The Effect of Diffuse Liver Diseases on the Occurrence of Liver Metastases in Cancer Patients: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Eligibility
2.2. Outcomes and Rationale of the Comparisons
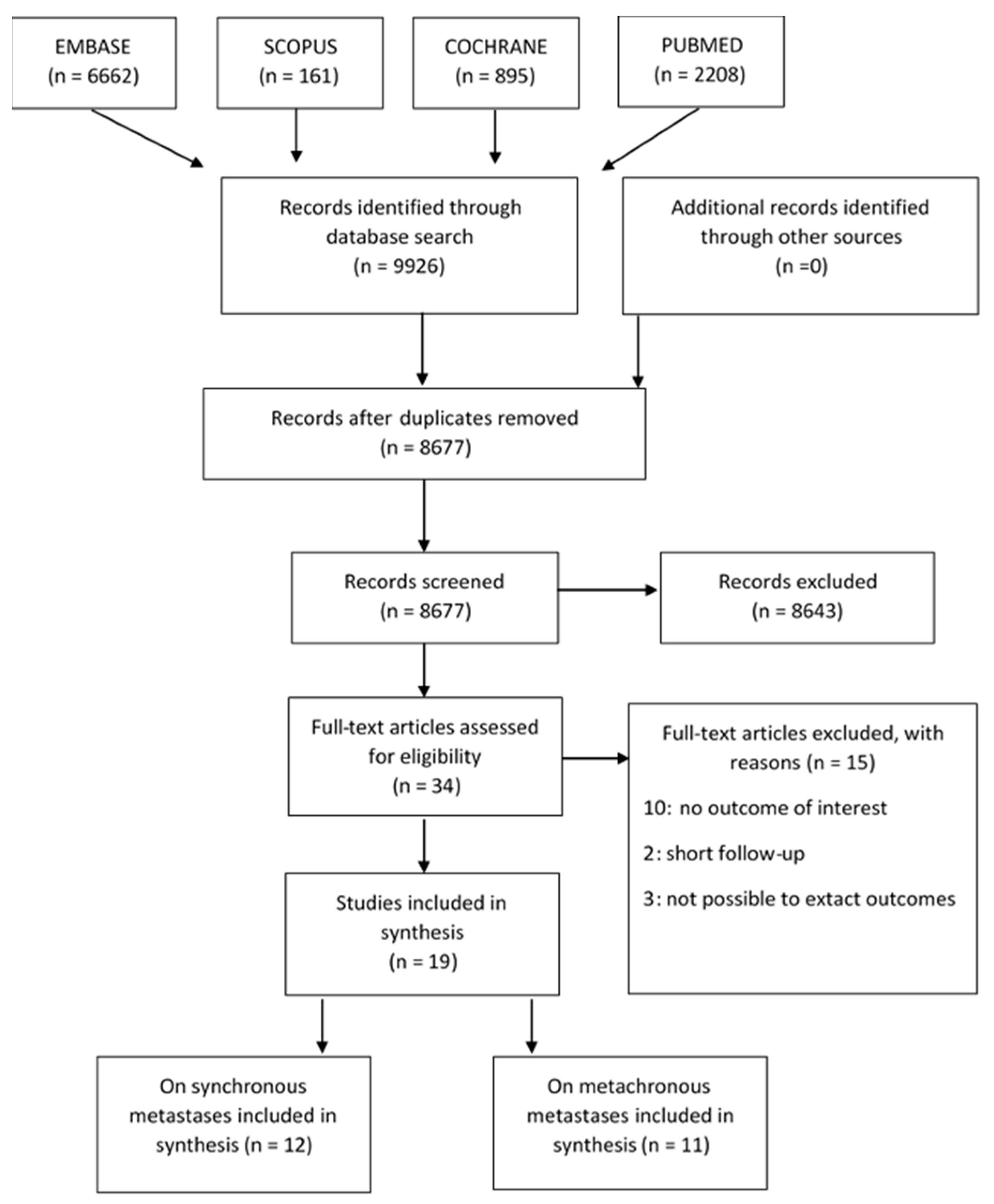
2.3. Study Search and Selection
2.4. Data Extraction and Synthesis
2.5. Statistical Analysis
3. Results
3.1. Study Selection and Characteristics of the Included Studies
3.2. Risk of Bias
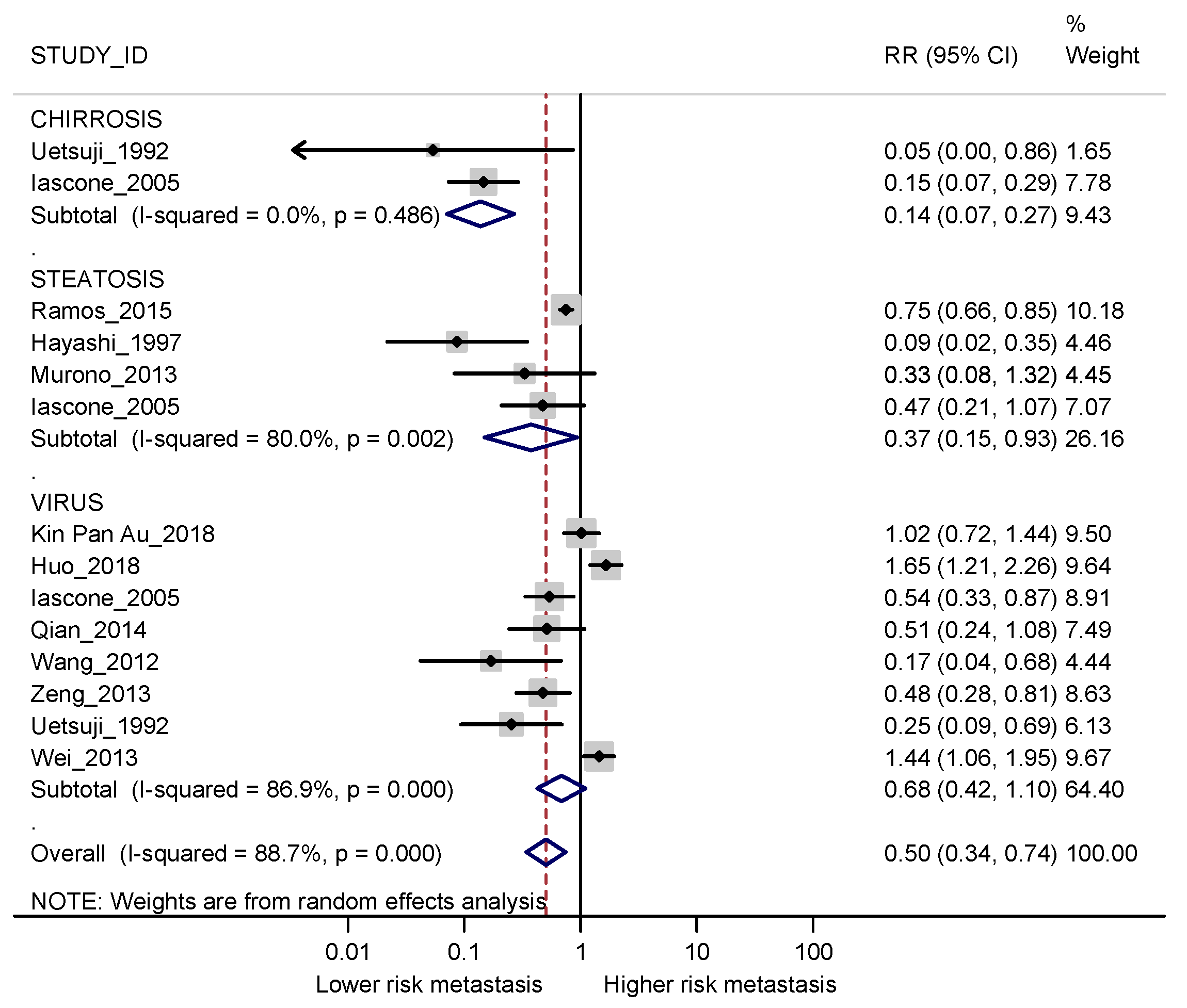
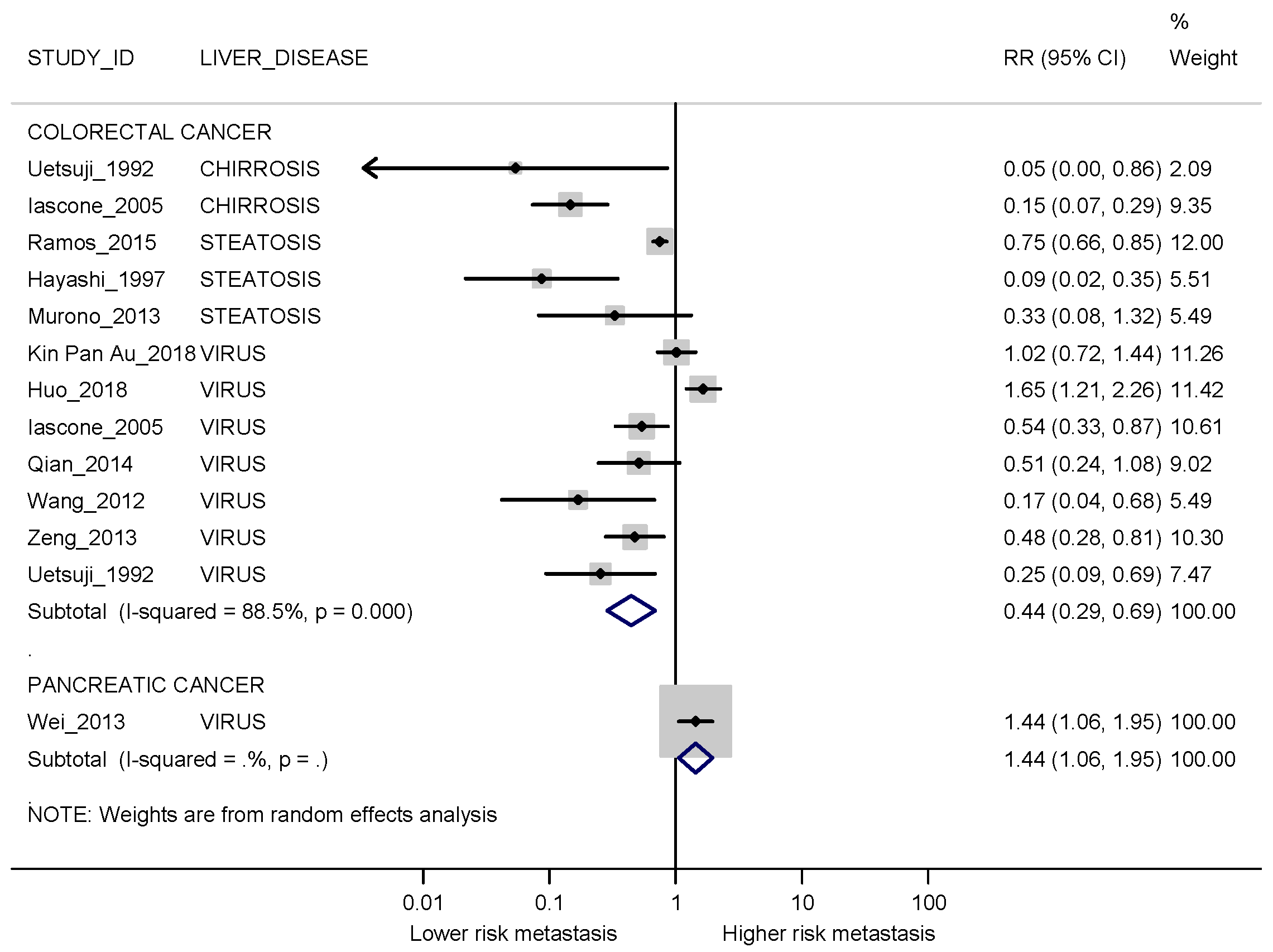
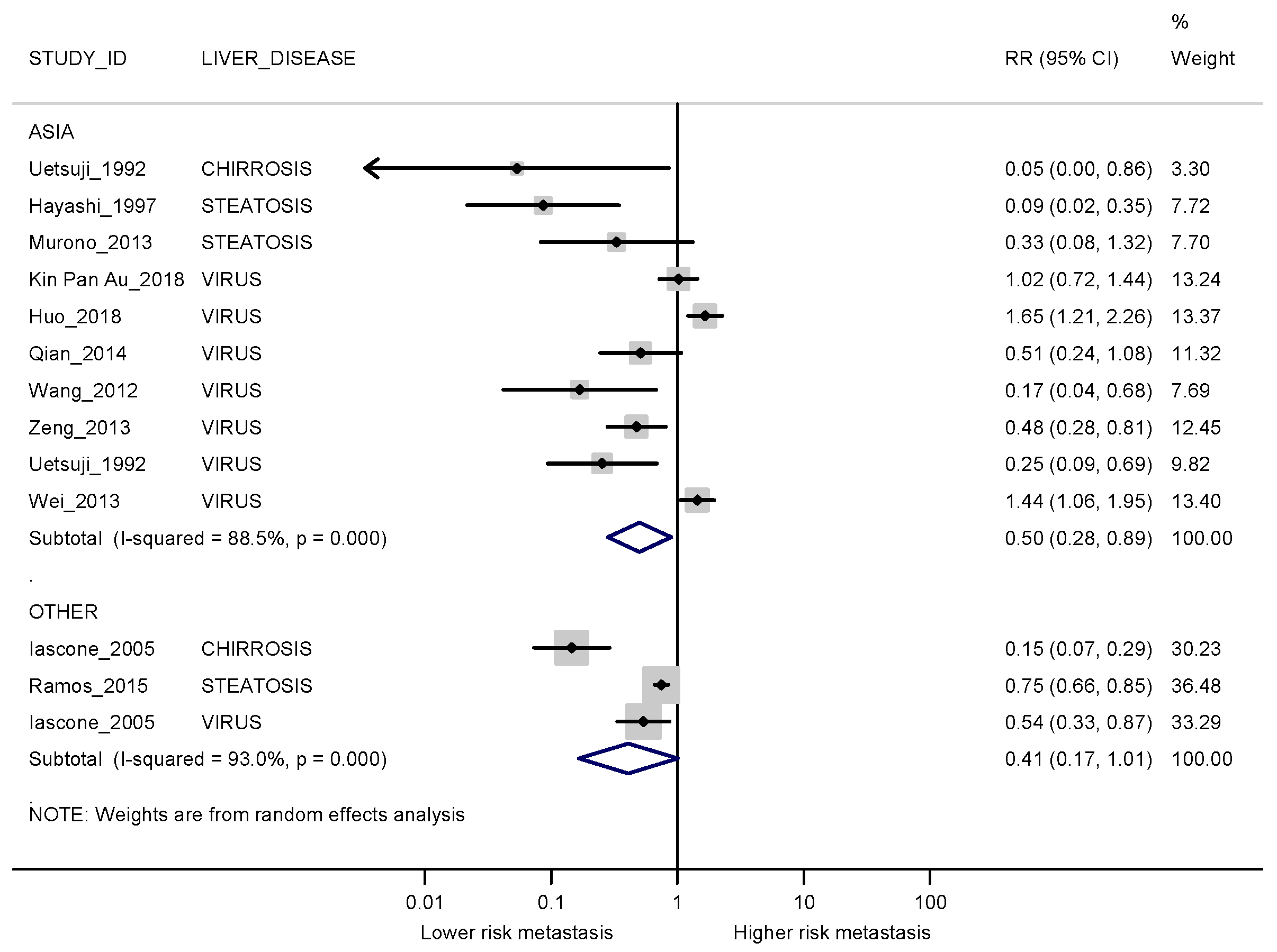
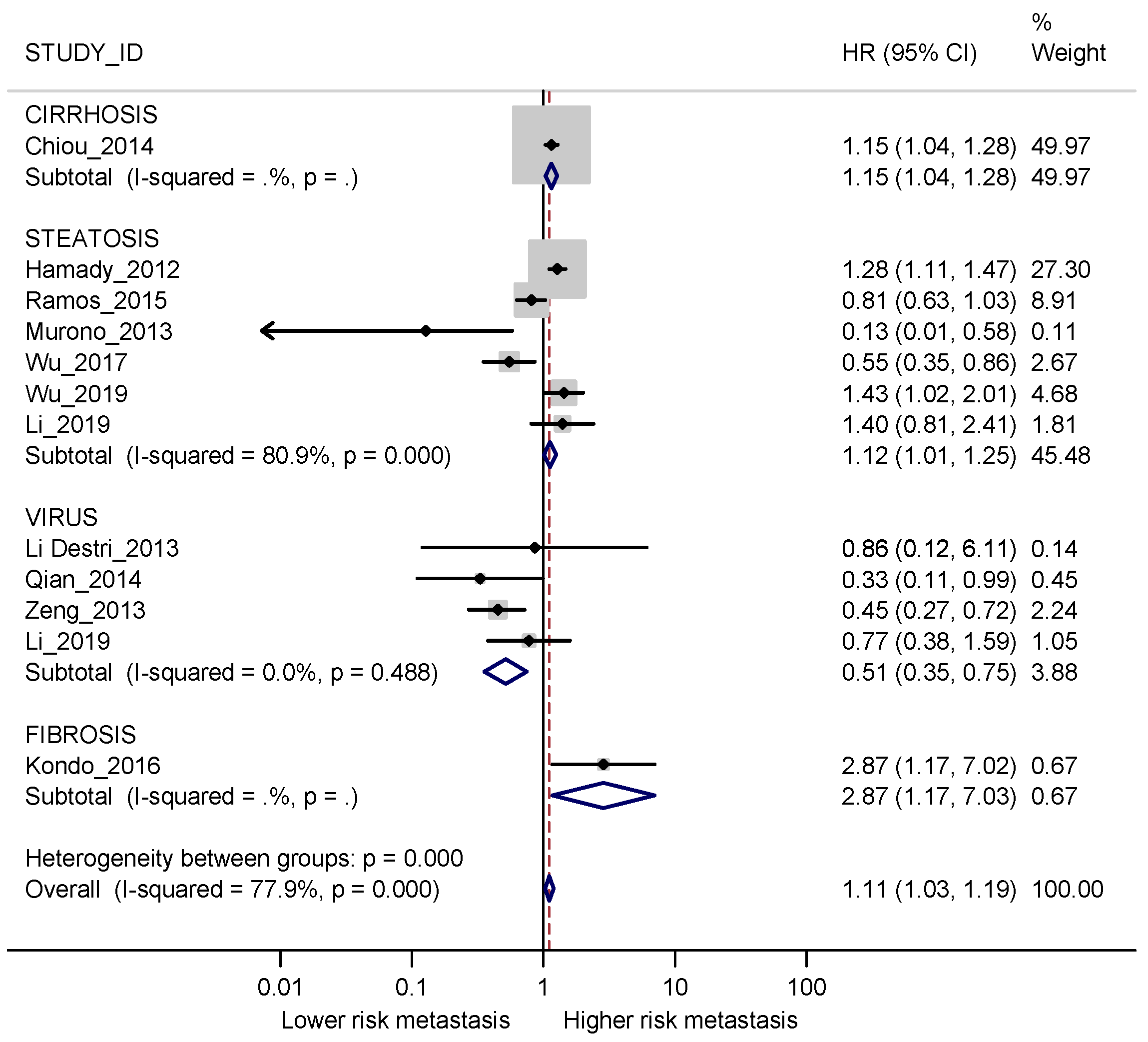
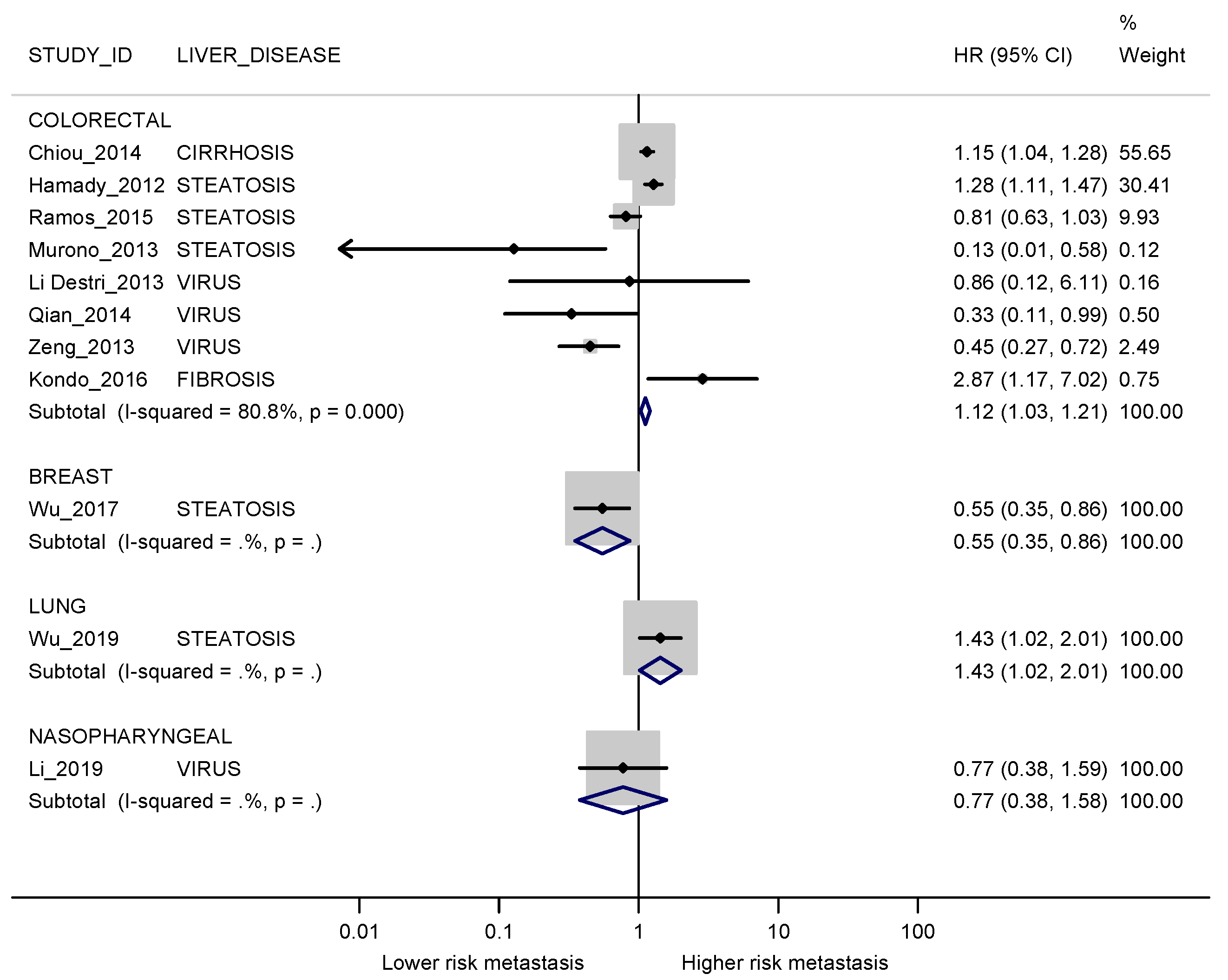
3.3. Meta-Analyses
3.3.1. Synchronous Metastases
3.3.2. Metachronous Metastases
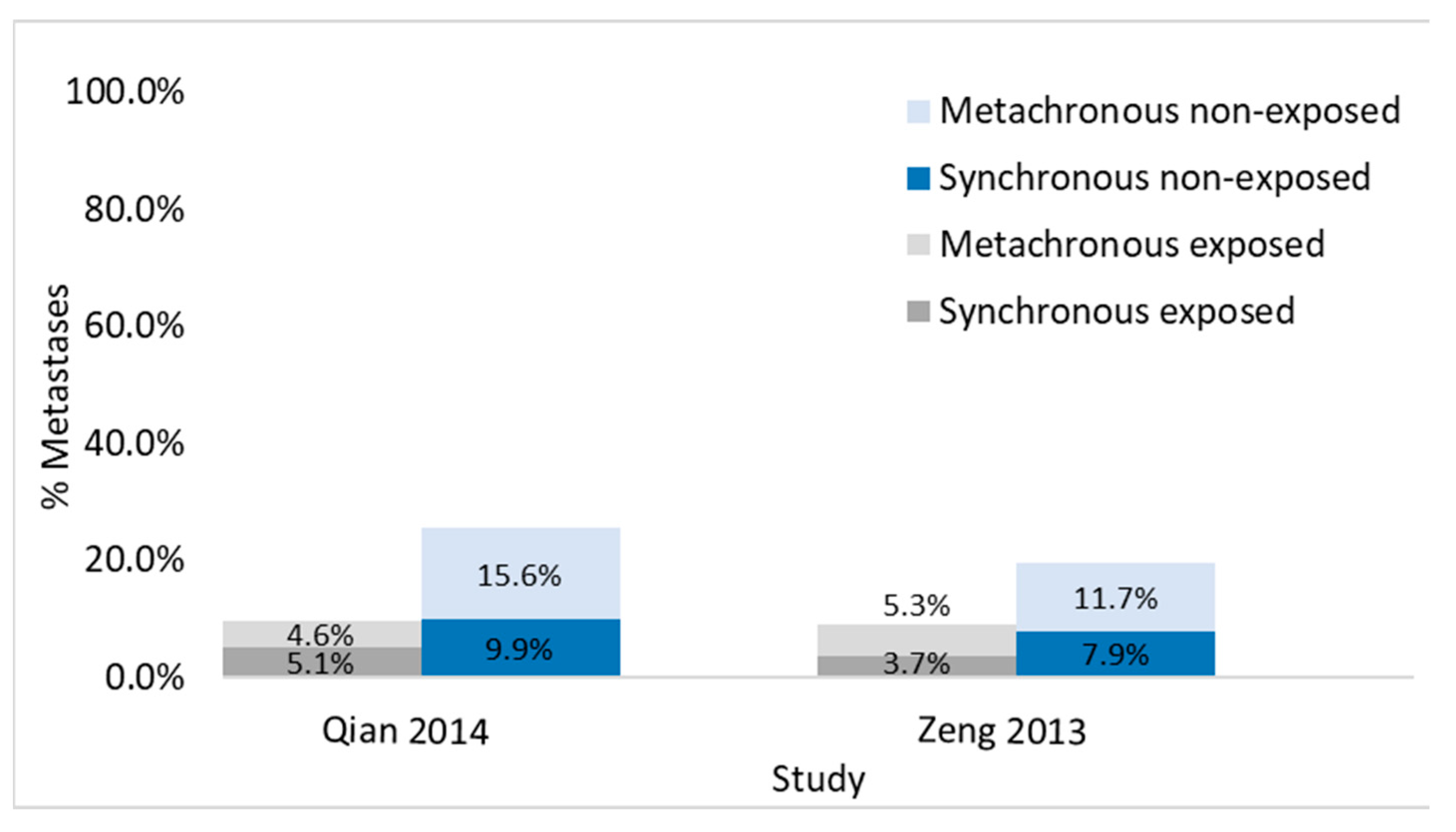
3.3.3. Studies Evaluating Synchronous and Metachronous Liver Metastases in the Same Population
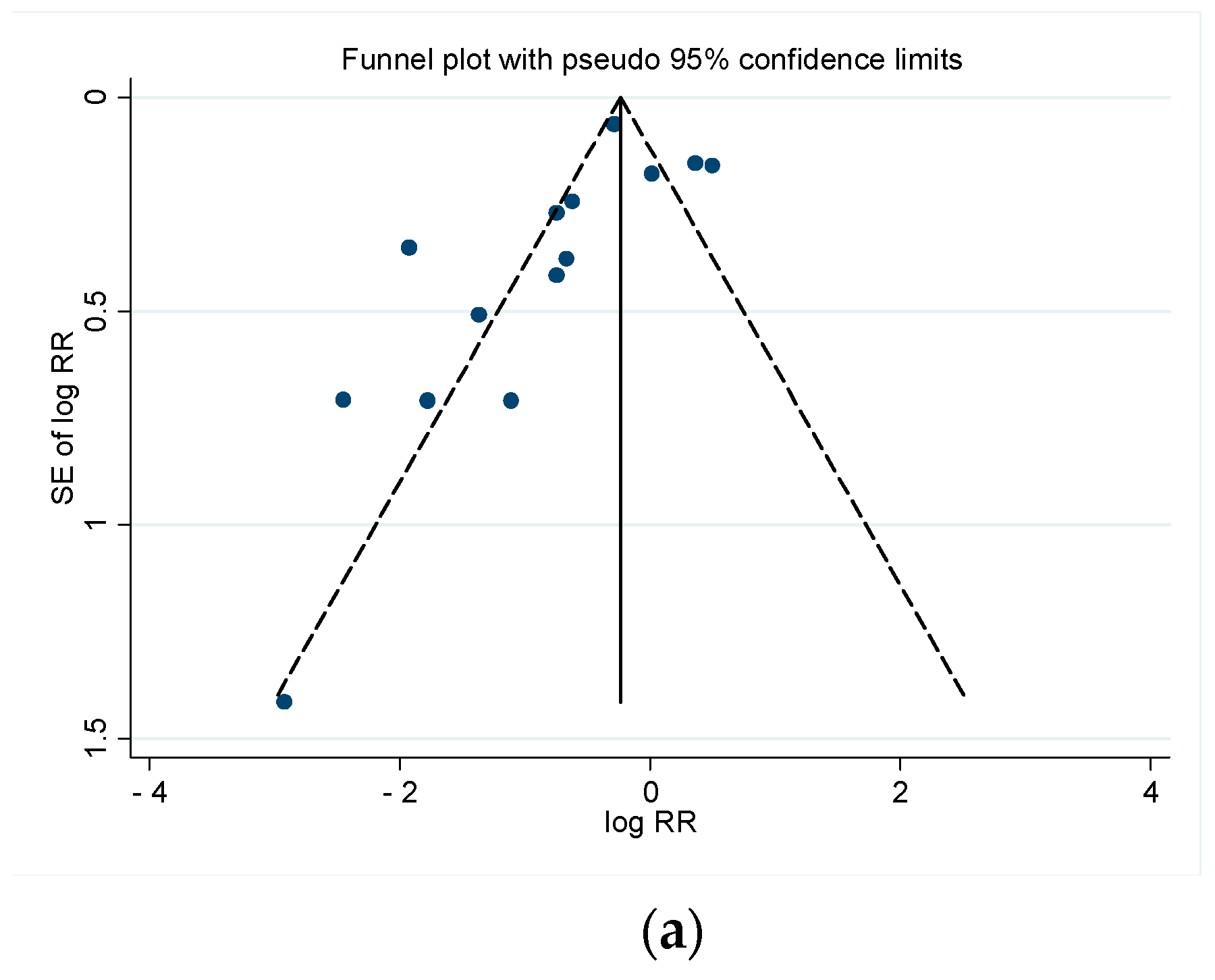
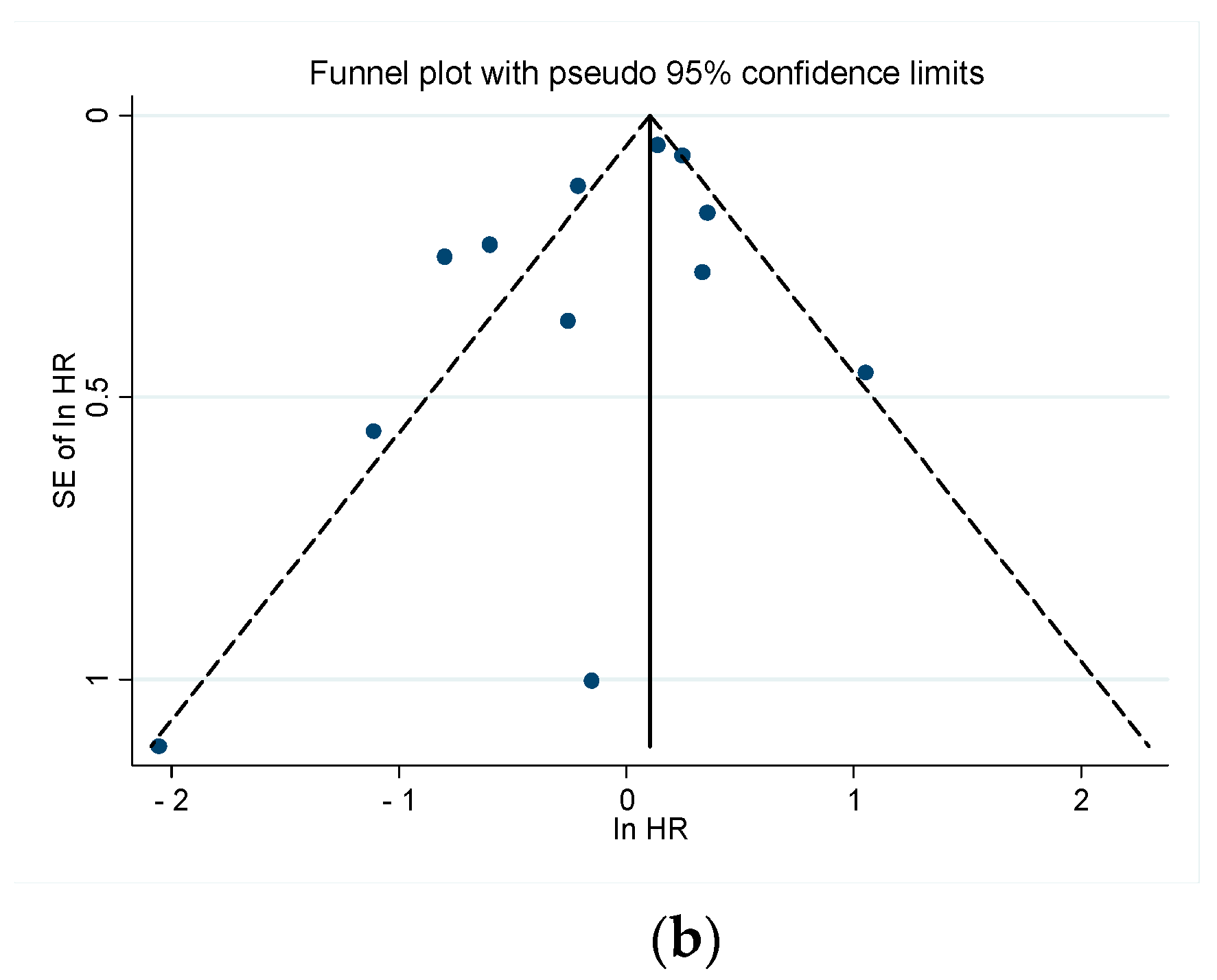
3.3.4. Publication Bias
3.3.5. Overall Survival in Studies Reporting Liver Disease Free Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Helling, T.S.; Martin, M. Cause of death from liver metastases in colorectal cancer. Ann. Surg. Oncol. 2014, 21, 501–506. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of death among cancer patients. Ann. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef]
