Allogenic Stem Cell Transplantation Abrogates Negative Impact on Outcome of AML Patients with KMT2A Partial Tandem Duplication
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Outcome Parameters
2.3. Treatments
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Overall Outcomes
3.3. Molecular Characteristics of KMT2A-PTD Patients and Their Impact on Intensively Treated Patients Outcome
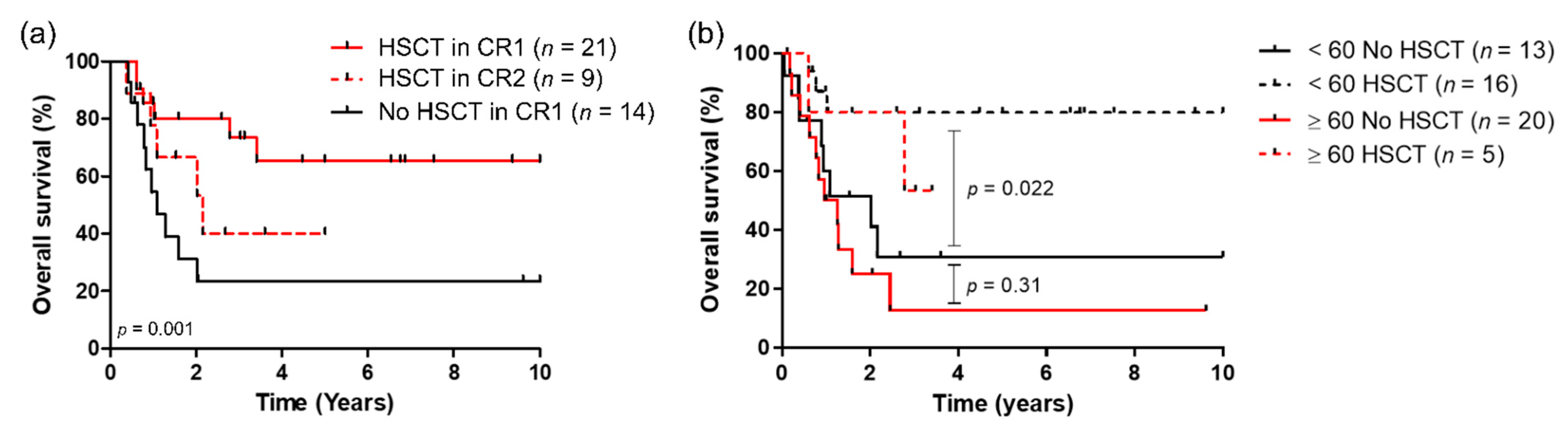
3.4. Impact of HSCT on Outcome
3.5. Univariate and Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Huet, S.; Paubelle, E.; Lours, C.; Grange, B.; Courtois, L.; Chabane, K.; Charlot, C.; Mosnier, I.; Simonet, T.; Hayette, S.; et al. Validation of the prognostic value of the knowledge bank approach to determine AML prognosis in real life. Blood 2018, 132, 865–867. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Campbell, P.J.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Schoch, C.; Schnittger, S.; Klaus, M.; Kern, W.; Hiddemann, W.; Haferlach, T. AML with 11q23/MLL abnormalities as defined by the WHO classification: Incidence, partner chromosomes, FAB subtype, age distribution, and prognostic impact in an unselected series of 1897 cytogenetically analyzed AML cases. Blood 2003, 102, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Vetro, C.; Haferlach, T.; Meggendorfer, M.; Stengel, A.; Jeromin, S.; Kern, W.; Haferlach, C. Cytogenetic and molecular genetic characterization of KMT2A-PTD positive acute myeloid leukemia in comparison to KMT2A-Rearranged acute myeloid leukemia. Cancer Genet. 2020, 240, 15–22. [Google Scholar] [CrossRef]
- Steudel, C.; Wermke, M.; Schaich, M.; Schäkel, U.; Illmer, T.; Ehninger, G.; Thiede, C. Comparative analysis of MLL partial tandem duplication and FLT3 internal tandem duplication mutations in 956 adult patients with acute myeloid leukemia. Genes Chromosom. Cancer 2003, 37, 237–251. [Google Scholar] [CrossRef]
- Schnittger, S.; Kinkelin, U.; Schoch, C.; Heinecke, A.; Haase, D.; Haferlach, T.; Büchner, T.; Wörmann, B.; Hiddemann, W.; Griesinger, F. Screening for MLL tandem duplication in 387 unselected patients with AML identify a prognostically unfavorable subset of AML. Leukemia 2000, 14, 796–804. [Google Scholar] [CrossRef]
- Dorrance, A.M.; Liu, S.; Yuan, W.; Becknell, B.; Arnoczky, K.J.; Guimond, M.; Strout, M.P.; Feng, L.; Nakamura, T.; Yu, L.; et al. Mll partial tandem duplication induces aberrant Hox expression in vivo via specific epigenetic alterations. J. Clin. Investig. 2006, 116, 2707–2716. [Google Scholar] [CrossRef]
- Hinai, A.S.A.A.; Pratcorona, M.; Grob, T.; Kavelaars, F.G.; Bussaglia, E.; Sanders, M.A.; Nomdedeu, J.; Valk, P.J.M. The landscape of KMT2A -PTD AML: Concurrent mutations, gene expression signatures, and clinical outcome. HemaSphere 2019, 3, 2–6. [Google Scholar] [CrossRef]
