Decreasing Postoperative Pulmonary Complication Following Laparoscopic Surgery in Elderly Individuals with Colorectal Cancer: A Competing Risk Analysis in a Propensity Score–Weighted Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Variables
2.2. Short-Term Outcome and Long-Term Follow-Up
2.3. Statistical Analysis
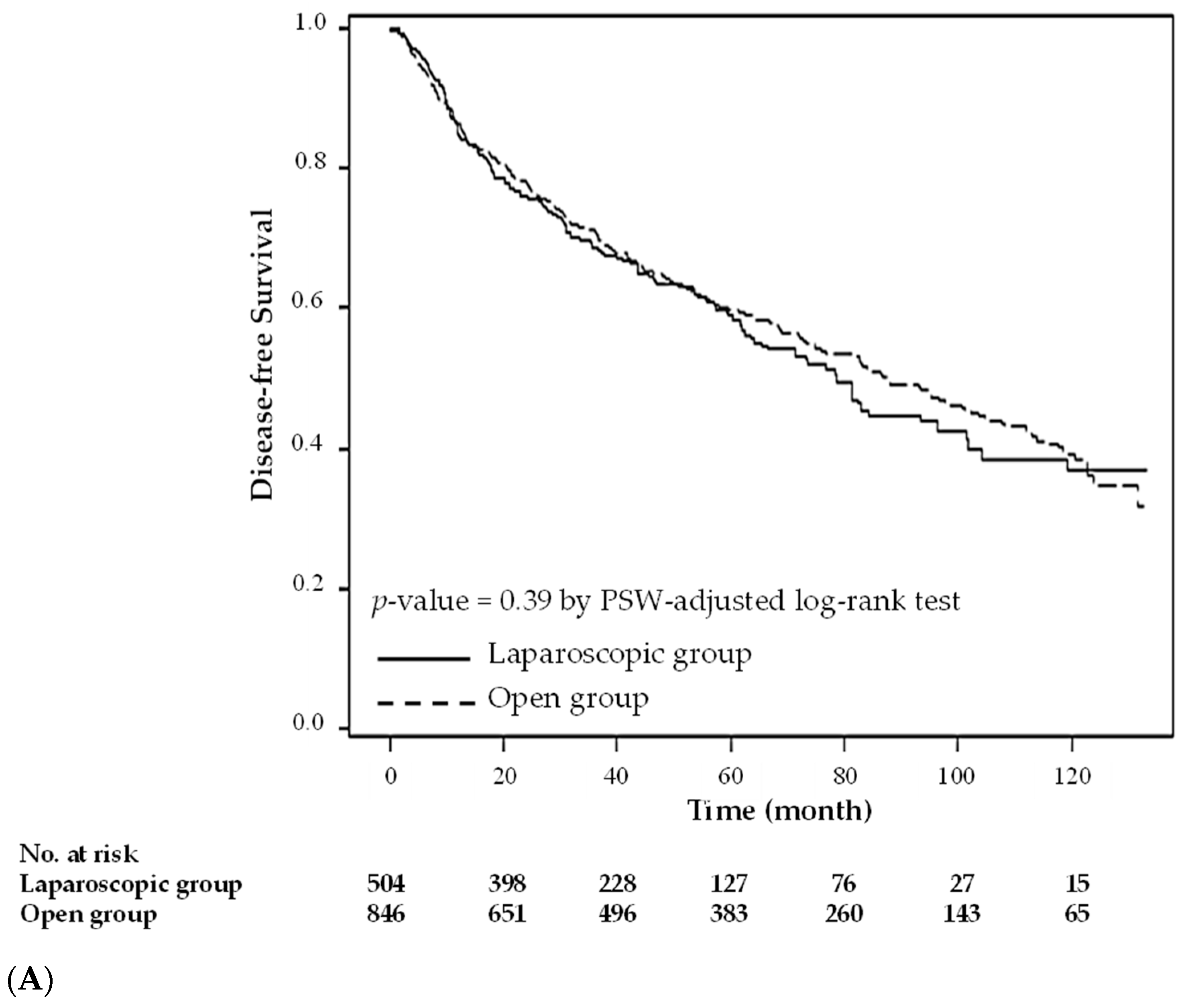
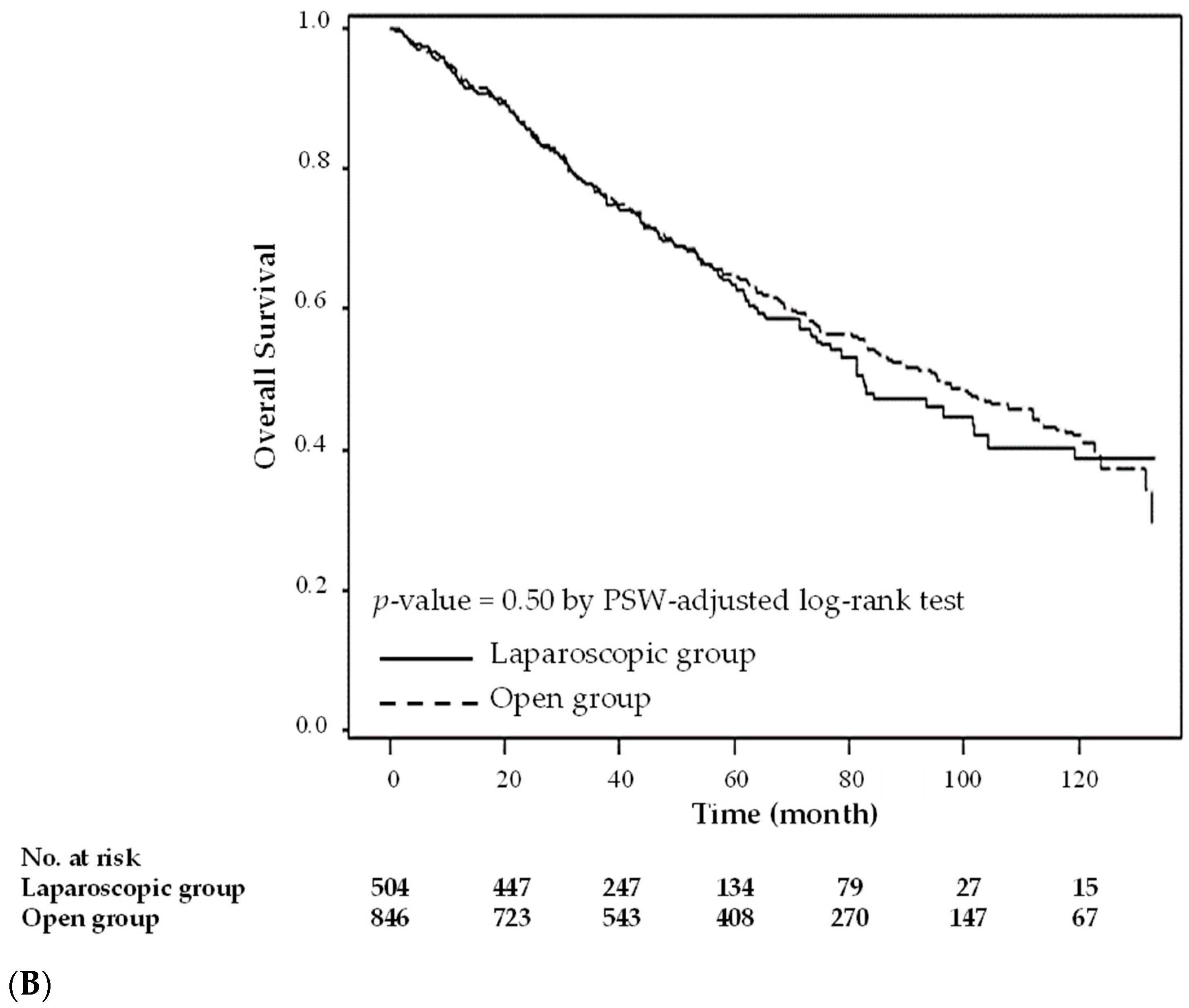
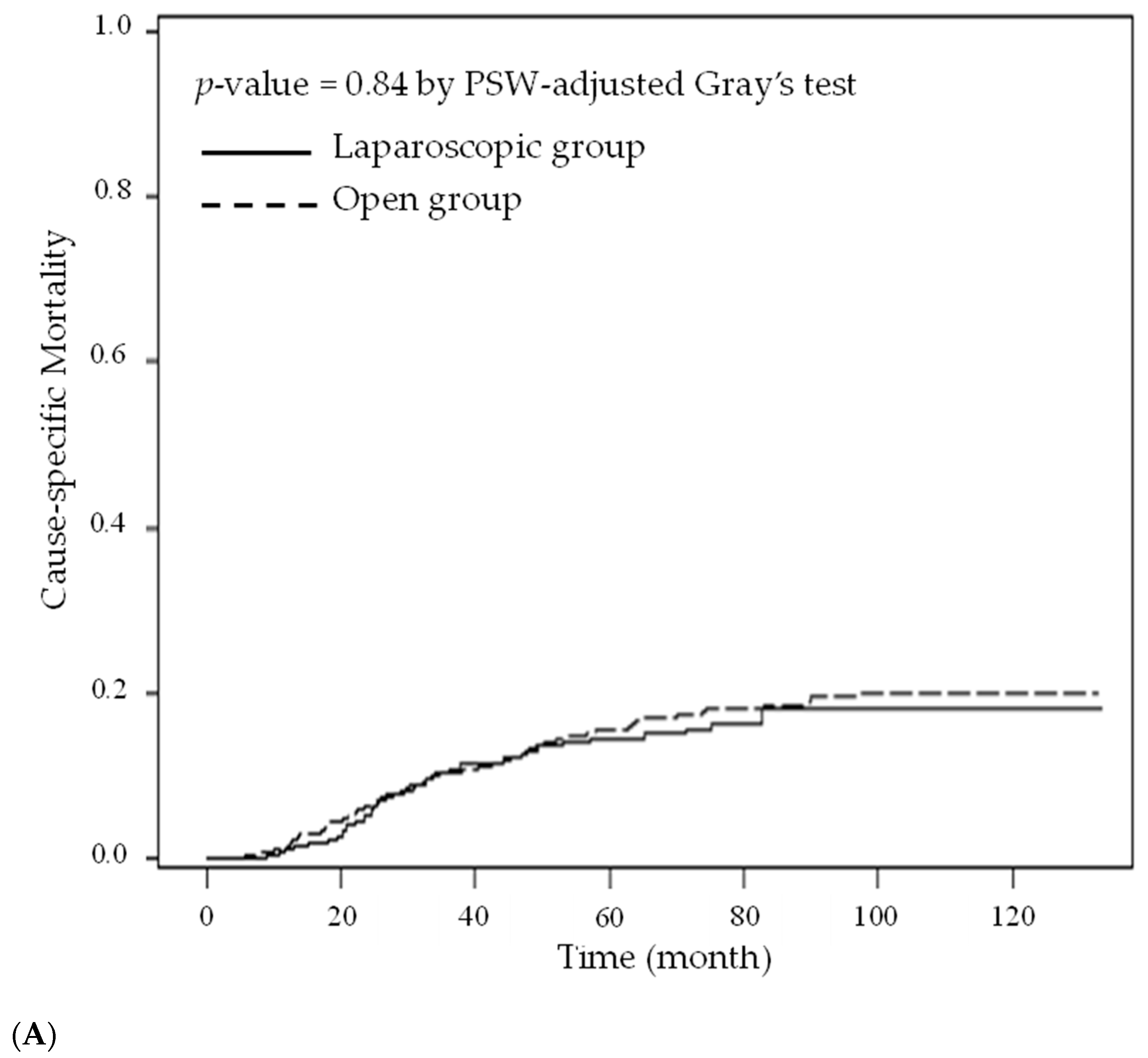
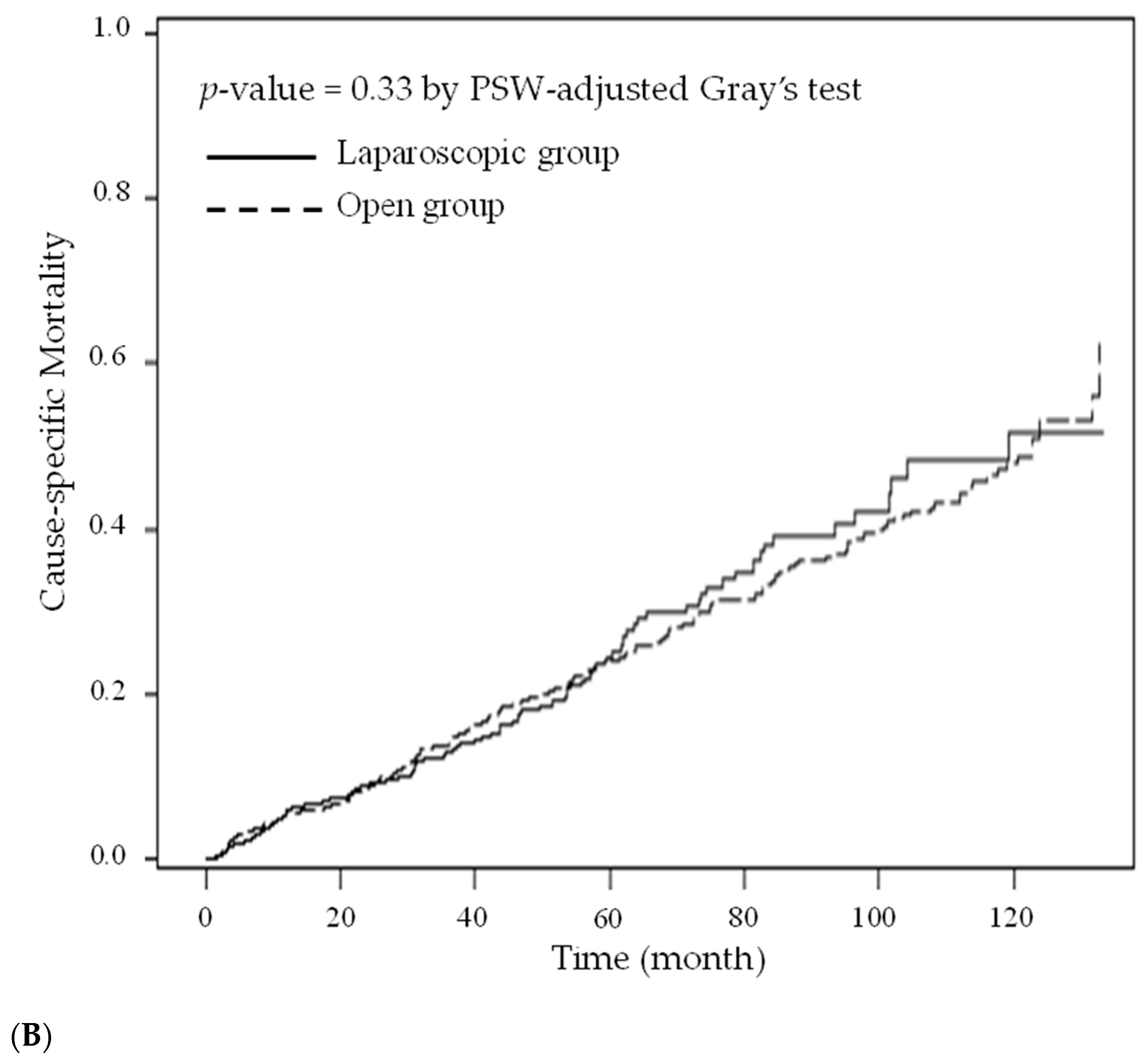
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chouhan, V.; Mansoor, E.; Parasa, S.; Cooper, G.S. Rates of Prevalent Colorectal Cancer Occurrence in Persons 75 Years of Age and Older: A Population-Based National Study. Dig. Dis. Sci. 2018, 63, 1929–1936. [Google Scholar] [CrossRef]
- Khan, M.R.; Bari, H.; Zafar, S.N.; Raza, S.A. Impact of age on outcome after colorectal cancer surgery in the elderly—A developing country perspective. BMC Surg. 2011, 11, 17. [Google Scholar] [CrossRef]
- Al-Refaie, W.B.; Parsons, H.M.; Habermann, E.B.; Kwaan, M.; Spencer, M.P.; Henderson, W.G.; Rothenberger, D.A. Operative Outcomes Beyond 30-day Mortality: Colorectal Cancer Surgery in Oldest Old. Ann. Surg. 2011, 253, 947–952. [Google Scholar] [CrossRef]
- Pérez Domínguez, L.; Cáceres Alvarado, N.; Toscano Novella, Á.; Casal Núñez, J.E. Results of colon cancer surgery in patients over 75 years old. ANZ J. Surg. 2018, 88, E11–E15. [Google Scholar] [CrossRef] [PubMed]
- Clinical Outcomes of Surgical Therapy Study, G. A comparison of laparoscopically assisted and open colectomy for colon cancer. N. Engl. J. Med. 2004, 350, 2050–2059. [Google Scholar] [CrossRef]
- Green, B.L.; Marshall, H.C.; Collinson, F.; Quirke, P.; Guillou, P.; Jayne, D.G.; Brown, J.M. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br. J. Surg. 2013, 100, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-Y.; Park, J.W.; Nam, B.H.; Kim, S.; Kang, S.-B.; Lim, S.-B.; Choi, H.S.; Kim, D.-W.; Chang, H.J.; Kim, D.Y.; et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): Survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014, 15, 767–774. [Google Scholar] [CrossRef]