- Stewart, C.L.; Warner, S.; Ito, K.; Raoof, M.; Wu, G.X.; Kessler, J.; Kim, J.Y.; Fong, Y. Cytoreduction for colorectal metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr. Probl. Surg. 2018, 55, 330–379. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Razavi, H. Global Epidemiology of Viral Hepatitis. Gastroenterol. Clin. N. Am. 2020, 49, 179–189. [Google Scholar] [CrossRef]
- Uzunlulu, M.; Telci Caklili, O.; Oguz, A. Association between Metabolic Syndrome and Cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer. 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Brodt, P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin. Cancer Res. 2016, 22, 5971–5982. [Google Scholar] [CrossRef] [PubMed]
- Milette, S.; Sicklick, J.K.; Lowy, A.M.; Brodt, P. Molecular Pathways: Targeting the Microenvironment of Liver Metastases. Clin. Cancer Res. 2017, 23, 6390–6399. [Google Scholar] [CrossRef]
- Masaki, S.; Hashimoto, Y.; Kunisho, S.; Kimoto, A.; Kitadai, Y. Fatty change of the liver microenvironment influences the metastatic potential of colorectal cancer. Int. J. Exp. Pathol. 2020, 101, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; De Rosa, G.; Toso, C.; Andres, A.; Ferrero, A.; Roth, A.; Sperti, E.; Majno, P.; Rubbia-Brandt, L. Reversibility of chemotherapy-related liver injury. J. Hepatol. 2017, 67, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Leoni, S.; Alswat, K.A.; Fouad, Y. History of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 5888. [Google Scholar] [CrossRef] [PubMed]
