New Perspectives for Eye-Sparing Treatment Strategies in Primary Uveal Melanoma
Simple Summary
Abstract
1. Introduction
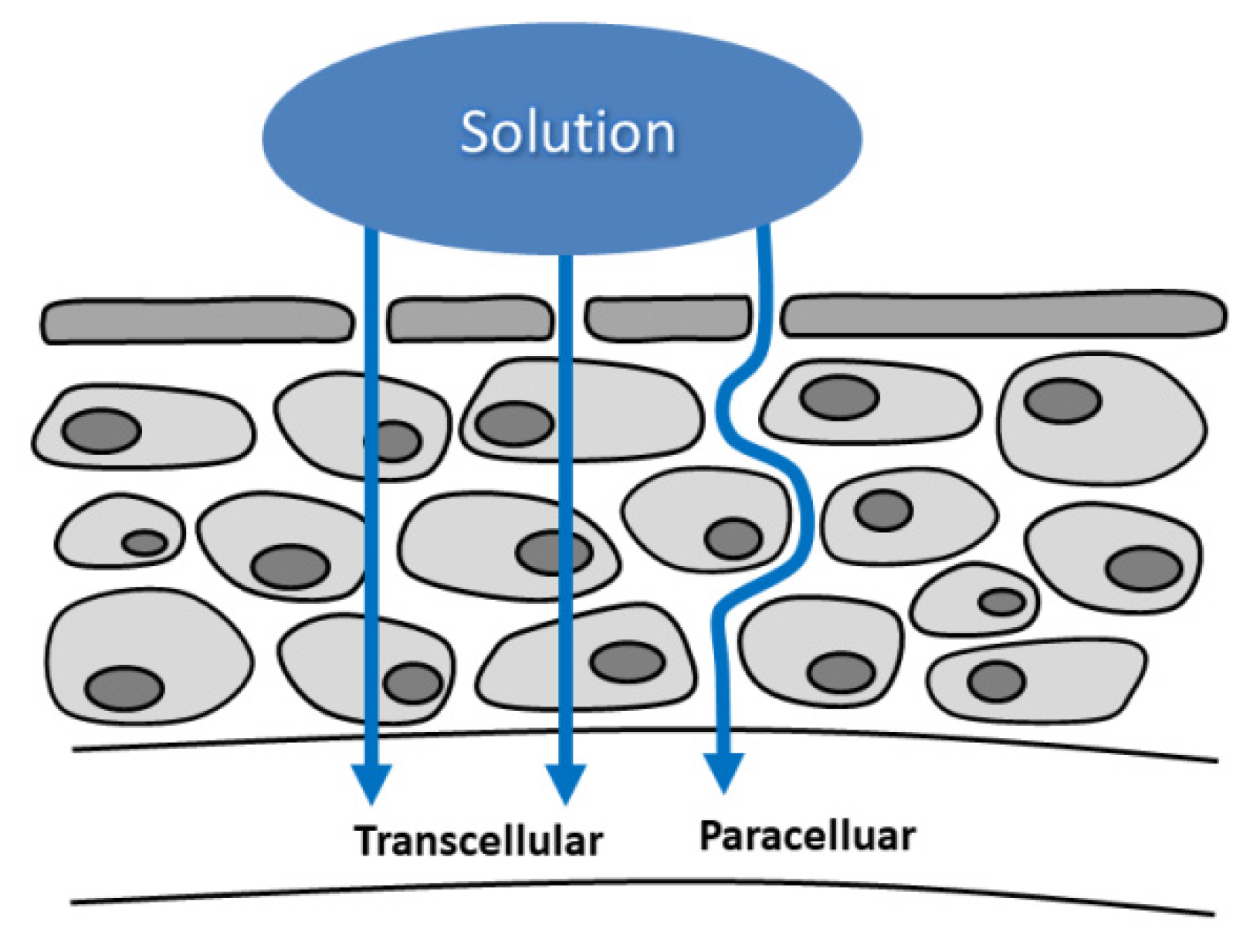
2. Ocular Pharmacology
3. Radiotherapy
3.1. Brachytherapy
3.2. Stereotactic Body Radiotherapy
3.3. Charged-Particle Radiotherapy
3.4. Ocular Complications of RT
4. Eye-Preserving Surgical Resection
5. Photodynamic Therapy
6. High Intensity Focused Ultrasound Ablative Technology
7. Sonodynamic Therapy
8. Electrically Enhanced Drug Delivery
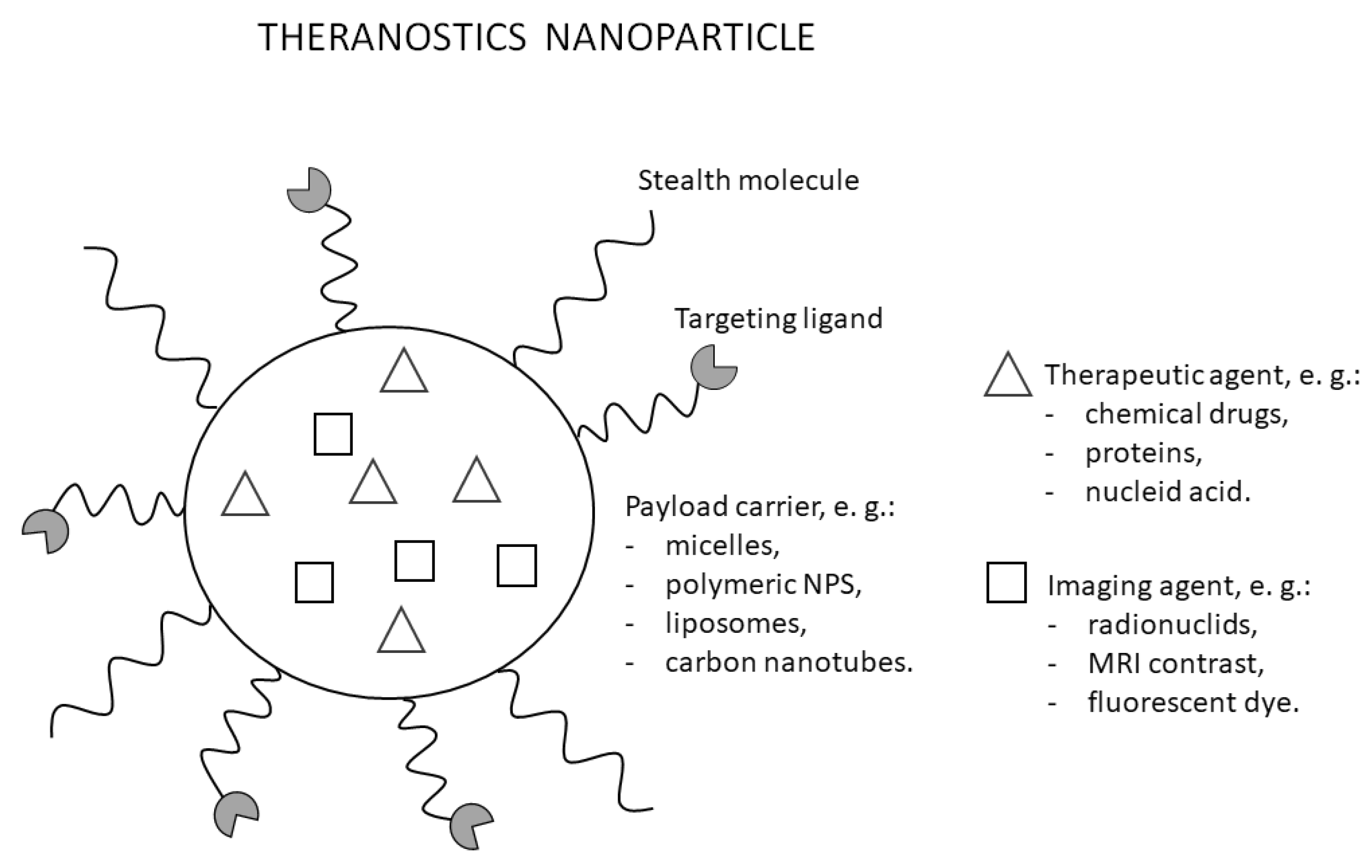
9. Theranostics
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Gozzo, L.; Tracia, L.; Cicciù, M.; Drago, F.; Bucolo, C.; Avitabile, T.; Rejdak, R.; Nowomiejska, K.; Zweifel, S.; et al. New Therapeutic Perspectives in the Treatment of Uveal Melanoma: A Systematic Review. Biomedicines 2021, 9, 1311. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Raciti, G.; Longo, A.; Reibaldi, M.; Bonfiglio, V.; Russo, A.; Caltabiano, R.; Gattuso, G.; Falzone, L.; Avitabile, T. Current molecular and clinical insights into uveal melanoma (Review). Int. J. Oncol. 2021, 58. [Google Scholar] [CrossRef] [PubMed]
- Aronow, M.E.; Topham, A.K.; Singh, A.D. Uveal melanoma: 5-year update on incidence, treatment, and survival (SEER 1973-2013). Ocul. Oncol. Pathol. 2018, 4, 145–151. [Google Scholar] [CrossRef]
- Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin. Ophthalmol. 2017, 11, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Måkitie, T.; Kivelä, T. Very Long-Term Prognosis of Patients with Malignant Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Diener-West, M.; Earle, J.D.; Fine, S.L.; Hawkins, B.S.; Moy, C.S.; Reynolds, S.M.; Schachat, A.P.; Straatsma, B.R. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch. Ophthalmol. 2001, 119, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, M.; Barbagallo, C.; Statello, L.; Caltabiano, R.; Russo, A.; Puzzo, L.; Avitabile, T.; Longo, A.; Toro, M.D.; Barbagallo, D.; et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol. Ther. 2015, 16, 1387–1396. [Google Scholar] [CrossRef]
- De Vita, V.T., Jr.; Lawrence, T.; Rosenberg, S.A. Cancer: Principles and Practice of Oncology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Van Poppelen, N.M.; de Bruyn, D.P.; Bicer, T.; Verdijk, R.; Naus, N.; Mensink, H.; Paridaens, D.; de Klein, A.; Brosens, E.; Kili, E. Genetics of Ocular Melanoma: Insights into Genetics, Inheritance and Testing. Int. J. Mol. Sci. 2020, 22, 336. [Google Scholar] [CrossRef]
- Chang, M.Y.; McCannel, T.A. Local treatment failure after globe-conserving therapy for choroidal melanoma. Br. J. Ophthalmol. 2013, 97, 804–811. [Google Scholar] [CrossRef]
- Chen, H. Recent developments in ocular drug delivery. J. Drug Target. 2015, 23, 597–604. [Google Scholar] [CrossRef]
- Awwad, S.; Mohamed Ahmed, A.H.A.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Occhiutto, M.L.; Freitas, F.R.; Maranhao, R.C.; Costa, V.P. Breakdown of the blood-ocular barrier as a strategy for the systemic use of nanosystems. Pharmaceutics 2012, 4, 252–275. [Google Scholar] [CrossRef]
- Tombran-Tink, J.; Barnstable, C. Ocular Transporters in Ophthalmic Diseases and Drug Delivery: Ophthalmology Research; Springer Science & Business Media: Basel, Switzerland, 2008. [Google Scholar]
- Geroski, D.H.; Edelhauser, H.F. Drug delivery for posterior segment eye disease. Investig. Ophthalmol. Vis. Sci. 2000, 41, 961–964. [Google Scholar]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef]
- Van den Aardweg, G.J.; Naus, N.C.; Verhoeven, A.C.; de Klein, A.; Luyten, G.P. Cellular radiosensitivity of primary and metastatic human uveal melanoma cell lines. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2561–2565. [Google Scholar]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef]
- Ciernik, I.F.; Wösle, M.; Krause, L.; Krayenbuehl, J. Optimizing radiosurgery with photons for ocular melanoma. Phys. Imaging Radiat. Oncol. 2018, 6, 83–88. [Google Scholar] [CrossRef]
- Hawkins, B.S. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am. J. Ophthalmol. 2004, 138, 936–951. [Google Scholar] [CrossRef]
- Vrabec, T.R.; Augsburger, J.J.; Gamel, J.W.; Brady, L.W.; Hernandez, C.; Woodleigh, R. Impact of local tumor relapse on patient survival after cobalt 60 plaque radiotherapy. Ophthalmology 1991, 98, 984–988. [Google Scholar] [CrossRef]
- Harbour, J.W.; Char, D.H.; Kroll, S.; Quivey, J.M.; Castro, J. Metastatic risk for distinct patterns of postirradiation local recurrence of posterior uveal melanoma. Ophthalmology 1997, 104, 1785–1792. [Google Scholar] [CrossRef]