- Schnittger, B.S.; Wo, B.; Hiddemann, W.; Griesinger, F. Partial Tandem Duplications of the MLL Gene Are Detectable in Peripheral Blood and Bone Marrow of Nearly All Healthy Donors. Blood 1998, 92, 1728–1734. [Google Scholar] [CrossRef]
- Döhner, K.; Tobis, K.; Ulrich, R.; Fröhling, S.; Benner, A.; Schlenk, R.F.; Döhner, H. Prognostic Significance of Partial Tandem Duplications of the MLL Gene in Adult Patients 16 to 60 Years Old with Acute Myeloid Leukemia and Normal Cytogenetics: A Study of the Acute Myeloid Leukemia Study Group Ulm. J. Clin. Oncol. 2002, 20, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, C.; Haferlach, T. Another piece of the AML puzzle. Blood J. Am. Soc. Hematol. 2013, 122, 2532–2534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simons, A.; Shaffer, L.G.; Hastings, R.J. Cytogenetic Nomenclature: Changes in the ISCN 2013 Compared to the 2009 Edition. Cytogenet. Genome Res. 2013, 141, 1–6. [Google Scholar] [CrossRef]
- Fattoum, J.; Cannas, G.; Elhamri, M.; Tigaud, I.; Plesa, A.; Heiblig, M.; Plesa, C.; Wattel, E.; Thomas, X. Effect of Age on Treatment Decision-Making in Elderly Patients with Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2015, 15, 477–483. [Google Scholar] [CrossRef]
- Shih, L.Y.; Liang, D.C.; Fu, J.F.; Wu, J.H.; Wang, P.N.; Lin, T.L.; Dunn, P.; Kuo, M.C.; Tang, T.C.; Lin, T.H.; et al. Characterization of fusion partner genes in 114 patients with de novo acute myeloid leukemia and MLL rearrangement. Leukemia 2006, 20, 218–223. [Google Scholar] [CrossRef]
- Shimada, A.; Taki, T.; Tabuchi, K.; Taketani, T.; Hanada, R.; Tawa, A.; Tsuchida, M.; Horibe, K.; Tsukimoto, I.; Hayashi, Y. Tandem Duplications of MLL and FLT3 Are Correlated with Poor Prognoses in Pediatric Acute Myeloid Leukemia: A Study of the Japanese Childhood AML Cooperative Study Group. Pediatr. Blood Cancer 2008, 50, 1018–1025. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Bug, G.; Baron, F.; Brissot, E.; Ciceri, F.; Dalle, I.A.; Döhner, H.; Esteve, J.; Floisand, Y.; Giebel, S.; et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3-internal tandem duplication: A position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow. Haematologica 2020, 105, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Kayser, S.; Bullinger, L. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood 2014, 124, 3441–3449. [Google Scholar] [CrossRef]