- Veldkamp, R.; Kuhry, E.; Hop, W.C.; Jeekel, J.; Kazemier, G.; Bonjer, H.J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; et al. Laparoscopic surgery versus open surgery for colon cancer: Short-term outcomes of a randomised trial. Lancet Oncol. 2005, 6, 477–484. [Google Scholar] [CrossRef]
- Zeng, W.G.; Zhou, Z.X.; Hou, H.R.; Liang, J.W.; Zhou, H.T.; Wang, Z.; Zhang, X.M.; Hu, J.J. Outcome of laparoscopic versus open resection for rectal cancer in elderly patients. J. Surg. Res. 2015, 193, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Gao, S.; Yang, C.; Yang, W.; Guo, S. Laparoscopic colorectal resection versus open colorectal resection in octogenarians: A systematic review and meta-analysis of safety and efficacy. Tech. Coloproctol. 2016, 20, 153–162. [Google Scholar] [CrossRef]
- Bos, A.; Kortbeek, D.; van Erning, F.N.; Zimmerman, D.D.E.; Lemmens, V.; Dekker, J.W.T.; Maas, H. Postoperative mortality in elderly patients with colorectal cancer: The impact of age, time-trends and competing risks of dying. Eur. J. Surg. Oncol. 2019, 45, 1575–1583. [Google Scholar] [CrossRef]
- Niemeläinen, S.; Huhtala, H.; Ehrlich, A.; Kössi, J.; Jämsen, E.; Hyöty, M. Long-term survival following elective colon cancer surgery in the aged. A population-based cohort study. Colorectal. Dis. 2020, 22, 1585–1596. [Google Scholar] [CrossRef]
- ROSENBAUM, P.R.; RUBIN, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat. Med. 2005, 24, 3089–3110. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T. Cumulative incidence in competing risks data and competing risks regression analysis. Clin. Cancer Res. 2007, 13, 559–565. [Google Scholar] [CrossRef]
- Lau, B.; Cole, S.R.; Gange, S.J. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 2009, 170, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.J. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Prentice, R.L.; Kalbfleisch, J.D.; Peterson, A.V.; Flournoy, N.; Farewell, V.T.; Breslow, N.E. The Analysis of Failure Times in the Presence of Competing Risks. Biometrics 1978, 34, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Frasson, M.; Braga, M.; Vignali, A.; Zuliani, W.; Di Carlo, V. Benefits of Laparoscopic Colorectal Resection Are More Pronounced in Elderly Patients. Dis. Colon Rectum 2008, 51, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Grailey, K.; Markar, S.R.; Karthikesalingam, A.; Aboud, R.; Ziprin, P.; Faiz, O. Laparoscopic versus open colorectal resection in the elderly population. Surg. Endosc. 2013, 27, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Ishibe, A.; Ota, M.; Yamagishi, S.; Watanabe, K.; Watanabe, J.; Kanazawa, A.; Ichikawa, Y.; Oba, M.; Morita, S.; et al. Short-term results of a randomized study between laparoscopic and open surgery in elderly colorectal cancer patients. Surg. Endosc. 2014, 28, 466–476. [Google Scholar] [CrossRef]
- Vallribera Valls, F.; Landi, F.; Espin Basany, E.; Sanchez Garcia, J.L.; Jimenez Gomez, L.M.; Marti Gallostra, M.; Salgado Cruz, L.; Armengol Carrasco, M. Laparoscopy-assisted versus open colectomy for treatment of colon cancer in the elderly: Morbidity and mortality outcomes in 545 patients. Surg. Endosc. 2014, 28, 3373–3378. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, H.; Hinoi, T.; Kawaguchi, Y.; Ohdan, H.; Hasegawa, H.; Suzuka, I.; Fukunaga, Y.; Yamaguchi, T.; Endo, S.; Tagami, S. Laparoscopic surgery for colorectal cancer is safe and has survival outcomes similar to those of open surgery in elderly patients with a poor performance status: Subanalysis of a large multicenter case–control study in Japan. J. Gastroenterol. 2016, 51, 43–54. [Google Scholar] [CrossRef]
- Nishikawa, T.; Ishihara, S.; Hata, K.; Murono, K.; Yasuda, K.; Otani, K.; Tanaka, T.; Kiyomatsu, T.; Kawai, K.; Nozawa, H.; et al. Short-term outcomes of open versus laparoscopic surgery in elderly patients with colorectal cancer. Surg. Endosc. 2016, 30, 5550–5557. [Google Scholar] [CrossRef]
