Long-Term Outcomes of Breast Cancer Patients Who Underwent Selective Neck Dissection for Metachronous Isolated Supraclavicular Nodal Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Treatment after miSLNM
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamirisa, N.P.; Ren, Y.; Campbell, B.M.; Thomas, S.M.; Fayanju, O.M.; Plichta, J.K.; Rosenberger, L.H.; Force, J.; Hyslop, T.; Hwang, E.S.; et al. Treatment Patterns and Outcomes of Women with Breast Cancer and Supraclavicular Nodal Metastases. Ann. Surg. Oncol. 2021, 28, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Borm, K.J.; Voppichler, J.; Düsberg, M.; Oechsner, M.; Vag, T.; Weber, W.; Combs, S.E.; Duma, M.N. FDG/PET-CT-Based Lymph Node Atlas in Breast Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Grotenhuis, B.A.; Klem, T.M.; Vrijland, W.W. Treatment outcome in breast cancer patients with ipsilateral supraclavicular lymph node metastasis at time of diagnosis: A review of the literature. Eur. J. Surg. Oncol. 2013, 39, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.A.; Chua, B.; Allan, S.J.; Speers, C.H.; Chia, S.; Ragaz, J. Long-term survival of patients with supraclavicular metastases at diagnosis of breast cancer. J. Clin. Oncol. 2003, 21, 851–854. [Google Scholar] [CrossRef] [PubMed]
- David Nathanson, S.; Leonard-Murali, S.; Burmeister, C.; Susick, L.; Baker, P. Clinicopathological Evaluation of the Potential Anatomic Pathways of Systemic Metastasis from Primary Breast Cancer Suggests an Orderly Spread Through the Regional Lymph Nodes. Ann. Surg. Oncol. 2020, 27, 4810–4818. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, A.; Rossi Saccarelli, C.; Morris, E.A.; Flynn, J.; Zhang, Z.; Khan, A.; Gillespie, E.; Cahlon, O.; Mueller, B.; Cuaron, J.J.; et al. Regional Lymph Node Involvement Among Patients With De Novo Metastatic Breast Cancer. JAMA Netw. Open 2020, 3, e2018790. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.H.; Strom, E.A.; Valero, V.; Fornage, B.; Perkins, G.H.; Oh, J.L.; Yu, T.-K.; Tereffe, W.; Woodward, W.A.; Hunt, K.K.; et al. Locoregional treatment outcomes for breast cancer patients with ipsilateral supraclavicular metastases at diagnosis. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 490–496. [Google Scholar] [CrossRef]
- Jung, J.; Kim, S.S.; Ahn, S.D.; Lee, S.W.; Ahn, S.H.; Son, B.H.; Lee, J.W.; Choi, E.K. Treatment Outcome of Breast Cancer with Pathologically Proven Synchronous Ipsilateral Supraclavicular Lymph Node Metastases. J. Breast Cancer 2015, 18, 167–172. [Google Scholar] [CrossRef]
- Sun, X.F.; Wang, Y.J.; Huang, T.; Niu, L.J.; Zhang, Q.; Liu, Z.Z. Comparison between surgery plus radiotherapy and radiotherapy alone in treating breast cancer patients with ipsilateral supraclavicular lymph node metastasis. Gland Surg. 2020, 9, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.T.; Zeng, L.Y.; Ouyang, D.J.; Zhao, P.; Zou, Q.Y.; Pei, L.; Luo, N.; Yi, W.-j. Surgery of the Primary Tumor Offers Survival Benefits of Breast Cancer with Synchronous Ipsilateral Supraclavicular Lymph Node Metastasis. World J. Surg. 2020, 44, 1163–1172. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.S.; Shin, K.H.; Kim, J.H.; Ahn, S.D.; Choi, D.H.; Park, W.; Lee, S.Y.; Chun, M.; Kim, J.H.; et al. Aggressive Surgical Excision of Supraclavicular Lymph Node Did Not Improve the Outcomes of Breast Cancer with Supraclavicular Lymph Node Involvement (KROG 16-14). Clin. Breast Cancer 2020, 20, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Chicago, IL, USA; American Joint Commission on Cancer: Chicago, IL, USA, 2017; Volume 22, p. 595. [Google Scholar]