- Bell, D.J.; Wilson, M.W. Choroidal Melanoma: Natural History and Management Options. Cancer Control 2004, 11, 296–303. [Google Scholar] [CrossRef]
- Hawkins, B.S. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef]
- Filì, M.; Trocme, E.; Bergman, L.; See, T.R.O.; André, H.; Bartuma, K.; Girnita, L.; All-Eriksson, C.; Seregard, S.; Stålhammar, G. Ruthenium-106 versus iodine-125 plaque brachytherapy of 571 choroidal melanomas with a thickness of ≥5.5 mm. Br. J. Ophthalmol. 2020, 104, 26–32. [Google Scholar] [CrossRef]
- Lommatzsch, P.K.; Werschnik, C.; Schuster, E. Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 129–137. [Google Scholar] [CrossRef]
- Marinkovic, M.; Horeweg, N.; Laman, M.S.; Bleeker, J.C.; Ketelaars, M.; Peters, F.P.; Luyten, G.P.M.; Creutzberg, C.L. Ruthenium-106 brachytherapy for iris and iridociliary melanomas. Br. J. Ophthalmol. 2018, 102, 1154–1159. [Google Scholar] [CrossRef]
- Ghassemi, F.; Sheibani, S.; Arjmand, M.; Poorbaygi, H.; Kouhestani, E.; Sabour, S.; Samiei, F.; Beiki-Ardakani, A.; Jabarvand, M.; Sadeghi Tari, A. Comparison of Iodide-125 and Ruthenium-106 Brachytherapy in the Treatment of Choroidal Melanomas. Clin. Ophthalmol. 2020, 14, 339–346. [Google Scholar] [CrossRef]
- Naseripour, M.; Jaberi, R.; Sedaghat, A.; Azma, Z.; Nojomi, M.; Falavarjani, K.G.; Nazari, H. Ruthenium-106 brachytherapy for thick uveal melanoma: Reappraisal of apex and base dose radiation and dose rate. J. Contemp. Brachytherapy 2016, 8, 66–73. [Google Scholar] [CrossRef]
- Badiyan, S.N.; Rao, R.C.; Apicelli, A.J.; Acharya, S.; Verma, V.; Garsa, A.A.; DeWees, T.; Speirs, C.K.; Garcia-Ramirez, J.; Esthappan, J.; et al. Outcomes of iodine-125 plaque brachytherapy for uveal melanoma with intraoperative ultrasonography and supplemental transpupillary thermotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 801–805. [Google Scholar] [CrossRef]
- Tabandeh, H.; Chaudhry, N.A.; Murray, T.G.; Ehlies, F.; Hughes, R.; Scott, I.U.; Markoe, A.M. Intraoperative echographic localization of iodine-125 episcleral plaque for brachytherapy of choroidal melanoma. Am. J. Ophthalmol. 2000, 129, 199–204. [Google Scholar] [CrossRef]
- Almony, A.; Breit, S.; Zhao, H.; Garcia-Ramirez, J.; Mansur, D.B.; Harbour, J.W. Tilting of radioactive plaques after initial accurate placement for treatment of uveal melanoma. Arch. Ophthalmol. 2008, 126, 65–70. [Google Scholar] [CrossRef]
- Simpson, E.R.; Gallie, B.; Laperrierre, N.; Beiki-Ardakani, A.; Kivelä, T.; Raivio, V.; American Brachytherapy Society-Ophthalmic Oncology Task Force. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy 2014, 13, 1–14. [Google Scholar] [CrossRef]
- Dunavoelgyi, R.; Dieckmann, K.; Gleiss, A.; Sacu, S.; Kircher, K.; Georgopoulos, M.; Georg, D.; Zehetmayer, M.; Poetter, R. Radiogenic side effects after hypofractionated stereotactic photon radiotherapy of choroidal melanoma in 212 patients treated between 1997 and 2007. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Saunders, W.M.; Char, D.H.; Quivey, J.M.; Castro, J.R.; Chen, G.T.; Collier, J.M.; Cartigny, A.; Blakely, E.A.; Lyman, J.T.; Zink, S.R.; et al. Precision, high dose radiotherapy: Helium ion treatment of uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 227–233. [Google Scholar] [CrossRef]
- Lane, A.M.; Kim, I.K.; Gragoudas, E.S. Long-term Risk of Melanoma-Related Mortality for Patients With Uveal Melanoma Treated With Proton Beam Therapy. JAMA Ophthalmol. 2015, 133, 792–796. [Google Scholar] [CrossRef]
- Tsuji, H.; Ishikawa, H.; Yanagi, T.; Hirasawa, N.; Kamada, T.; Mizoe, J.E.; Kanai, T.; Tsujii, H.; Ohnishi, Y. Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: A Phase I/II dose-escalation study. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Caujolle, J.P.; Mammar, H.; Chamorey, E.; Pinon, F.; Herault, J.; Gastaud, P. Proton beam radiotherapy for uveal melanomas at nice teaching hospital: 16 years’ experience. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 98–103. [Google Scholar] [CrossRef]
- Particle Therapy Co-Operative Group. Particle Therapy Facilities in Clinical Operation (Last Update: (November 2021). Available online: https://www.ptcog.ch/index.php/facilities-in-operation (accessed on 2 December 2021).
- Dendale, R.; Lumbroso-Le Rouic, L.; Noel, G.; Feuvret, L.; Levy, C.; Delacroix, S.; Meyer, A.; Nauraye, C.; Mazal, A.; Mammar, H.; et al. Proton beam radiotherapy for uveal melanoma: Results of Curie Institut–Orsay Proton Therapy Center (ICPO). Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 780–787. [Google Scholar] [CrossRef]