- Kazama, K.; Aoyama, T.; Hayashi, T.; Yamada, T.; Numata, M.; Amano, S.; Kamiya, M.; Sato, T.; Yoshikawa, T.; Shiozawa, M.; et al. Evaluation of short-term outcomes of laparoscopic-assisted surgery for colorectal cancer in elderly patients aged over 75 years old: A multi-institutional study (YSURG1401). BMC Surg. 2017, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Biondi, A.; Marventano, S.; Mistretta, A.; Calabrese, G.; Basile, F. Major postoperative complications and survival for colon cancer elderly patients. BMC Surg. 2012, 12, S20. [Google Scholar] [CrossRef] [PubMed]
- Arenal-Vera, J.J.; Tinoco-Carrasco, C.; del-Villar-Negro, A.; Labarga-Rodriguez, F.; Delgado-Mucientes, A.; Citores, M.A. Colorectal cancer in the elderly: Characteristics and short term results. Rev. Esp. Enferm. Dig. 2011, 103, 408–415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, W.S.; Chew, M.H.; Lim, I.A.L.; Ng, K.H.; Tang, C.L.; Eu, K.W. Evaluation of laparoscopic versus open colorectal surgery in elderly patients more than 70 years old: An evaluation of 727 patients. Int. J. Colorectal Dis. 2012, 27, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Shigeta, K.; Baba, H.; Yamafuji, K.; Asami, A.; Takeshima, K.; Nagasaki, K.; Okamoto, N.; Murata, T.; Arai, S.; Kubochi, K. Effects of laparoscopic surgery on the patterns of death in elderly colorectal cancer patients: Competing risk analysis compared with open surgery. Surg. Today 2016, 46, 422–429. [Google Scholar] [CrossRef]
- Law, W.L.; Chu, K.W.; Tung, P.H.M. Laparoscopic colorectal resection: A safe option for elderly patients. J. Am. Coll. Surg. 2002, 195, 768–773. [Google Scholar] [CrossRef]
- Folkesson, J.; Nilsson, J.; Påhlman, L.; Glimelius, B.; Gunnarsson, U. The circular stapling device as a risk factor for anastomotic leakage. Colorectal. Dis. 2004, 6, 275–279. [Google Scholar] [CrossRef]
- Jeong, D.H.; Hur, H.; Min, B.S.; Baik, S.H.; Kim, N.K. Safety and feasibility of a laparoscopic colorectal cancer resection in elderly patients. Ann. Coloproctol. 2013, 29, 22–27. [Google Scholar] [CrossRef]