- Van der Sangen, M.J.; Coebergh, J.W.; Roumen, R.M.; Rutten, H.J.; Vreugdenhil, G.; Voogd, A.C. Detection, treatment, and outcome of isolated supraclavicular recurrence in 42 patients with invasive breast carcinoma. Cancer 2003, 98, 11–17. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Adamo, V.; Russi, E.; Santacaterina, A.; Maisano, R.; Numico, G.; Palazzolo, C.; Ferraù, F.; Settiner, N.; Altavilla, G.; et al. Prospective multicenter study of combined treatment with chemotherapy and radiotherapy in breast cancer women with the rare clinical scenario of ipsilateral supraclavicular node recurrence without distant metastases. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 25–32. [Google Scholar] [CrossRef]
- Pedersen, A.N.; Møller, S.; Steffensen, K.D.; Haahr, V.; Jensen, M.; Kempel, M.M.; Jepsen, S.L.; Madsen, E.L.; Roslind, A.; Sandberg, E.; et al. Supraclavicular recurrence after early breast cancer: A curable condition? Breast Cancer Res. Treat. 2011, 125, 815–822. [Google Scholar] [CrossRef][Green Version]
- Fan, Y.; Xu, B.; Liao, Y.; Yao, S.; Sun, Y. A retrospective study of metachronous and synchronous ipsilateral supraclavicular lymph node metastases in breast cancer patients. Breast 2010, 19, 365–369. [Google Scholar] [CrossRef]
- Inari, H.; Teruya, N.; Kishi, M.; Horii, R.; Akiyama, F.; Takahashi, S.; Ito, Y.; Ueno, T.; Iwase, T.; Ohno, S. Clinicopathological features of breast cancer patients with internal mammary and/or supraclavicular lymph node recurrence without distant metastasis. BMC Cancer 2020, 20, 932. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Mao, Y.; Wang, H. Combined Therapy Can Improve the Outcomes of Breast Cancer with Isolated Supraclavicular Lymph Node Involvement. Cancer Manag. Res. 2020, 12, 11857–11869. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, M.F.; Hwang, T.L.; Chao, T.C.; Lo, Y.F.; Hsueh, S.; Chang, J.-T.C.; Leung, W.-M. Prediction of supraclavicular lymph node metastasis in breast carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 614–619. [Google Scholar] [CrossRef]
- Chen, S.C.; Chang, H.K.; Lin, Y.C.; Leung, W.M.; Tsai, C.S.; Cheung, Y.C.; Hsueh, S.; See, L.-C.; Chen, M.-F. Prognosis of breast cancer after supraclavicular lymph node metastasis: Not a distant metastasis. Ann. Surg. Oncol. 2006, 13, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chen, S.C.; Chang, H.K.; Hsueh, S.; Tsai, C.S.; Lo, Y.F.; Hwang, T.-L.; Chen, M.-F. Identifying good prognosis group of breast cancer patients with 1-3 positive axillary nodes for adjuvant cyclophosphamide, methotrexate and 5-fluorouracil (CMF) chemotherapy. Jpn. J. Clin. Oncol. 2005, 35, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, H.; Qian, M.; Mao, X.; Shi, G.; Ma, G.; Yu, M.; Xie, H.; Ling, L.; Ding, Q.; et al. Comparison of Survival Outcomes Among Patients With. Breast Cancer with Distant vs. Ipsilateral Supraclavicular Lymph Node Metastases. JAMA Netw. Open 2021, 4, e211809. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Li, J.; Guo, H.; Wang, C.; Tian, P.; Ma, Y. Correction to: Impact of Ipsilateral Supraclavicular Lymph Node Dissection (ISLND) for Breast Cancer Patients and a Nomogram for Predicting Ipsilateral Supraclavicular Pathological Complete Response (ispCR). Ann. Surg. Oncol. 2021, 28, 871. [Google Scholar] [CrossRef]
- Baek, J.Y.; Choi, D.H.; Park, W.; Kim, H.; Cho, W.K.; Yoo, G.S. Survival and Prognostic Factors for Breast Cancer Patients with Regional Oligo-Recurrence. J. Breast Cancer 2020, 23, 622–634. [Google Scholar] [CrossRef]
- Magnoni, F.; Colleoni, M.; Mattar, D.; Corso, G.; Bagnardi, V.; Frassoni, S.; Santomauro, G.; Jereczek-Fossa, B.A.; Veronesi, P.; Galimberti, V.; et al. Contralateral Axillary Lymph Node Metastases from Breast Carcinoma: Is it Time to Review TNM Cancer Staging? Ann. Surg. Oncol. 2020, 27, 4488–4499. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.A.; Valero, V.; Buzdar, A.U.; Booser, D.J.; Ames, F.; Strom, E.; Ross, M.; Theriault, R.L.; Frye, D.; Kau, S.-W.; et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: The University of Texas, M.D. Anderson Cancer Center experience. J. Clin. Oncol. 2001, 19, 628–633. [Google Scholar] [CrossRef]
- NCCN Guidelines Version 3.2021. Breast Cancer. 29 March 2021. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 25 May 2021).