- Egger, E.; Schalenbourg, A.; Zografos, L.; Bercher, L.; Boehringer, T.; Chamot, L.; Goitein, G. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 138–147. [Google Scholar] [CrossRef]
- Peddada, K.V.; Sangani, R.; Menon, H.; Verma, V. Complications and adverse events of plaque brachytherapy for ocular melanoma. J. Contemp. Brachytherapy 2019, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Giuliari, G.P.; Sadaka, A.; Hinkle, D.M.; Simpson, E.R. Current treatments for radiation retinopathy. Acta Oncol. 2011, 50, 6–13. [Google Scholar] [CrossRef]
- Mills, M.D.; Harbour, J.W. Lipid exudation following plaque radiotherapy for posterior uveal melanoma. Am. J. Ophthalmol. 2006, 141, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, M.M.; Tagliaferri, L.; Azario, L.; Lenkowicz, J.; Lanza, A.; Autorino, R.; Caputo, C.G.; Gambacorta, M.A.; Valentini, V.; Blasi, M.A. Ruthenium brachytherapy for uveal melanomas: Factors affecting the development of radiation complications. Brachytherapy 2018, 17, 432–438. [Google Scholar] [CrossRef]
- Miguel, D.; de Frutos-Baraja, J.M.; López-Lara, F.; Antonia Saornil, M.; García-Alvarez, C.; Alonso, P.; Diezhandino, P. Visual outcome after posterior uveal melanoma episcleral brachytherapy including radiobiological doses. J. Contemp. Brachytherapy 2018, 10, 123–131. [Google Scholar] [CrossRef] [PubMed]
- McCannel, T.A. Post-brachytherapy tumor endoresection for treatment of toxic maculopathy in choroidal melanoma. Eye 2013, 27, 984–988. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Pagliara, M.M.; Masciocchi, C.; Scupola, A.; Azario, L.; Grimaldi, G.; Autorino, R.; Gambacorta, M.A.; Laricchiuta, A.; Boldrini, L.; et al. Nomogram for predicting radiation maculopathy in patients treated with Ruthenium-106 plaque brachytherapy for uveal melanoma. J. Contemp. Brachytherapy 2017, 9, 540–547. [Google Scholar] [CrossRef]
- Finger, P.T.; Chin, K.J.; Semenova, E.A. Intravitreal anti-VEGF therapy for macular radiation retinopathy: A 10-year study. Eur. J. Ophthalmol. 2016, 26, 60–66. [Google Scholar] [CrossRef]
- Mishra, K.K.; Daftari, I.K.; Weinberg, V.; Cole, T.; Quivey, J.M.; Castro, J.R.; Phillips, T.L.; Char, D.H. Risk factors for neovascular glaucoma after proton beam therapy of uveal melanoma: A detailed analysis of tumor and dose-volume parameters. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 330–336. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; Karlsson, U.; Markoe, A.M.; Brady, L.W. Reasons for enucleation after plaque radiotherapy for posterior uveal melanoma. Clinical findings. Ophthalmology 1989, 96, 919–923. [Google Scholar] [CrossRef]
- Piirtola, A.; Puska, P.; Kivelä, T. Red laser cyclophotocoagulation in the treatment of secondary glaucoma in eyes with uveal melanoma. J. Glaucoma 2014, 23, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Nagendran, S.T.; Finger, P.T. Anti-VEGF intravitreal bevacizumab for radiation-associated neovascular glaucoma. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; Caltabiano, R.; et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging-Part II: Treatment indications and complications. Insights Imaging 2021, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Tarlan, B.; Kıratlı, H. Uveal Melanoma: Current Trends in Diagnosis and Management. Turk. J. Ophthalmol. 2016, 46, 123–137. [Google Scholar] [CrossRef]
- Bechrakis, N.E.; Bornfeld, N.; Zöller, I.; Foerster, M.H. Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology 2002, 109, 1855–1861. [Google Scholar] [CrossRef]
- Caminal, J.M.; Padrón-Pérez, N.; Arias, L.; Masuet-Aumatell, C.; Gutiérrez, C.; Piulats, J.M.; Pera, J.; Català, J.; Rubio, M.J.; Arruga, J. Transscleral resection without hypotensive anaesthesia vs iodine-125 plaque brachytherapy in the treatment of choroidal melanoma. Eye 2016, 30, 833–842. [Google Scholar] [CrossRef]
- Willerding, G.D.; Cordini, D.; Moser, L.; Krause, L.; Foerster, M.H.; Bechrakis, N.E. Neoadjuvant proton beam irradiation followed by transscleral resection of uveal melanoma in 106 cases. Br. J. Ophthalmol. 2016, 100, 463–467. [Google Scholar] [CrossRef]
- Newman, D.K. Photodynamic therapy: Current role in the treatment of chorioretinal conditions. Eye 2016, 30, 202–210. [Google Scholar] [CrossRef]
- Blasi, M.A.; Pagliara, M.M.; Lanza, A.; Sammarco, M.G.; Caputo, C.G.; Grimaldi, G.; Scupola, A. Photodynamic Therapy in Ocular Oncology. Biomedicines 2018, 6, 17. [Google Scholar] [CrossRef]