- Tan, Y.-T.; Sun, Y.; Zhu, S.-H.; Ye, L.; Zhao, C.-J.; Zhao, W.-L.; Chen, Z.; Chen, S.-J.; Liu, H. Deregulation of HOX genes by DNMT3A and MLL mutations converges on BMI1. Leukemia 2016, 30, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Oran, B.; de Lima, M.; Garcia-Manero, G.; Thall, P.F.; Lin, R.; Popat, U.; Alousi, M.; Hosing, C.; Giralt, S.; Rondon, G.; et al. A phase 3 randomized study of 5-azacitidine maintenance vs. observation after transplant in high-risk AML and MDS patients. Blood Adv. 2020, 4, 5580–5588. [Google Scholar] [CrossRef] [PubMed]
- Bug, G.; Burchert, A.; Wagner, E.M.; Kröger, N.; Berg, T.; Güller, S.; Metzelder, S.K.; Wolf, A.; Hünecke, S.; Bader, B.; et al. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST trial). Leukemia 2017, 31, 2523–2525. [Google Scholar] [CrossRef]
- Oshikawa, G.; Kakihana, K.; Saito, M.; Aoki, J.; Najima, Y.; Kobayashi, T. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br. J. Haematol. 2015, 169, 756–759. [Google Scholar] [CrossRef]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- Kühn, M.W.; Hadler, M.J.; Daigle, S.R.; Koche, R.P.; Krivtsov, A.V.; Olhava, E.J.; Caligiuri, M.A.; Huang, G.; Bradner, J.E.; Pollock, M.; et al. MLL partial tandem duplication leukemia cells are sensitive to small molecule DOT1L inhibition. Haematologica 2015, 100, e190–e193. [Google Scholar] [CrossRef][Green Version]
- Lonetti, A.; Indio, V.; Laginestra, M.A.; Tarantino, G.; Chiarini, F.; Astolfi, A.; Bertuccio, S.N.; Martelli, A.M.; Locatelli, F.; Pession, A.; et al. Inhibition of Methyltransferase DOT1L Sensitizes to Sorafenib Treatment AML Cells Irrespective of MLL-Rearrangements: A Novel Therapeutic Strategy for Pediatric AML. Cancers 2020, 12, 1972. [Google Scholar] [CrossRef]
- Liu, W.; Deng, L.; Song, Y.; Redell, M. DOT1L inhibition sensitizes MLL-rearranged AML to chemotherapy. PLoS ONE 2014, 9, e98270. [Google Scholar] [CrossRef]
- Stein, E.M.; Garcia-Manero, G.; Rizzieri, D.A.; Tibes, R.; Berdeja, J.G.; Savona, M.R.; Jongen-Lavrenic, M.; Altman, J.K.; Thomson, B.; Blakemore, S.J.; et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood 2018, 131, 2661–2669. [Google Scholar] [CrossRef]
- Brzezinka, K.; Nevedomskaya, E.; Lesche, R.; Steckel, M.; Eheim, A.L.; Haegebarth, A.; Stresemann, C. Functional diversity of inhibitors tackling the differentiation blockage of MLL-rearranged leukemia. J. Hematol. Oncol. 2019, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.; Dai, B.; Gentles, A.J.; Majeti, R.; Feinberg, A.P. An LSC epigenetic signature is largely mutation independent and implicates the HOXA cluster in AML pathogenesis. Nat. Commun. 2015, 6, 8489. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A.; Stevens, B.M.; Jones, C.L.; Winters, A.; Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Culp-Hill, R.; Riemondy, K.A.; Gillen, A.E.; et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat. Med. 2018, 24, 1859–1866. [Google Scholar] [CrossRef] [PubMed]