- Reddy, J.P.; Levy, L.; Oh, J.L.; Strom, E.A.; Perkins, G.H.; Buchholz, T.A.; Woodward, W.A. Long-term outcomes in patients with isolated supraclavicular nodal recurrence after mastectomy and doxorubicin-based chemotherapy for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1453–1457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dellapasqua, S.; Bagnardi, V.; Balduzzi, A.; Iorfida, M.; Rotmensz, N.; Santillo, B.; Viale, G.; Ghisini, R.; Veronesi, P.; Luini, A.; et al. Outcomes of patients with breast cancer who present with ipsilateral supraclavicular or internal mammary lymph node metastases. Clin. Breast Cancer 2014, 14, 53–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poortmans, P.M.; Weltens, C.; Fortpied, C.; Kirkove, C.; Peignaux-Casasnovas, K.; Budach, V.; van der Leij, F.; Vonk, E.; Weidner, N.; Rivera, S.; et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1602–1610. [Google Scholar] [CrossRef]
- Aebi, S.; Gelber, S.; Anderson, S.J.; Láng, I.; Robidoux, A.; Martín, M.; Nortier, J.W.R.; Paterson, A.H.G.; Rimawi, M.F.; Baena-Cañada, J.M.; et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): A randomised trial. Lancet Oncol. 2014, 15, 156–163. [Google Scholar] [CrossRef]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.R.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Van Loo, P.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell 2017, 32, 169–184.e7. [Google Scholar] [CrossRef]
- De Bock, G.H.; Bonnema, J.; van der Hage, J.; Kievit, J.; van de Velde, C.J. Effectiveness of routine visits and routine tests in detecting isolated locoregional recurrences after treatment for early-stage invasive breast cancer: A meta-analysis and systematic review. J. Clin. Oncol. 2004, 22, 4010–4018. [Google Scholar] [CrossRef]