- Leviskas, B.; Valyi-Nagy, T.; Munirathinam, G.; Bork, M.; Valyi-Nagy, K.; Skwor, T. Metalloporphyrin Pd(T4) Exhibits Oncolytic Activity and Cumulative Effects with 5-ALA Photodynamic Treatment against C918 Cells. Int. J. Mol. Sci. 2020, 21, 669. [Google Scholar] [CrossRef]
- Kawczyk-Krupka, A.; Bugaj, A.M.; Latos, W.; Zaremba, K.; Sieroń, A. Photodynamic therapy in treatment of cutaneous and choroidal melanoma. Photodiagnosis Photodyn. Ther. 2013, 10, 503–509. [Google Scholar] [CrossRef]
- Juan, L.; Diandian, W.; Jianfeng, W.; Ning, L.; Yuchen, F.; Na, L.; Sijie, Z.; Kun, L.; Fengyuan, S. Efficient Anticancer Effect on Choroidal Melanoma Cells Induced by Tanshinone IIA Photosensitization. Photochem. Photobiol. 2021, 97, 841–850. [Google Scholar] [CrossRef]
- Fabian, I.D.; Stacey, A.W.; Papastefanou, V.; Al Harby, L.; Arora, A.K.; Sagoo, M.S.; Cohen, V.M. Primary photodynamic therapy with verteporfin for small pigmented posterior pole choroidal melanoma. Eye 2017, 31, 519–528. [Google Scholar] [CrossRef]
- Turkoglu, E.B.; Pointdujour-Lim, R.; Mashayekhi, A.; Shields, C.L. PHOTODYNAMIC THERAPY AS PRIMARY TREATMENT FOR SMALL CHOROIDAL MELANOMA. Retina 2019, 39, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Rundle, P. Treatment of posterior uveal melanoma with multi-dose photodynamic therapy. Br. J. Ophthalmol. 2014, 98, 494. [Google Scholar] [CrossRef]
- Rundle, P. Photodynamic Therapy for Eye Cancer. Biomedicines 2017, 5, 69. [Google Scholar] [CrossRef]
- Roelofs, K.A.; Fabian, I.D.; Arora, A.K.; Cohen, V.M.L.; Sagoo, M.S. Long-term Outcomes of Small Pigmented Choroidal Melanoma Treated with Primary Photodynamic Therapy. Ophthalmol. Retin. 2021, 5, 468–478. [Google Scholar] [CrossRef]
- Yordi, S.; Soto, H.; Bowen, R.C.; Singh, A.D. Photodynamic therapy for choroidal melanoma: What is the response rate? Surv. Ophthalmol. 2021, 66, 552–559. [Google Scholar] [CrossRef]
- Chaussy, C.G.; Thüroff, S. High-Intensity Focused Ultrasound for the Treatment of Prostate Cancer: A Review. J. Endourol. 2017, 31, S30–S37. [Google Scholar] [CrossRef] [PubMed]
- Illing, R.O.; Kennedy, J.E.; Wu, F.; ter Haar, G.R.; Protheroe, A.S.; Friend, P.J.; Gleeson, F.V.; Cranston, D.W.; Phillips, R.R.; Middleton, M.R. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br. J. Cancer 2005, 93, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, Y.; Zhu, J.; Zhu, L.; Zhu, Y.; Hu, K.; Zhao, H. High intensity focused ultrasound (HIFU) for primary hepatocellular carcinoma: A single center experience. Int. J. Clin. Exp. Med. 2017, 10, 15432–15438. [Google Scholar]
- Kim, S.H.; Jung, S.E.; Kim, H.L.; Hahn, S.T.; Park, G.S.; Park, W.C. The potential role of dynamic MRI in assessing the effectiveness of high-intensity focused ultrasound ablation of breast cancer. Int. J. Hyperth. 2010, 26, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, Z.B.; Cao, Y.D.; Chen, W.Z.; Bai, J.; Zou, J.Z.; Zhu, H. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br. J. Cancer 2003, 89, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Fornage, B.D.; Hwang, R.F. Current status of imaging-guided percutaneous ablation of breast cancer. AJR. Am. J. Roentgenol. 2014, 203, 442–448. [Google Scholar] [CrossRef]
- Schmitz, A.C.; Gianfelice, D.; Daniel, B.L.; Mali, W.P.; van den Bosch, M.A. Image-guided focused ultrasound ablation of breast cancer: Current status, challenges, and future directions. Eur. Radiol. 2008, 18, 1431–1441. [Google Scholar] [CrossRef]
- Bohlmann, M.K.; Hoellen, F.; Hunold, P.; David, M. High-Intensity Focused Ultrasound Ablation of Uterine Fibroids—Potential Impact on Fertility and Pregnancy Outcome. Geburtshilfe Frauenheilkd. 2014, 74, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef]
- Zhou, Y.F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. [Google Scholar] [CrossRef]
- Ebbini, E.S.; ter Haar, G. Ultrasound-guided therapeutic focused ultrasound: Current status and future directions. International journal of hyperthermia: The official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. Int. J. Hyperth. 2015, 31, 77–89. [Google Scholar] [CrossRef]
- Ter Haar, G. Ultrasound focal beam surgery. Ultrasound Med. Biol. 1995, 21, 1089–1100. [Google Scholar] [CrossRef]
- Alkins, R.D.; Mainprize, T.G. High-Intensity Focused Ultrasound Ablation Therapy of Gliomas. Prog. Neurol. Surg. 2018, 32, 39–47. [Google Scholar] [CrossRef]
- Haut, J.; Colliac, J.P.; Falque, L.; Renard, Y. [Indications and results of Sonocare (ultrasound) in the treatment of ocular hypertension. A preliminary study of 395 cases]. Ophtalmol. Organe Soc. Fr. D’ophtalmol. 1990, 4, 138–141. [Google Scholar]