- Chern, Y.J.; Tsai, W.S.; Hung, H.Y.; Chen, J.S.; Tang, R.; Chiang, J.M.; Yeh, C.Y.; You, Y.T.; Hsieh, P.S.; Chiang, S.F.; et al. The dark side of laparoscopic surgery for colorectal cancer patients aged 75 years or older. Int. J. Colorectal. Dis. 2018, 33, 1367–1371. [Google Scholar] [CrossRef]
- She, W.-H.; Poon, J.T.-C.; Fan, J.K.-M.; Lo, O.S.-H.; Law, W.-L. Outcome of laparoscopic colectomy for cancer in elderly patients. Surg. Endosc. 2013, 27, 308–312. [Google Scholar] [CrossRef]
- Hinoi, T.; Kawaguchi, Y.; Hattori, M.; Okajima, M.; Ohdan, H.; Yamamoto, S.; Hasegawa, H.; Horie, H.; Murata, K.; Yamaguchi, S.; et al. Laparoscopic versus open surgery for colorectal cancer in elderly patients: A multicenter matched case-control study. Ann. Surg. Oncol. 2015, 22, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Chern, Y.-J.; Hung, H.-Y.; You, J.-F.; Hsu, Y.-J.; Chiang, J.-M.; Hsieh, P.-S.; Tsai, W.-S. Advantage of laparoscopy surgery for elderly colorectal cancer patients without compromising oncologic outcome. BMC Surg. 2020, 20, 294. [Google Scholar] [CrossRef] [PubMed]

| Variables | Open Group | Laparoscopic Group | p-Value | |
|---|---|---|---|---|
| n = 846 | n = 504 | Before | After | |
| Age, No. (%) | 0.85 | 0.95 | ||
| 80 years | 415 (49.05) | 250 (49.60) | ||
| 80 years | 431 (50.95) | 254 (50.40) | ||
| Gender, No. (%) | 0.73 | 0.92 | ||
| Female | 378 (44.68) | 230 (45.63) | ||
| Male | 468 (55.32) | 274 (54.37) | ||
| BMI, No. (%) | <0.01 | 0.75 | ||
| 25 | 596 (70.45) | 315 (62.50) | ||
| 25 | 250 (29.55) | 189 (37.50) | ||
| Previous abdominal operation, No. (%) | ||||
| Appendectomy | 97 (11.47) | 54 (10.71) | 0.67 | 0.02 |
| Cholecystectomy | 59 (6.97) | 36 (7.14) | 0.91 | 0.01 |
| Hysterectomy | 50 (5.91) | 20 (3.97) | 0.12 | 0.09 |
| Oophorectomy | 14 (1.65) | 6 (1.19) | 0.49 | 0.03 |
| Colon-rectal operation | 44 (5.20) | 12 (2.38) | 0.01 | 0.10 |
| total | 584 (69.03) | 336 (66.67) | 0.37 | <0.01 |
| Operation, No. (%) | ||||
| Hartmann resection | 38 (4.49) | 16 (3.17) | 0.23 | 0.59 |
| Abdomino-peritoneal | 18 (2.13) | 14 (2.78) | 0.45 | 0.08 |
| Anterior resection | 473 (55.91) | 295 (58.53) | 0.35 | 0.92 |