- Kuo, G.; Choo, Q.L.; Alter, H.J.; Gitnick, G.L.; Redeker, A.G.; Purcell, R.H.; Miyamura, T.; Dienstag, J.L.; Alter, M.J.; Stevens, C.E.; et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 1989, 244, 362–364. [Google Scholar] [CrossRef]
- Aach, R.D.; Stevens, C.E.; Hollinger, F.B.; Mosley, J.W.; Peterson, D.A.; Taylor, P.E.; Johnson, R.G.; Barbosa, L.H.; Nemo, G.J. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N. Engl. J. Med. 1991, 325, 1325–1329. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.L.; Peterson, J.; Welch; Losos, M.; Tugwell, P.; Ga, S.W.; Zello, G.; Petersen, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Health Research Institute: Ottawa, ON, Canada, 2014; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 6 May 2021).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Harris, R.J.; Deeks, J.J.; Altman, D.G.; Bradburn, M.J.; Harbord, R.M.; Sterne, J.A.C. Metan: Fixed- and Random-Effects Meta-Analysis. Stata J. 2008, 8, 3–28. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Updated February 2021; Cochrane, 2021; Available online: www.training.cochrane.org/handbook (accessed on 15 March 2021).
- Chiou, W.Y.; Chang, C.M.; Tseng, K.C.; Hung, S.K.; Lin, H.Y.; Chen, Y.C.; Su, Y.C.; Tseng, C.W.; Tsai, S.J.; Lee, M.S.; et al. Effect of liver cirrhosis on metastasis in colorectal cancer patients: A nationwide population-based cohort study. Jpn. J. Clin. Oncol. 2015, 45, 160–168. [Google Scholar] [CrossRef][Green Version]