- Muratore, R. A History of the Sonocare CSt 100: The First FDa approved HIFU Device. AIP Conf. Proc. 2006, 829, 508–512. [Google Scholar]
- Aptel, F.; Charrel, T.; Palazzi, X.; Chapelon, J.-Y.; Denis, P.; Lafon, C. Histologic Effects of a New Device for High-Intensity Focused Ultrasound Cyclocoagulation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5092–5098. [Google Scholar] [CrossRef] [PubMed]
- Costley, D.; Mc Ewan, C.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating cancer with sonodynamic therapy: A review. Int. J. Hyperth. 2015, 31, 107–117. [Google Scholar] [CrossRef]
- Qian, X.; Zheng, Y.; Chen, Y. Micro/Nanoparticle-Augmented Sonodynamic Therapy (SDT): Breaking the Depth Shallow of Photoactivation. Adv. Mater. 2016, 28, 8097–8129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Gao, Y.; Zheng, B.; Tang, F.; Huang, J. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov. Today 2014, 19, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Rengeng, L.; Qianyu, Z.; Yuehong, L.; Zhongzhong, P.; Libo, L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017, 19, 159–166. [Google Scholar] [CrossRef] [PubMed]
- McEwan, C.; Nesbitt, H.; Nicholas, D.; Kavanagh, O.N.; McKenna, K.; Loan, P.; Jack, I.G.; McHale, A.P.; Callan, J.F. Comparing the efficacy of photodynamic and sonodynamic therapy in non-melanoma and melanoma skin cancer. Bioorg. Med. Chem. 2016, 24, 3023–3028. [Google Scholar] [CrossRef]
- Dai, S.; Xu, C.; Tian, Y.; Cheng, W.; Li, B. In vitro stimulation of calcium overload and apoptosis by sonodynamic therapy combined with hematoporphyrin monomethyl ether in C6 glioma cells. Oncol. Lett. 2014, 8, 1675–1681. [Google Scholar] [CrossRef]
- Su, X.; Wang, P.; Wang, X.; Cao, B.; Li, L.; Liu, Q. Apoptosis of U937 cells induced by hematoporphyrin monomethyl ether-mediated sonodynamic action. Cancer Biother. Radiopharm. 2013, 28, 207–217. [Google Scholar] [CrossRef]
- Su, X.; Li, Y.; Wang, P.; Wang, X.; Liu, Q. Protoporphyrin IX-mediated sonodynamic action induces apoptosis of K562 cells. Ultrasonics 2014, 54, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Yumita, N.; Nishigaki, R.; Umemura, K.; Umemura, S. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn. J. Cancer Res. Gann 1989, 80, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, H.; Wang, S.; Sun, X.; Wang, L.; Wang, W.; Shen, H.; Liu, H. Sonodynamic therapy (SDT): A novel strategy for cancer nanotheranostics. Sci. China. Life Sci. 2018, 61, 415–426. [Google Scholar] [CrossRef]
- Harada, Y.; Ogawa, K.; Irie, Y.; Endo, H.; Feril, L.B., Jr.; Uemura, T.; Tachibana, K. Ultrasound activation of TiO2 in melanoma tumors. J. Control. Release Off. J. Control. Release Soc. 2011, 149, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Khan-Lim, D.; Berry, M. Still confused about rose bengal? Curr. Eye Res. 2004, 29, 311–317. [Google Scholar] [CrossRef]
- Mousavi, H.; Zhang, X.; Gillespie, S.; Wachter, E.; Hersey, P. Rose Bengal induces dual modes of cell death in melanoma cells and has clinical activity against melanoma. Melanoma Res. 2006, 16, S8. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Mujtaba, S.F.; Dwivedi, A.; Amar, S.K.; Goyal, S.; Verma, A.; Kushwaha, H.N.; Chaturvedi, R.K.; Ray, R.S. Photosensitized rose Bengal-induced phototoxicity on human melanoma cell line under natural sunlight exposure. J. Photochem. Photobiol. B Biol. 2016, 156, 87–99. [Google Scholar] [CrossRef]
- Wan, G.Y.; Liu, Y.; Chen, B.W.; Liu, Y.Y.; Wang, Y.S.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef]
- Hoogenboom, M.; Eikelenboom, D.; den Brok, M.H.; Heerschap, A.; Fütterer, J.J.; Adema, G.J. Mechanical high-intensity focused ultrasound destruction of soft tissue: Working mechanisms and physiologic effects. Ultrasound Med. Biol. 2015, 41, 1500–1517. [Google Scholar] [CrossRef]
- Huang, D.; Chen, Y.S.; Rupenthal, I.D. Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Deliv. Rev. 2018, 126, 96–112. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Pe’er, J.; Domb, A.J. New techniques for drug delivery to the posterior eye segment. Pharm. Res. 2010, 27, 530–543. [Google Scholar] [CrossRef]
- Gower, N.J.D.; Barry, R.J.; Edmunds, M.R.; Titcomb, L.C.; Denniston, A.K. Drug discovery in ophthalmology: Past success, present challenges, and future opportunities. BMC Ophthalmol. 2016, 16, 11. [Google Scholar] [CrossRef]
- EyeGate Awarded New U.S. Patent for Iontophoretic Delivery of Corticosteroids to the Eye. Available online: https://kiorapharma.com/eyegate-awarded-new-u-s-patent-for-iontophoretic-delivery-of-corticosteroids-to-the-eye/ (accessed on 22 December 2021).