| Left hemicolectomy | 55 (6.50) | 35 (6.94) | 0.75 | 0.17 |
| Right hemicolectomy | 225 (26.60) | 139 (27.58) | 0.69 | 0.48 |
| Segmental resection | 18 (2.13) | 2 (0.40) | 0.01 | <0.01 |
| Subtotal colectomy | 19 (2.25) | 3 (0.60) | 0.02 | <0.01 |
| Comorbidity, No. (%) | ||||
| Hypertension | 479 (56.62) | 327 (64.88) | <0.01 | 0.89 |
| Cardiac disease | 151 (17.85) | 90 (17.86) | 1.00 | 0.99 |
| Cerebrovascular accident | 70 (8.27) | 32 (6.35) | 0.20 | 0.59 |
| Asthma | 39 (4.61) | 23 (4.56) | 0.97 | 0.91 |
| Diabetes mellitus | 214 (25.30) | 131 (25.99) | 0.78 | 0.98 |
| Liver Cirrhosis | 96 (11.35) | 56 (11.11) | 0.89 | 0.98 |
| others | 277 (32.74) | 186 (36.90) | 0.12 | 0.81 |
| Carcinoembryonic antigen, No. (%) | <0.01 | 0.65 | ||
| 5 ng/mL | 537 (63.48) | 365 (72.42) | ||
| 5 ng/mL | 309 (36.52) | 139 (27.58) | ||
| Hemoglobin, No. (%) | 1.00 | 1.00 | ||
| 10 mg/mL | 225 (26.60) | 134 (26.59) | ||
| 10 mg/mL | 621 (73.40) | 370 (73.41) | ||
| Albumin, No. (%) | <0.01 | 0.78 | ||
| 3.5 mg/dL | 200 (23.64) | 82 (16.27) | ||
| 3.5 mg/dL | 646 (76.36) | 422 (83.73) | ||
| Total bilirubin, No. (%) | 0.78 | 0.77 | ||
| 1.3 | 831 (98.23) | 494 (98.02) | ||
| 1.3 | 15 (1.77) | 10 (1.98) | ||
| Creatinine, No. (%) | 0.55 | 0.75 | ||
| 1.3 | 709 (83.81) | 416 (82.54) | ||
| 1.3 | 137 (16.19) | 88 (17.46) | ||
| Tumor stage, No. (%) | <0.01 | 0.96 | ||
| 1 | 111 (13.12) | 100 (19.84) | ||
| 2 | 369 (43.62) | 224 (44.44) | ||
| 3 | 366 (43.26) | 180 (35.71) | ||
| Histological type, No. (%) | 0.36 | 0.60 | ||
| Adenocarcinoma | 794 (93.85) | 479 (95.04) | ||
| Mucinous adenocarcinoma & Signet ring cell | 52 (6.15) | 25 (4.96) | ||
| Histology grade, No. (%) | 0.01 | 0.80 | ||
| Poorly differentiated | 85 (10.05) | 30 (5.95) | ||
| Moderately differentiated | 682 (80.61) | 412 (81.75) | ||
| Well differentiated | 79 (9.34) | 62 (12.30) | ||
| Retrieved lymph node (+) number, No. (%) | 0.01 | 0.18 | ||
| <12 | 47 (5.56) | 13 (2.58) | ||
| 12 | 799 (94.44) | 491 (97.42) | ||
| Tumor site, No. (%) | 0.52 | 0.91 | ||