- Moon, H.J.; Kim, M.J.; Kim, E.K.; Park, B.W.; Youk, J.H.; Kwak, J.Y.; Sohn, J.; Kim, S.-I. US surveillance of regional lymph node recurrence after breast cancer surgery. Radiology 2009, 252, 673–681. [Google Scholar] [CrossRef]
- Schoppmann, S.F.; Bayer, G.; Aumayr, K.; Taucher, S.; Geleff, S.; Rudas, M.; Kubista, E.; Hausmaninger, H.; Samonigg, H.; Gnant, M.; et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann. Surg. 2004, 240, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Hunt, K.K.; Ballman, K.V.; Beitsch, P.D.; Whitworth, P.W.; Blumencranz, P.W.; Leitch, M.A.; Saha, S.; McCall, L.M.; Morrow, M. Axillary dissection vs. no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA 2011, 305, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Mougalian, S.S.; Adelson, K.B.; Young, M.R.; Yu, J.B. Association between prolonged metastatic free interval and recurrent metastatic breast cancer survival: Findings from the SEER database. Breast Cancer Res. Treat. 2019, 173, 209–216. [Google Scholar] [CrossRef] [PubMed]
| Variables | Neck Dissection (n = 61) | No Dissection (n = 78) | p Value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 47 (15) | 45 (16) | 0.516 | |
| Tumor size, cm, median (IQR) | 2.5 (1.1) | 3.0 (2.0) | 0.053 | |
| Operation procedure | Total mastectomy | 57 (93.4) | 67 (85.9) | 0.155 |
| Partial mastectomy | 4 (6.6) | 11 (14.1) | ||
| Axillary involvement | Yes | 46 (75.4) | 59 (75.6) | 0.975 |
| No | 15 (24.6) | 19 (24.4) | ||
| Estrogen receptor status | Positive | 33 (54.1) | 42 (53.8) | 0.266 |
| Negative | 27 (44.3) | 30 (38.5) | ||
| Unknown | 1 (1.6) | 6 (7.7) | ||
| Progesterone receptor status | Positive | 30 (49.2) | 39 (50.0) | 0.270 |
| Negative | 30 (49.2) | 33 (42.3) | ||