- Hayden, B.; Jockovich, M.-E.; Murray, T.G.; Kralinger, M.T.; Voigt, M.; Hernandez, E.; Feuer, W.; Parel, J.-M. Iontophoretic Delivery of Carboplatin in a Murine Model of Retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3717–3721. [Google Scholar] [CrossRef]
- Hayden, B.; Jockovich, M.-E.; Murray, T.; Voigt, M.; Milne, P.; Kralinger, M.; Feuer, W.; Hernandez, E.; Parel, J.-M. Pharmacokinetics of Systemic Versus Focal Carboplatin Chemotherapy in the Rabbit Eye: Possible Implication in the Treatment of Retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3644–3649. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Chiang, B.; Grossniklaus, H.E.; Prausnitz, M.R. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J. Control. Release Off. J. Control. Release Soc. 2018, 277, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.G.; Testori, A.; Curatolo, P.; Quaglino, P.; Mocellin, S.; Framarini, M.; Borgognoni, L.; Ascierto, P.A.; Mozzillo, N.; Guida, M.; et al. Treatment efficacy with electrochemotherapy: A multi-institutional prospective observational study on 376 patients with superficial tumors. Eur. J. Surg. Oncol. 2016, 42, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Maglietti, F.; Tellado, M.; Olaiz, N.; Michinski, S.; Marshall, G. Minimally Invasive Electrochemotherapy Procedure for Treating Nasal Duct Tumors in Dogs using a Single Needle Electrode. Radiol. Oncol. 2017, 51, 422–430. [Google Scholar] [CrossRef]
- De Vry, J.; Martinez-Martinez, P.; Losen, M.; Temel, Y.; Steckler, T.; Steinbusch, H.W.; De Baets, M.H.; Prickaerts, J. In vivo electroporation of the central nervous system: A non-viral approach for targeted gene delivery. Prog. Neurobiol. 2010, 92, 227–244. [Google Scholar] [CrossRef]
- Cadossi, R.; Ronchetti, M.; Cadossi, M. Locally enhanced chemotherapy by electroporation: Clinical experiences and perspective of use of electrochemotherapy. Future Oncol. 2014, 10, 877–890. [Google Scholar] [CrossRef]
- Fiorentzis, M.; Katopodis, P.; Kalirai, H.; Seitz, B.; Viestenz, A.; Coupland, S.E. Image Analysis of 3D Conjunctival Melanoma Cell Cultures Following Electrochemotherapy. Biomedicines 2020, 8, 158. [Google Scholar] [CrossRef]
- Fiorentzis, M.; Viestenz, A.; Siebolts, U.; Seitz, B.; Coupland, S.E.; Heinzelmann, J. The Potential Use of Electrochemotherapy in the Treatment of Uveal Melanoma: In Vitro Results in 3D Tumor Cultures and In Vivo Results in a Chick Embryo Model. Cancers 2019, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Fiorentzis, M.; Katopodis, P.; Kalirai, H.; Seitz, B.; Viestenz, A.; Coupland, S.E. Conjunctival melanoma and electrochemotherapy: Preliminary results using 2D and 3D cell culture models in vitro. Acta Ophthalmol. 2019, 97, e632–e640. [Google Scholar] [CrossRef] [PubMed]