- Uetsuji, S.; Yamamura, M.; Yamamichi, K.; Okuda, Y.; Takada, H.; Hioki, K. Absence of colorectal cancer metastasis to the cirrhotic liver. Am. J. Surg. 1992, 164, 176–177. [Google Scholar] [CrossRef]
- Iascone, C.; Ruperto, M.; Barillari, P. Metastasi epatiche sincrone da carcinoma colo-rettale in pazienti con cirrosi epatica o steatosi [Occurrence of synchronous colorectal cancer metastasis in the cirrhotic or fatty liver]. Minerva Chir. 2005, 60, 185–190. (In Italian) [Google Scholar]
- Hamady, Z.Z.; Rees, M.; Welsh, F.K.; Toogood, G.J.; Prasad, K.R.; John, T.K.; Lodge, J.P. Fatty liver disease as a predictor of local recurrence following resection of colorectal liver metastases. Br. J. Surg. 2013, 100, 820–826. [Google Scholar] [CrossRef]
- Ramos, E.; Torras, J.; Lladó, L.; Rafecas, A.; Serrano, T.; Lopez-Gordo, S.; Busquets, J.; Fabregat, J. The influence of steatosis on the short- and long-term results of resection of liver metastases from colorectal carcinoma. HPB 2016, 18, 389–396. [Google Scholar] [CrossRef]
- Hayashi, S.; Masuda, H.; Shigematsu, M. Liver metastasis rare in colorectal cancer patients with fatty liver. Hepatogastroenterology 1997, 44, 1069–1075. [Google Scholar]