| Unknown | 1 (1.6) | 6 (7.6) | ||
| HER-2/neu | Positive | 17 (27.9) | 21 (26.9) | 0.749 |
| Negative | 19 (31.1) | 29 (37.2) | ||
| Unknown | 25 (41.0) | 28 (35.9) | ||
| SBR grade | 1 | 8 (13.1) | 7 (8.9) | 0.623 |
| 2 | 17 (27.9) | 29 (37.2) | ||
| 3 | 23 (37.7) | 25 (32.1) | ||
| Unknown | 13 (21.3) | 17 (21.8) | ||
| Initial axillary level II dissection | Yes | 52 (85.2) | 66 (84.6) | 0.918 |
| No | 9 (14.8) | 12 (15.4) | ||
| Chemotherapy | Yes | 54 (88.5) | 70 (89.7) | 0.818 |
| No | 7 (11.5) | 8 (10.3) | ||
| Radiotherapy | Yes | 9 (14.8) | 23 (29.5) | 0.041 |
| No | 52 (85.2) | 55 (70.5) | ||
| Hormonal therapy | Yes | 32 (52.5) | 41 (52.6) | >0.999 |
| No | 29 (47.5) | 37 (47.4) |
| Variables | Neck Dissection (n = 61) | No Dissection (n = 78) | p Value | |
|---|---|---|---|---|
| Time interval from primary surgery, months, median (IQR) | 28.7 (35.5) | 24.0 (27.0) | 0.131 | |
| Time interval from primary surgery | ||||
| ≤24 months | 25 (41.0) | 39 (50.0) | 0.309 | |
| >24 months | 36 (59.0) | 39 (50.0) | ||
| Age at relapse, years, median (IQR) | 52 (13) | 49 (17) | 0.233 | |
| Largest size of neck node, cm, median (IQR) | 1.3 (2.0) | 1.3 (1.0) | 0.843 | |
| Treatment after relapse | ||||
| Systemic therapy + radiotherapy | 30 (49.2) | 26 (33.3) | 0.021 | |
| Systemic therapy | 30 (49.2) | 42 (53.8) | ||
| None | 1 (1.6) | 10 (12.8) | ||
| Variables | No. | Median Survival Time (Months) | 95% CI *of Median | p ∆Value | HR # | 95% CI of HR | p Value | |
|---|---|---|---|---|---|---|---|---|
| Initial clinical features | ||||||||
| Age (years) | ≤40 | 42 | 22.1 | 16.6–27.5 | 0.589 | – | ||
| >40 | 97 | 18.1 | 11.7–24.6 | |||||
| Tumor size (cm) | ≤3 | 92 | 22.9 | 17.7–28.1 | 0.544 | – | ||