- Mandel, Y.; Rubinsky, B. Treatment of Uveal Melanoma by Nonthermal Irreversible Electroporation: Electrical and Bioheat Finite Element Model of the Human Eye. J. Heat Transf. 2012, 134, 111101. [Google Scholar] [CrossRef]
- Mandel, Y.; Frenkel, S.; Laufer, S.; Rubinsky, B.; Belkin, M.; Pe’er, J. Treatment Of Uveal Melanoma By Non-thermal Irreversible Electroporation—Mathematical Model, Animal And Preliminary Ex-vivo Human Experiments. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3284. [Google Scholar]
- Walia, S.; Acharya, A. Theragnosis: Nanoparticles as a Tool for Simultaneous Therapy and Diagnosis. In Nanoscale Materials in Targeted Drug Delivery, Theragnosis and Tissue Regeneration; Yadav, S.K., Ed.; Springer: Singapore, 2016; pp. 127–152. [Google Scholar]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjugate Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Siddiqui, R. Application and Importance of Theranostics in the Diagnosis and Treatment of Cancer. Arch. Med. Res. 2021, 52, 131–142. [Google Scholar] [CrossRef]
- Bhujwalla, Z.M.; Kakkad, S.; Chen, Z.; Jin, J.; Hapuarachchige, S.; Artemov, D.; Penet, M.F. Theranostics and metabolotheranostics for precision medicine in oncology. J. Magn. Reson. 2018, 291, 141–151. [Google Scholar] [CrossRef]
- De Jong, M.; Breeman, W.A.; Kwekkeboom, D.J.; Valkema, R.; Krenning, E.P. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc. Chem. Res. 2009, 42, 873–880. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.H. An introduction to the clinical practice of theranostics in oncology. Br. J. Radiol. 2018, 91, 20180440. [Google Scholar] [CrossRef]
- Turner, J.H. Recent advances in theranostics and challenges for the future. Br. J. Radiol. 2018, 91, 20170893. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Liao, J.; Qi, T.; Chu, B.; Peng, J.; Luo, F.; Qian, Z. Multifunctional nanostructured materials for multimodal cancer imaging and therapy. J. Nanosci. Nanotechnol. 2014, 14, 175–189. [Google Scholar] [CrossRef]
- Chen, F.; Ehlerding, E.B.; Cai, W. Theranostic nanoparticles. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2014, 55, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Mintri, S.; Menon, A.V.; Lee, H.Y.; Choi, H.S.; Kim, J. Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale 2015, 7, 18848–18862. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.W.; Kim, I.S.; et al. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. J. Control. Release Off. J. Control. Release Soc. 2010, 146, 219–227. [Google Scholar] [CrossRef]