| Variable | Total (n = 79) | |
|---|---|---|
Age at diagnosis, median (min–max)
| 66 (19–87) 9/79 (11.4%) 21/79 (26.6%) 49/79 (62%) | |
| PS ≥ 2, n (%) | 12/50 (24%) | |
| Sex ratio M/F | 1.1 | |
| Normal karyotype, n (%) | 47/72 (65.3%) | |
| FLT3-ITD, n (%) | 20/70 (28.6%) | |
| NPM1, n (%) | 12/75 (16%) | |
| IDH1/2, n (%) | 20/40 (50%) | |
ELN 2013 classification
| 8/70 (11.4%) 54/70 (77.2%) 8/70 (11.4%) | |
| Secondary AML, n (%) | 20/79 (25.2%) | |
| NGS at diagnosis, n (%) | 24/79 (30.4%) | |
| Molecular subgroups | DNMT3A, n (%) | 12/24 (50%) |
| ASXL1, n (%) | 2/24 (8.3%) | |
| RUNX1, n (%) | 6/24 (25%) | |
| TET2, n (%) | 5/24 (20.8%) | |
| TP53, n (%) | 1/24 (4.2%) | |
| SRSF2, n (%) | 5/24 (20.8%) | |
| STAG2, n (%) | 5/24 (20.8%) | |
| RAS/PTPN11, n (%) | 4/24 (16.7%) | |
| Variables | OS | LFS | ||||
|---|---|---|---|---|---|---|
| HR (IC 95%) | Range | p-Value | HR (IC 95%) | Range | p-Value | |
| Age < 60 vs. ≥60 years old | 1.74 | (0.77–3.91) | 0.180 | 2.15 | (0.99–4.67) | 0.053 |
| HSCT (yes vs. no) | 2.35 | (1.06–5.19) | 0.034 | 2.36 | (1.08–5.13) | 0.031 |
| FLT3-ITD (mut vs. wt) | 0.29 | (0.11–0.78) | 0.014 | 0.27 | (0.11–0.70) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antherieu, G.; Bidet, A.; Huet, S.; Hayette, S.; Migeon, M.; Boureau, L.; Sujobert, P.; Thomas, X.; Ghesquières, H.; Pigneux, A.; et al. Allogenic Stem Cell Transplantation Abrogates Negative Impact on Outcome of AML Patients with KMT2A Partial Tandem Duplication. Cancers 2021, 13, 2272. https://doi.org/10.3390/cancers13092272
Antherieu G, Bidet A, Huet S, Hayette S, Migeon M, Boureau L, Sujobert P, Thomas X, Ghesquières H, Pigneux A, et al. Allogenic Stem Cell Transplantation Abrogates Negative Impact on Outcome of AML Patients with KMT2A Partial Tandem Duplication. Cancers. 2021; 13(9):2272. https://doi.org/10.3390/cancers13092272
Chicago/Turabian StyleAntherieu, Gabriel, Audrey Bidet, Sarah Huet, Sandrine Hayette, Marina Migeon, Lisa Boureau, Pierre Sujobert, Xavier Thomas, Hervé Ghesquières, Arnaud Pigneux, and et al. 2021. "Allogenic Stem Cell Transplantation Abrogates Negative Impact on Outcome of AML Patients with KMT2A Partial Tandem Duplication" Cancers 13, no. 9: 2272. https://doi.org/10.3390/cancers13092272
APA StyleAntherieu, G., Bidet, A., Huet, S., Hayette, S., Migeon, M., Boureau, L., Sujobert, P., Thomas, X., Ghesquières, H., Pigneux, A., & Heiblig, M. (2021). Allogenic Stem Cell Transplantation Abrogates Negative Impact on Outcome of AML Patients with KMT2A Partial Tandem Duplication. Cancers, 13(9), 2272. https://doi.org/10.3390/cancers13092272