- Murono, K.; Kitayama, J.; Tsuno, N.H.; Nozawa, H.; Kawai, K.; Sunami, E.; Akahane, M.; Watanabe, T. Hepatic steatosis is associated with lower incidence of liver metastasis from colorectal cancer. Int. J. Colorectal. Dis. 2013, 28, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Au, K.P.; Chok, K.S.H.; Chan, A.C.Y.; Dai, W.C.; Cheung, T.T.; Lo, C.M. Impact of Hepatitis B Carrier Status on the Outcomes of Surgical Treatment of Colorectal Liver Metastases. World J. Surg. 2018, 42, 2642–2650. [Google Scholar] [CrossRef]
- Huo, T.; Cao, J.; Tian, Y.; Shi, X.; Wu, L.; Zhang, M.; Wong, L.L.; Zhao, L. Effect of Concomitant Positive Hepatitis B Surface Antigen on the Risk of Liver Metastasis: A Retrospective Clinical Study of 4033 Consecutive Cases of Newly Diagnosed Colorectal Cancer. Clin. Infect. Dis. 2018, 66, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Iascone, C.; Ruperto, M.; Barillari, P. Metastasi epatiche da carcinoma colo-rettale in pazienti con infezione da epatite B o C [Colorectal carcinoma metastasis in livers infected with hepatitis B or C virus]. Minerva Chir. 2005, 60, 77–81. (In Italian) [Google Scholar] [PubMed]
- Li Destri, G.; Castaing, M.; Ferlito, F.; Minutolo, V.; Di Cataldo, A.; Puleo, S. Rare hepatic metastases of colorectal cancer in livers with symptomatic HBV and HCV hepatitis. Ann. Ital. Chir. 2013, 84, 323–327. [Google Scholar]
- Qian, H.G.; Hao, C.Y. Hepatitis B virus infection is an independent factor influencing the occurrence of liver metastasis in colorectal cancer: A retrospective analysis of 1413 cases. Hepatogastroenterology 2014, 61, 1908–1914. [Google Scholar]
