging for rhinosinusitis. Otolaryngol. Head Neck Surg. 1997, 117, S35–S40. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Hamilos, D.L.; Hadley, J.A.; Lanza, D.C.; Marple, B.F.; Nicklas, R.A.; Bachert, C.; Baraniuk, J.; Baroody, F.M.; Benninger, M.S.; et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. Otolaryngol. Head Neck Surg. 2004, 131, S1–S62. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.; Walgama, E.; Thamboo, A.; Nayak, J.V.; Hwang, P.H. Efficacy of endoscopic sinus surgery for chronic rhinosinusitis following primary radiotherapy and concurrent chemotherapy for nasopharyngeal carcinoma. Int. Forum Allergy Rhinol. 2017, 7, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Bassiouny, A.; Abd El Raouf, M.; Atef, A.; Nasr, S.; Talaat, S.; Nasr, M.; Ayad, E. A comparative study between ciliary count and the degree of opacity of paranasal sinus CT scans in chronic sinusitis pre and post FESS. J. Laryngol. Otol. 2005, 119, 950–954. [Google Scholar] [CrossRef]
- Tarp, B.; Fiirgaard, B.; Christensen, T.; Jensen, J.J.; Black, F.T. The prevalence and significance of incidental paranasal sinus abnormalities on MRI. Rhinology 2000, 38, 33–38. [Google Scholar]
- Lim, W.K.; Ram, B.; Fasulakis, S.; Kane, K.J. Incidental magnetic resonance image sinus abnormalities in asymptomatic Australian children. J. Laryngol. Otol. 2003, 117, 969–972. [Google Scholar] [CrossRef]
- Cooke, L.D.; Hadley, D.M. MRI of the paranasal sinuses: Incidental abnormalities and their relationship to symptoms. J. Laryngol. Otol. 1991, 105, 278–281. [Google Scholar] [CrossRef]
- Ashraf, N.; Bhattacharyya, N. Determination of the “incidental” Lund score for the staging of chronic rhinosinusitis. Otolaryngol. Head Neck Surg. 2001, 125, 483–486. [Google Scholar] [CrossRef]
- Fokkens, W.; Lund, V.; Mullol, J. European position paper on rhinosinusitis and nasal polyps group [in Chinese]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2008, 43, 317–320. [Google Scholar]
- Wabnitz, D.A.; Nair, S.; Wormald, P.J. Correlation between preoperative symptom scores, quality-of-life questionnaires, and staging with computed tomography in patients with chronic rhinosinusitis. Am. J. Rhinol. 2005, 19, 91–96. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Y.; Lv, D.; Liu, Y.; Qiao, X.; An, P.; Wang, D. Correlation between computed tomography staging and quality of life instruments in patients with chronic rhinosinusitis. Am. J. Rhinol. Allergy 2010, 24, e41–e45. [Google Scholar] [CrossRef]

| IMPT (n = 53) No. of Patients (%) * | VMAT (n = 54) No. of Patients (%) * | p-Value | ||
|---|---|---|---|---|
| Age (years) | 48.28 ± 13.38 | 50.31 ± 10.36 | 0.38 | |
| Gender | Male | 39 (73.6) | 45 (83.3) | 0.82 |
| Female | 14 (26.4) | 9 (16.6) | ||
| Pre-RT L–M scores | 0.49 ± 0.93 | 0.33 ± 0.73 | 0.33 | |
| Tumor classification | T1 | 22 (41.5) | 25 (46.3) | 0.86 |
| T2 | 8 (15.1) | 6 (11.1) | ||