| >3 | 47 | 16.8 | 8.1–25.5 | |||||
| Axillary involvement | Yes | 105 | 20.8 | 14.9–26.8 | 0.180 | – | ||
| No | 34 | 23.7 | 9.8–37.6 | |||||
| Estrogen receptor status | Positive | 75 | 17.5 | 9.7–25.3 | 0.617 | – | ||
| Negative | 57 | 23.7 | 16.9–30.5 | |||||
| Progesterone receptor status | Positive | 69 | 21.8 | 12.5–31.1 | 0.692 | – | ||
| Negative | 63 | 21.4 | 13.6–29.1 | |||||
| HER-2/neu | Positive | 38 | 25.6 | 24.1–27.2 | 0.823 | – | ||
| Negative | 48 | 20.8 | 13.5–28.2 | |||||
| SBR grade | 1 | 15 | 41.0 | 19.8–62.2 | 0.087 | – | ||
| 2 | 46 | 18.1 | 8.6–27.7 | |||||
| 3 | 48 | 16.8 | 9.6–23.9 | |||||
| Axillary level II dissection | Yes | 118 | 22.9 | 16.8–29.0 | 0.608 | – | ||
| No | 21 | 14.9 | 9.1–20.6 | |||||
| Adjuvant therapy before relapse | ||||||||
| Chemotherapy | Yes | 124 | 21.8 | 15.9–27.8 | 0.886 | – | ||
| No | 15 | 14.9 | 4.4–25.3 | |||||
| Hormonal therapy | Yes | 73 | 23.3 | 15.5–31.1 | 0.247 | – | ||
| No | 66 | 20.8 | 14.4–27.3 | |||||
| Radiotherapy | Yes | 32 | 23.7 | 14.4–33.0 | 0.650 | – | ||
| No | 107 | 20.8 | 14.5–27.2 | |||||
| Clinical features after relapse | ||||||||
| Age at relapse | ≤50 | 70 | 20.8 | 13.5–28.2 | 0.475 | – | ||
| >50 | 69 | 22.9 | 14.0–31.9 | |||||
| Clinical neck node size (cm) | ≤1.3 | 47 | 20.8 | 11.4–30.3 | 0.403 | – | ||
| >1.3 | 46 | 21.3 | 13.4–29.3 | |||||
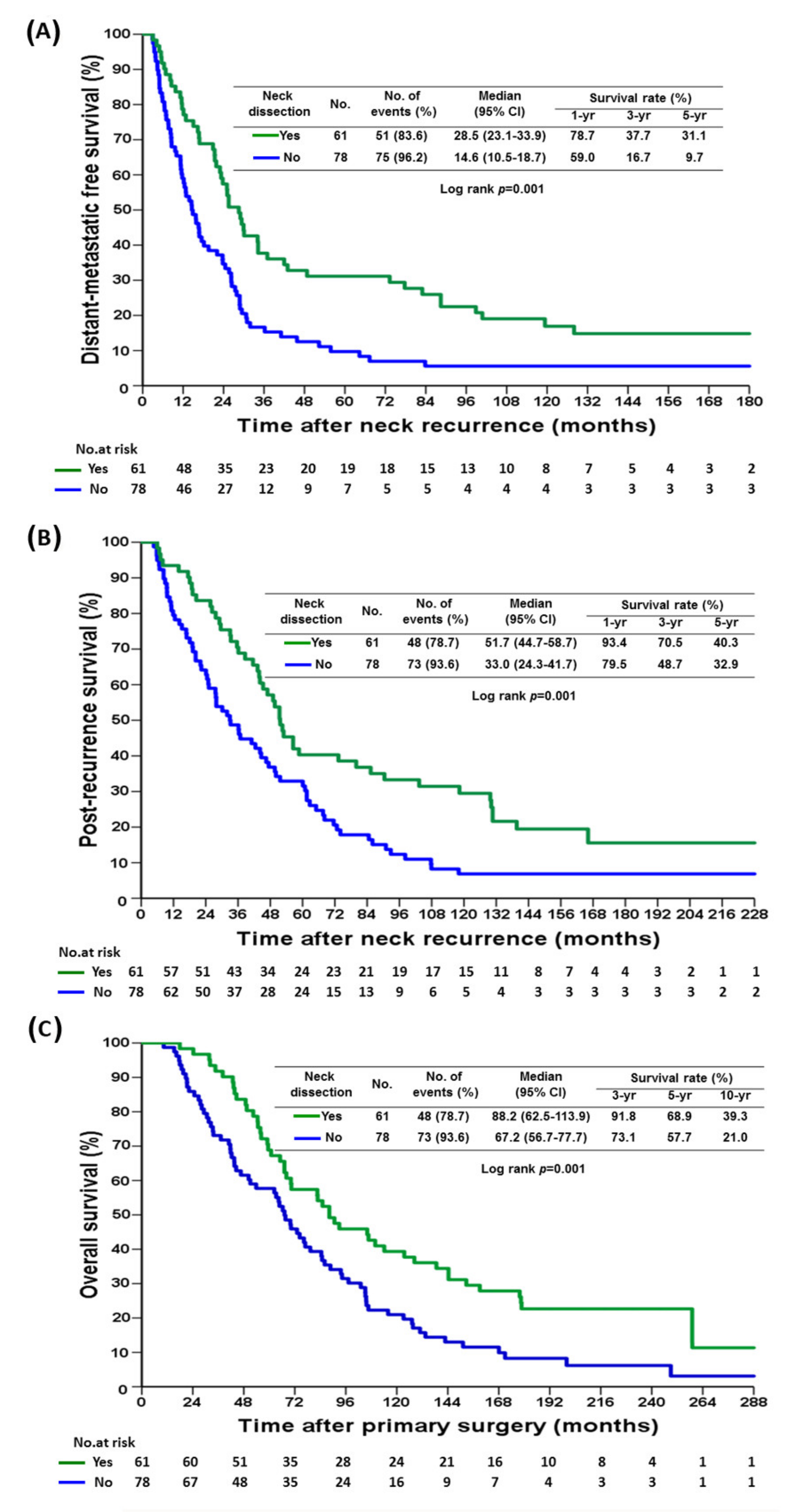
| Selective neck dissection | Yes | 61 | 28.5 | 23.1–33.9 | 0.001 | 1 | ||
| No | 78 | 14.6 | 10.5–18.7 | 1.77 | 1.23–2.56 | 0.002 | ||
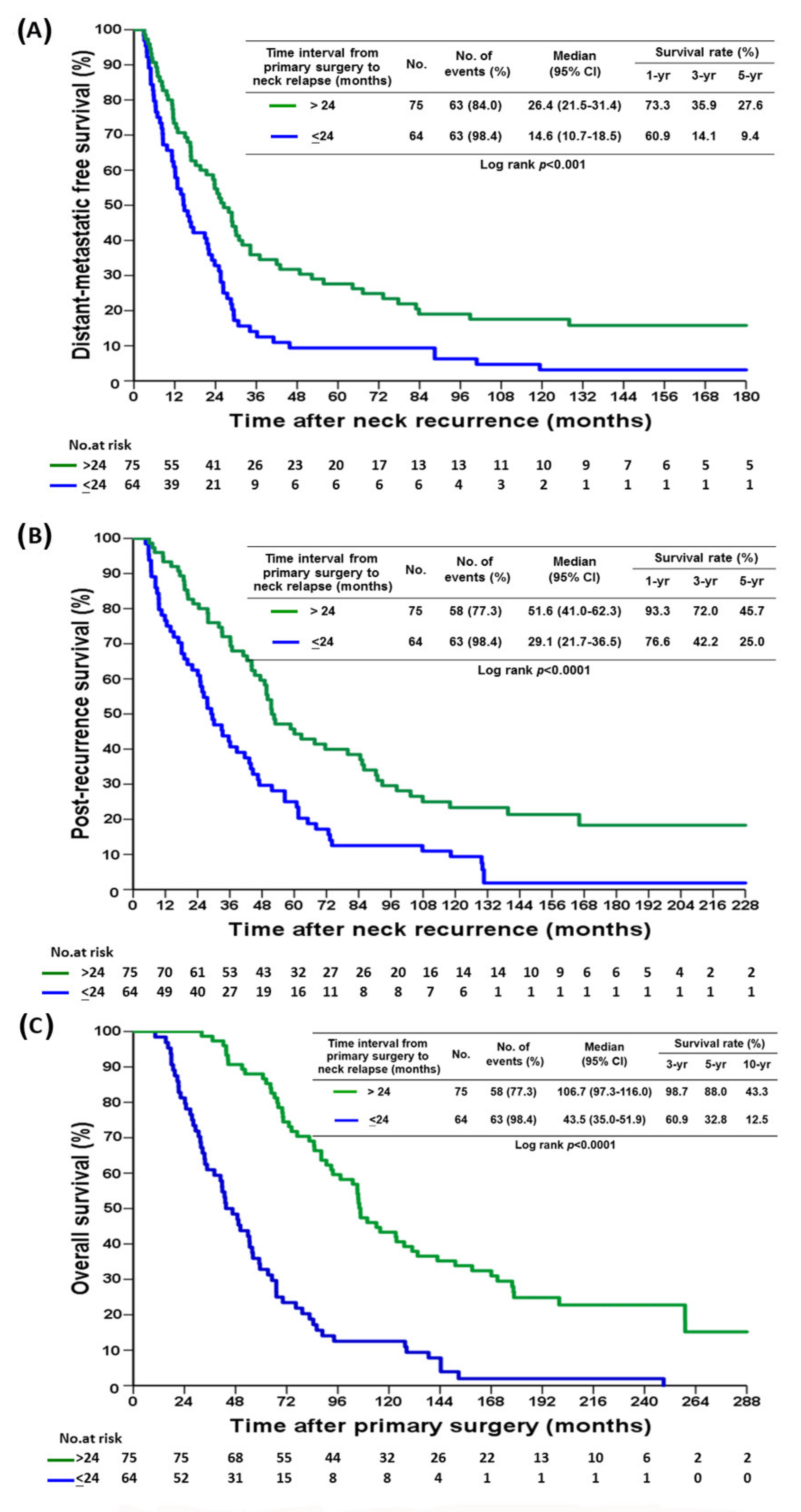
| Time interval from primary tumor surgery to neck relapse (months) | ≤24 | 64 | 14.6 | 10.7–18.5 | <0.001 | 1.76 | 1.23–2.52 | 0.002 |
| >24 | 75 | 26.4 | 21.5–31.4 | 1 | ||||