- Wang, F.S.; Shao, Z.G.; Zhang, J.L.; Liu, Y.F. Colorectal liver metastases rarely occur in patients with chronic hepatitis virus infection. Hepatogastroenterology 2012, 59, 1390–1392. [Google Scholar] [CrossRef]
- Zeng, Y.; He, X.W.; He, X.S.; Wu, X.J.; Ma, J.P.; Wang, J.P.; Lan, P. Chronic hepatic viral infection could be a protective factor for colorectal cancer liver metastases: Analysis in a single institute. Hepatogastroenterology 2013, 60, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Okabayashi, K.; Hasegawa, H.; Tsuruta, M.; Shigeta, K.; Kitagawa, Y. The impact of hepatic fibrosis on the incidence of liver metastasis from colorectal cancer. Br. J. Cancer 2016, 115, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, J.; Ye, W.; Li, X.; Zhang, J. Fatty liver decreases the risk of liver metastasis in patients with breast cancer: A two-center cohort study. Breast Cancer Res. Treat. 2017, 166, 289–297. [Google Scholar] [CrossRef]
- Wu, W.; Liao, H.; Ye, W.; Li, X.; Zhang, J.; Bu, J. Fatty liver is a risk factor for liver metastasis in Chinese patients with non-small cell lung cancer. PeerJ 2019, 7, e6612. [Google Scholar] [CrossRef]
- Wei, X.L.; Qiu, M.Z.; Chen, W.W.; Jin, Y.; Ren, C.; Wang, F.; Luo, H.Y.; Wang, Z.Q.; Zhang, D.S.; Wang, F.H.; et al. The status of HBV infection influences metastatic pattern and survival in Chinese patients with pancreatic cancer. J. Transl. Med. 2013, 11, 249. [Google Scholar] [CrossRef]