- Jha, A.; Viswanadh, M.K.; Burande, A.S.; Mehata, A.K.; Poddar, S.; Yadav, K.; Mahto, S.K.; Parmar, A.S.; Muthu, M.S. DNA biodots based targeted theranostic nanomedicine for the imaging and treatment of non-small cell lung cancer. Int. J. Biol. Macromol. 2020, 150, 413–425. [Google Scholar] [CrossRef]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G.P.; Valapala, M.; Kerur, N.; Passaglia, C.L.; et al. Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef] [PubMed]
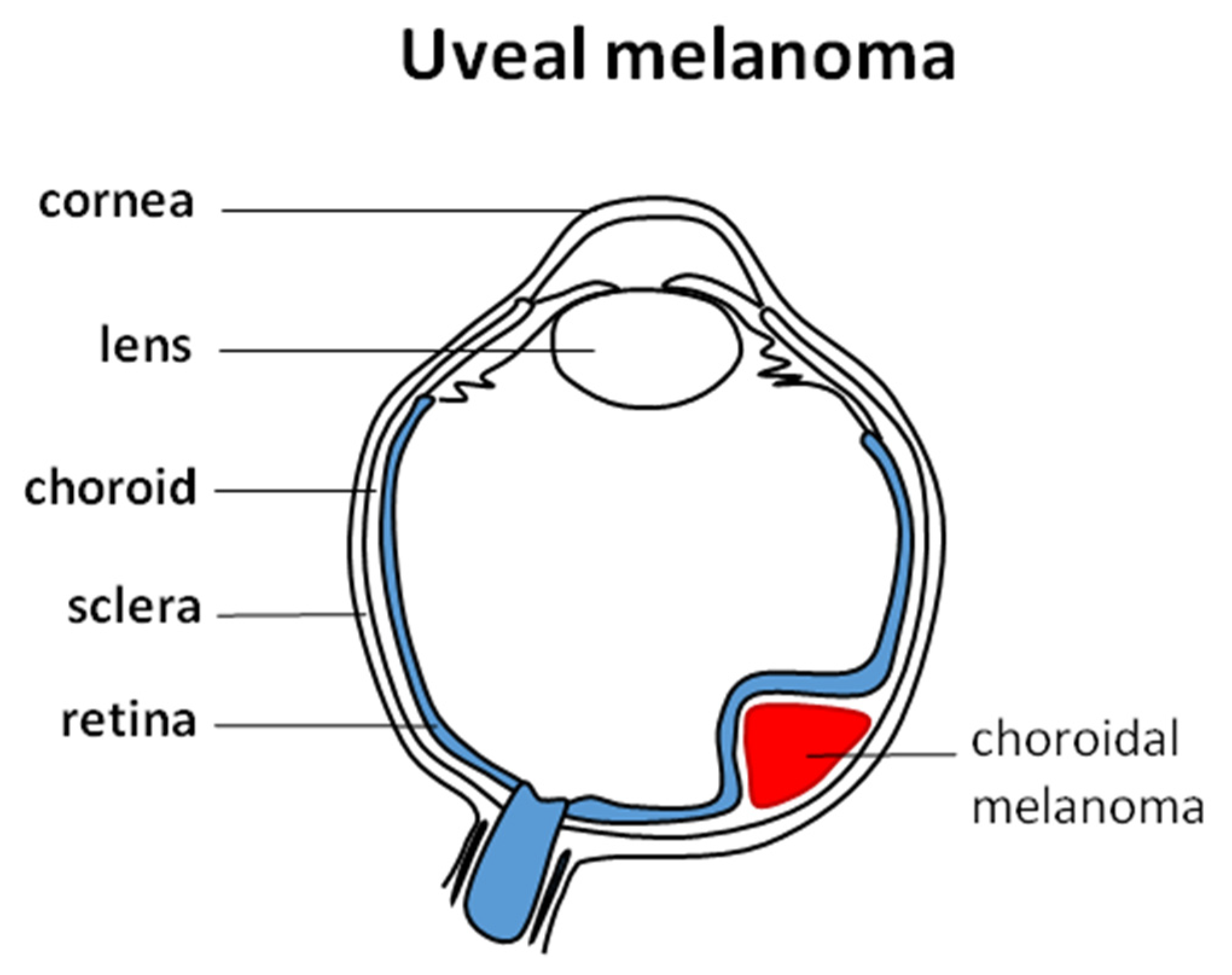
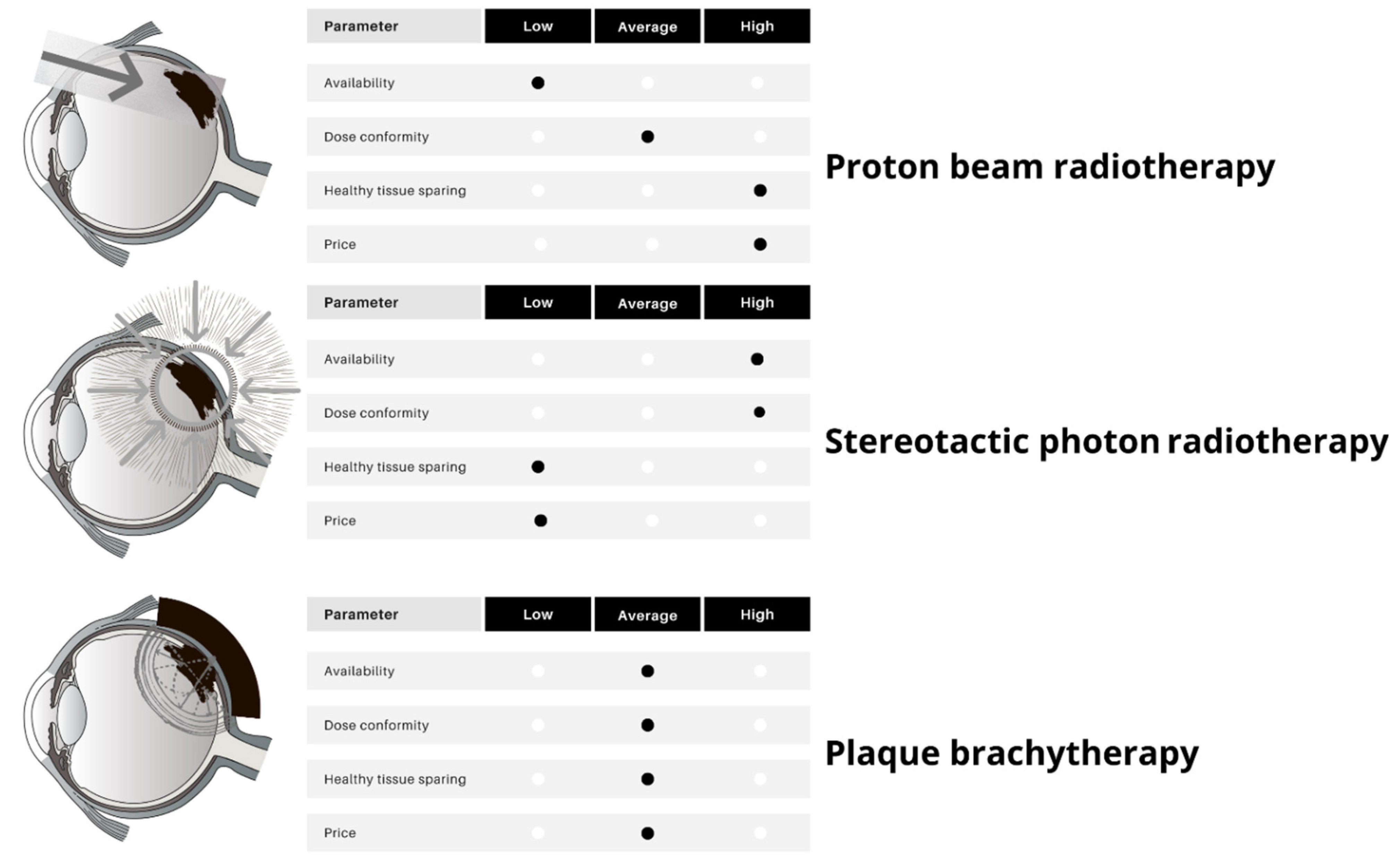
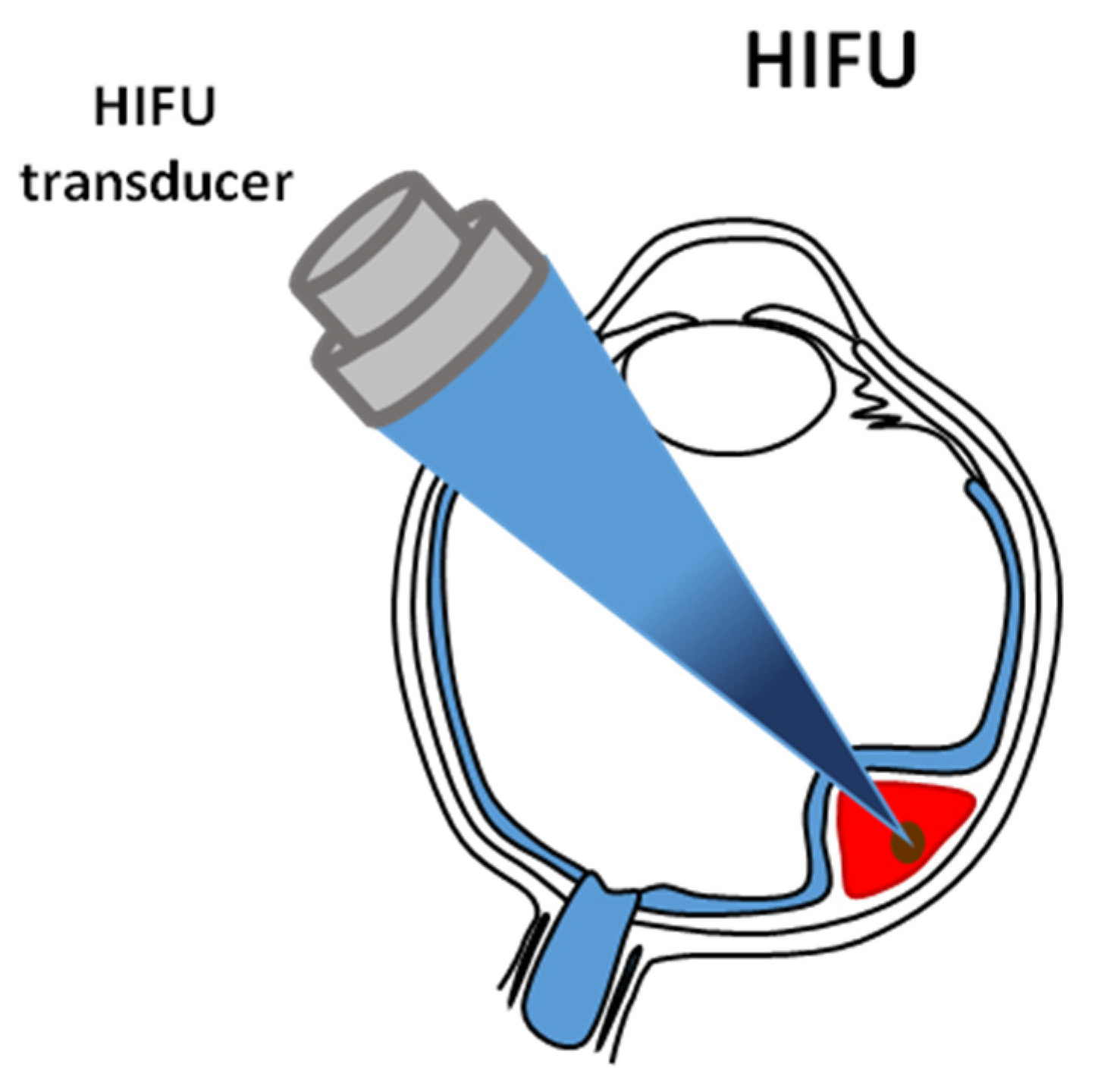
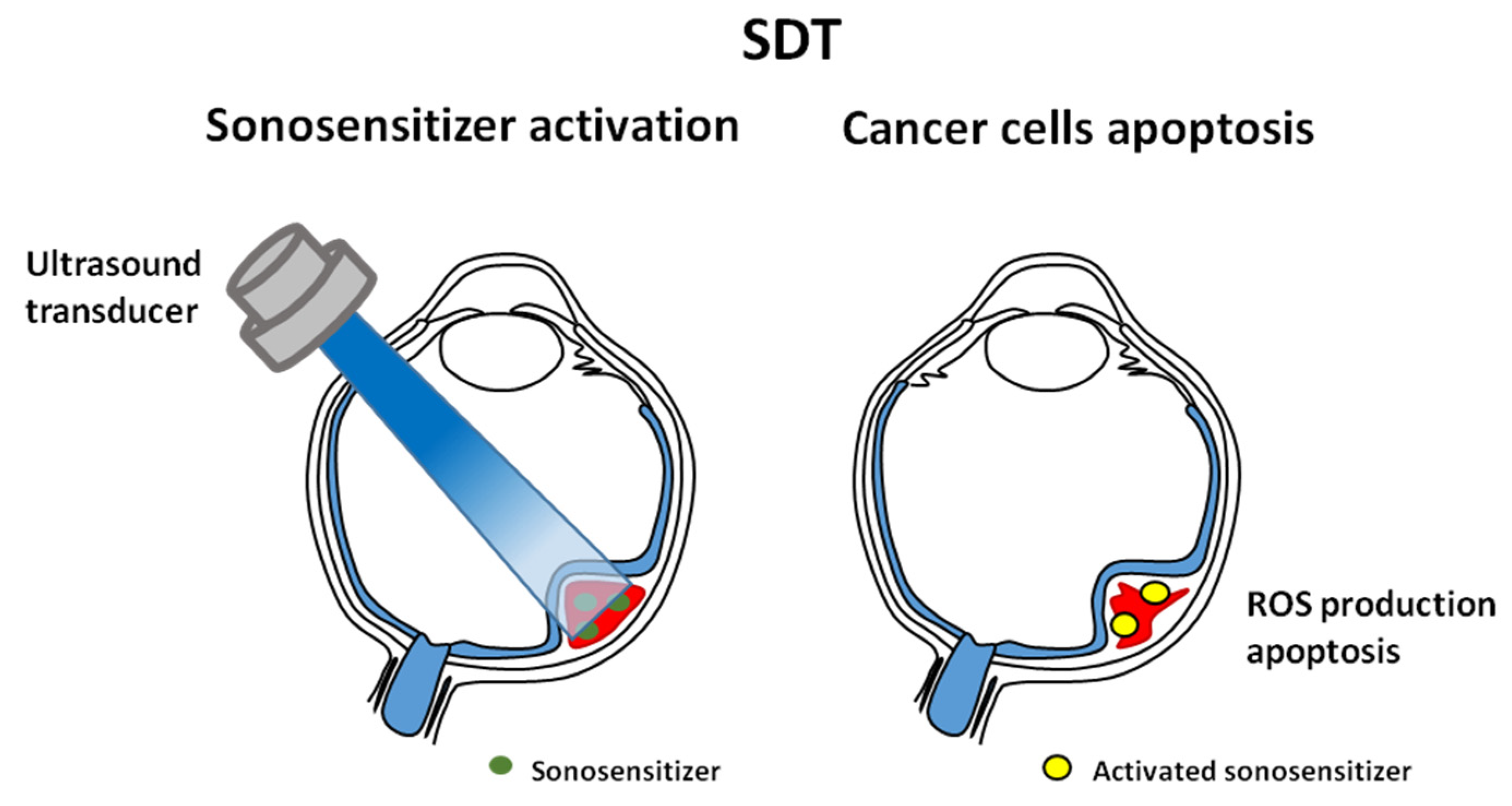
| Brachytherapy | Particle Therapy | Photon Stereotactic Body Radiotherapy | |
|---|---|---|---|
| Availability | Moderate | Low | High |
| Cost | Moderate | High | Low |
| Tumor size | Small, medium | Medium, large | Medium, large |
| Specific toxicity | Visual acuity loss, immediate procedural discomfort | Anterior eye complications | |
| Indications | Majority of uveal melanomas (also with limited extrascleral extension) | Tumors surrounding the optic disk and fovea; an attempt of eye-sparing treatment in large tumors | Rapidly growing tumors |
| Particular contraindications | Gross orbital extension, blind painful eyes, no light perception | None | Young age predicted long survival (higher late complications rate) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilmin, K.; Synoradzki, K.J.; Czarnecka, A.M.; Spałek, M.J.; Kujawska, T.; Solnik, M.; Merks, P.; Toro, M.D.; Rejdak, R.; Fiedorowicz, M. New Perspectives for Eye-Sparing Treatment Strategies in Primary Uveal Melanoma. Cancers 2022, 14, 134. https://doi.org/10.3390/cancers14010134
Bilmin K, Synoradzki KJ, Czarnecka AM, Spałek MJ, Kujawska T, Solnik M, Merks P, Toro MD, Rejdak R, Fiedorowicz M. New Perspectives for Eye-Sparing Treatment Strategies in Primary Uveal Melanoma. Cancers. 2022; 14(1):134. https://doi.org/10.3390/cancers14010134
Chicago/Turabian StyleBilmin, Krzysztof, Kamil J. Synoradzki, Anna M. Czarnecka, Mateusz J. Spałek, Tamara Kujawska, Małgorzata Solnik, Piotr Merks, Mario Damiano Toro, Robert Rejdak, and Michał Fiedorowicz. 2022. "New Perspectives for Eye-Sparing Treatment Strategies in Primary Uveal Melanoma" Cancers 14, no. 1: 134. https://doi.org/10.3390/cancers14010134
APA StyleBilmin, K., Synoradzki, K. J., Czarnecka, A. M., Spałek, M. J., Kujawska, T., Solnik, M., Merks, P., Toro, M. D., Rejdak, R., & Fiedorowicz, M. (2022). New Perspectives for Eye-Sparing Treatment Strategies in Primary Uveal Melanoma. Cancers, 14(1), 134. https://doi.org/10.3390/cancers14010134