| Left side colon | 325 (38.42) | 182 (36.11) | ||
| Anus & rectum | 266 (31.44) | 173 (34.33) | ||
| Right side colon | 255 (30.14) | 149 (29.56) | ||
| Tumor size, No. (%) | <0.01 | 0.99 | ||
| 4 cm | 422 (49.88) | 297 (58.93) | ||
| 4 cm | 424 (50.12) | 207 (41.07) | ||
| Stomy type, No. (%) | 0.07 | 0.96 | ||
| No | 671 (79.31) | 425 (84.33) | ||
| Diverting stomy | 103 (12.17) | 46 (9.13) | ||
| End stomy | 72 (8.51) | 33 (6.55) | ||
| Variables | Open Group | Laparoscopic Group | p-Value | |
|---|---|---|---|---|
| n = 846 | n = 504 | Before | After | |
| Postoperative mortality, No. (%) | 16 (1.89) | 4 (0.79) | 0.106 | 0.033 |
| Pulmonary | 9 (1.06) | 1 (0.20) | 0.071 | 0.004 |
| Cardiovascular event | 3 + (0.35) | 1 (0.20) | 0.610 | 0.646 |
| Abdominal abscess | 1 (0.12) | 0 (0.00) | 0.440 | 0.220 |
| Anastomosis | 1 (0.12) | 1 (0.20) | 0.711 | 0.306 |
| Others | 2 (0.24) | 1 (0.20) | 0.886 | 0.657 |
| Postoperative morbidity, No. (%) | 159 (18.79) | 82 (16.27) | 0.241 | 0.218 |
| Wound | 41 * (4.85) | 14 (2.78) | 0.063 | 0.061 |
| Pulmonary | 30 (3.55) | 9 (1.79) | 0.062 | 0.003 |
| Cardiovascular event | 3 + (0.35) | 2 ++ (0.40) | 0.902 | 0.565 |
| Bladder dysfunction | 23 (2.72) | 10 (1.98) | 0.398 | 0.224 |
| Ileus | 30 (3.55) | 15 (2.98) | 0.573 | 0.373 |
| Abdominal abscess | 6 (0.71) | 8 (1.59) | 0.124 | 0.015 |
| Anastomosis | 7 # (0.83) | 16 ## (3.17) | 0.001 | 0.001 |
| Others | 19 (2.25) | 8 (1.59) | 0.403 | 0.748 |
| Clavien-Dindo Classification, No. (%) | 0.391 | 0.378 | ||
| Grade I, II | 113 (13.36) | 54 (10.71) | ||
| Grade III, IV, V | 46 (5.44) | 28 (5.56) | ||
| Conversion, No. (%) | - | 8 (1.59) | - | - |
| Length of hospital stay, median (IQR), day | 10.5 (9–15) | 8 (7–11) | <0.001 | <0.001 |
| Operation Methods | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| Crude HR [95% CI] | PSW-Adjusted HR [95% CI] | Crude HR [95% CI] | PSW-Adjusted HR [95% CI] | |
| Laparoscopic group | 0.939 [0.789–1.117] | 1.081 [0.965–1.211] | 0.940 [0.781–1.132] | 1.069 [0.946–1.207] |
| Open group | - | - | - | - |