- Li, X.; Wu, W.; Chen, J.; Ye, W.; Zhang, J.; Wei, T. Effect of hepatitis B virus infection status on liver metastasis of nasopharyngeal carcinoma: A cohort study. Transl. Cancer Res. 2019, 8, 262–272. [Google Scholar] [CrossRef]
- Cai, B.; Liao, K.; Song, X.Q.; Wei, W.Y.; Zhuang, Y.; Zhang, S. Patients with chronically diseased livers have lower incidence of colorectal liver metastases: A meta-analysis. PLoS ONE 2014, 9, e108618. [Google Scholar] [CrossRef]
- Barsky, S.H.; Gopalakrishna, R. High metalloproteinase inhibitor content of human cirrhosis and its possible conference of metastasis resistance. J. Natl. Cancer Inst. 1988, 80, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Karube, H.; Masuda, H.; Hayashi, S.; Ishii, Y.; Nemoto, N. Fatty liver suppressed the angiogenesis in liver metastatic lesions. Hepatogastroenterology 2000, 47, 1541–1545. [Google Scholar]
- Okuno, K.; Hirai, N.; Lee, Y.S.; Kawai, I.; Shigeoka, H.; Yasutomi, M. Involvement of liver-associated immunity in hepatic metastasis formation. J. Surg. Res. 1998, 75, 148–152. [Google Scholar] [CrossRef]
- Mareel, M.; Leroy, A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol. Rev. 2003, 83, 337–376. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]