| Chemotherapy | Yes | 113 | 22.9 | 17.4–28.4 | 0.614 | – | ||
| No | 26 | 14.5 | 9.7–19.3 | |||||
| Hormonal therapy ** | Yes | 78 | 24.5 | 19.5–29.5 | 0.058 | – | ||
| No | 12 | 9.6 | 4.5–14.8 | |||||
| Radiotherapy | Yes | 57 | 26.3 | 19.5–33.1 | 0.103 | – | ||
| No | 82 | 16.8 | 11.2–22.4 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-C.; Shen, S.-C.; Yu, C.-C.; Huang, T.-S.; Lo, Y.-F.; Chang, H.-K.; Lin, Y.-C.; Kuo, W.-L.; Tsai, H.-P.; Chou, H.-H.; et al. Long-Term Outcomes of Breast Cancer Patients Who Underwent Selective Neck Dissection for Metachronous Isolated Supraclavicular Nodal Metastasis. Cancers 2022, 14, 164. https://doi.org/10.3390/cancers14010164
Chen S-C, Shen S-C, Yu C-C, Huang T-S, Lo Y-F, Chang H-K, Lin Y-C, Kuo W-L, Tsai H-P, Chou H-H, et al. Long-Term Outcomes of Breast Cancer Patients Who Underwent Selective Neck Dissection for Metachronous Isolated Supraclavicular Nodal Metastasis. Cancers. 2022; 14(1):164. https://doi.org/10.3390/cancers14010164
Chicago/Turabian StyleChen, Shin-Cheh, Shih-Che Shen, Chi-Chang Yu, Ting-Shuo Huang, Yung-Feng Lo, Hsien-Kun Chang, Yung-Chang Lin, Wen-Ling Kuo, Hsiu-Pei Tsai, Hsu-Huan Chou, and et al. 2022. "Long-Term Outcomes of Breast Cancer Patients Who Underwent Selective Neck Dissection for Metachronous Isolated Supraclavicular Nodal Metastasis" Cancers 14, no. 1: 164. https://doi.org/10.3390/cancers14010164
APA StyleChen, S.-C., Shen, S.-C., Yu, C.-C., Huang, T.-S., Lo, Y.-F., Chang, H.-K., Lin, Y.-C., Kuo, W.-L., Tsai, H.-P., Chou, H.-H., Lee, L.-Y., & Huang, Y.-T. (2022). Long-Term Outcomes of Breast Cancer Patients Who Underwent Selective Neck Dissection for Metachronous Isolated Supraclavicular Nodal Metastasis. Cancers, 14(1), 164. https://doi.org/10.3390/cancers14010164