| T3 | 13 (24.5) | 11 (20.4) | ||
| T4 | 10 (18.9) | 12 (22.2) | ||
| Nodal classification | N0 | 10 (18.9) | 10 (18.5) | 0.56 |
| N1 | 29 (54.7) | 24 (44.4) | ||
| N2 | 5 (9.4) | 10 (18.5) | ||
| N3 | 9 (17.0) | 10 (18.5) | ||
| Stage group | I | 6 (11.3) | 5 (29.3) | 0.92 |
| II | 17 (32.1) | 16 (29.6) | ||
| III | 10 (18.9) | 13 (24.1) | ||
| IV | 20 (37.7) | 20 (37.0) |
| IMPT | VMAT | p-Value | ||
|---|---|---|---|---|
| 3 m post-RT | L–M score | 0.72 ± 1.61 | 4.02 ± 2.73 | <0.001 |
| Endoscopic score | 1.42 ± 0.82 | 3.02 ± 0.81 | <0.001 | |
| 1 y post-RT | L–M score | 0.70 ± 1.48 | 2.98 ± 2.52 | <0.001 |
| Endoscopic score | 1.25 ± 0.96 | 3.00 ± 0.95 | <0.001 | |
| 2 y post-RT | L–M score | 0.68 ± 1.58 | 1.54 ± 1.94 | <0.001 |
| Endoscopic score | 1.08 ± 1.03 | 2.41 ± 1.11 | <0.001 |
| Maxillary | Ant. Ethmoid | Post. Ethmoid | Sphenoid | Frontal | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IMPT | VMAT | IMPT | VMAT | IMPT | VMAT | IMPT | VMAT | IMPT | VMAT | |
| Pre-RT | 11(10.3) | 7(6.5) | 6(5.7) | 2(1.9) | 3(2.8) | 0(0) | 3(2.8) | 6(5.7) | 2(1.9) | 0(0) |
| 3 m post-RT | 24(22.6) ** | 66(61.1) *** | 18(17.0) ** | 77(71.3) *** | 9(8.4) | 34(31.5) *** | 3(2.8) | 14(13.2) | 3(2.8) | 3(2.8) |
| 1 y post-RT | 23(21.7) ** | 53(49.1) *** | 12(11.3) | 61(56.5) *** | 2(1.9) | 28(25.9) *** | 3(2.8) | 9(8.5) | 3(2.8) | 3(2.8) |
| 2 y post-RT | 17(16.0) | 39(36.1) *** | 8(7.5) | 26(24.1) *** | 2(1.9) | 6(5.6) * | 1(0.9) | 7(6.6) | 2(1.9) | 1(1.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-W.; Huang, C.-C.; Lee, Y.-S.; Chou, Y.-C.; Fan, K.-H.; Lin, C.-Y.; Huang, B.-S.; Yang, S.-W.; Huang, C.-C.; Chang, P.-H.; et al. Post-Irradiation Sinus Mucosa Disease in Nasopharyngeal Carcinoma Patients Treated with Intensity-Modulated Proton Therapy. Cancers 2022, 14, 225. https://doi.org/10.3390/cancers14010225
Wu P-W, Huang C-C, Lee Y-S, Chou Y-C, Fan K-H, Lin C-Y, Huang B-S, Yang S-W, Huang C-C, Chang P-H, et al. Post-Irradiation Sinus Mucosa Disease in Nasopharyngeal Carcinoma Patients Treated with Intensity-Modulated Proton Therapy. Cancers. 2022; 14(1):225. https://doi.org/10.3390/cancers14010225
Chicago/Turabian StyleWu, Pei-Wen, Chien-Chia Huang, Yun-Shien Lee, Yung-Chih Chou, Kang-Hsing Fan, Chien-Yu Lin, Bing-Shen Huang, Shih-Wei Yang, Chi-Che Huang, Po-Hung Chang, and et al. 2022. "Post-Irradiation Sinus Mucosa Disease in Nasopharyngeal Carcinoma Patients Treated with Intensity-Modulated Proton Therapy" Cancers 14, no. 1: 225. https://doi.org/10.3390/cancers14010225
APA StyleWu, P.-W., Huang, C.-C., Lee, Y.-S., Chou, Y.-C., Fan, K.-H., Lin, C.-Y., Huang, B.-S., Yang, S.-W., Huang, C.-C., Chang, P.-H., Lee, T.-J., & Chang, J. T.-C. (2022). Post-Irradiation Sinus Mucosa Disease in Nasopharyngeal Carcinoma Patients Treated with Intensity-Modulated Proton Therapy. Cancers, 14(1), 225. https://doi.org/10.3390/cancers14010225