| Operation Methods | Cause-Specific Hazard Model | Subdistribution Hazard Model | ||
|---|---|---|---|---|
| Death from This Cancer PSW-Adjusted HR [95% CI] | Death from Other Causes PSW-Adjusted HR [95% CI] | Death from This Cancer PSW-Adjusted HR [95% CI] | Death from Other Causes PSW-Adjusted HR [95% CI] | |
| Laparoscopic group | 0.966 [0.782–1.194] | 1.123 [0.968–1.303] | 0.925 [0.750–1.141] | 1.074 [0.927–1.244] |
| Open group | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chern, Y.-J.; You, J.-F.; Cheng, C.-C.; Jhuang, J.-R.; Yeh, C.-Y.; Hsieh, P.-S.; Tsai, W.-S.; Liao, C.-K.; Hsu, Y.-J. Decreasing Postoperative Pulmonary Complication Following Laparoscopic Surgery in Elderly Individuals with Colorectal Cancer: A Competing Risk Analysis in a Propensity Score–Weighted Cohort Study. Cancers 2022, 14, 131. https://doi.org/10.3390/cancers14010131
Chern Y-J, You J-F, Cheng C-C, Jhuang J-R, Yeh C-Y, Hsieh P-S, Tsai W-S, Liao C-K, Hsu Y-J. Decreasing Postoperative Pulmonary Complication Following Laparoscopic Surgery in Elderly Individuals with Colorectal Cancer: A Competing Risk Analysis in a Propensity Score–Weighted Cohort Study. Cancers. 2022; 14(1):131. https://doi.org/10.3390/cancers14010131
Chicago/Turabian StyleChern, Yih-Jong, Jeng-Fu You, Ching-Chung Cheng, Jing-Rong Jhuang, Chien-Yuh Yeh, Pao-Shiu Hsieh, Wen-Sy Tsai, Chun-Kai Liao, and Yu-Jen Hsu. 2022. "Decreasing Postoperative Pulmonary Complication Following Laparoscopic Surgery in Elderly Individuals with Colorectal Cancer: A Competing Risk Analysis in a Propensity Score–Weighted Cohort Study" Cancers 14, no. 1: 131. https://doi.org/10.3390/cancers14010131
APA StyleChern, Y.-J., You, J.-F., Cheng, C.-C., Jhuang, J.-R., Yeh, C.-Y., Hsieh, P.-S., Tsai, W.-S., Liao, C.-K., & Hsu, Y.-J. (2022). Decreasing Postoperative Pulmonary Complication Following Laparoscopic Surgery in Elderly Individuals with Colorectal Cancer: A Competing Risk Analysis in a Propensity Score–Weighted Cohort Study. Cancers, 14(1), 131. https://doi.org/10.3390/cancers14010131