| Author/Year | Primary Cancer Site | Diffuse Liver Disease | Study Design | Outcome | Primary/R0 | Hospital/Population-Based | Events/ Total | Events/ Exposed | Events/ Non-Exposed | LD Diagnosis Method | LD Diagnosis Timing | Follow-Up Exposed | Follow-Up Non-Exposed | Age (Years) Exposed | Age (Years) Non-Exposed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uetsuji 1992 [21] | CRC | Cirr | Cr-Sec | S | PR | HOS | 40/250 | 0/46 | 40/204 | Bilirubin > 1.5, Albumin < 3.5, Cholinesterase< 3500, GGT > 46, IG retention rate at 15′ > 15% | At PR surgery | - | - | - | - |
| Iascone 2005 [22] | CRC | Cirrs | Cr-Sec | S | PR | HOS | 182/576 | 8/171 | 174/405 | Biopsy | At PR surgery | - | - | 71.2 | 65.8 |
| Iascone 2005 [22] | CRC | Steat | Cr-Sec | S | PR | HOS | 179/576 | 5/33 | 174/543 | Biopsy | At PR surgery | - | - | - | - |
| Uetsuji 1992 [21] | CRC | Vir | Cr-Sec | S | PR | HOS | 40/250 | 4/76 | 36/174 | Blood Test | At PR surgery | - | - | - | - |
| Huo 2018 [28] | CRC | Vir | Cr-Sec | S | PR | HOS | 364/4033 | 38/244 | 326/3789 | Blood Test | At PR diagnosis | - | - | 57.3 | 62.7 |
| Iascone 2005 [29] | CRC | Vir | Cr-Sec | S | PR | HOS | 189/630 | 15/87 | 174/543 | Blood Test | Before PR surgery | - | - | 69.8 | 65.8 |
| Wang 2012 [32] | CRC | Vir | Cr-Sec | S | PR | HOS | 50/354 | 2/70 | 48/284 | Blood Test | - | - | - | 52.9 | 56.2 |
| Chiou 2014 [20] | CRC | Cirr | Cohort | M | PR | POP | 2529/14865 | 516/2973 | 2013/11892 | NatReg: ICD-9-CM: 571.2, 571.5, 571.6 | Before PR surgery | - | - | 67.6 | 67.6 |
| Hamady 2012 [23] | CRC | Steat | Cohort | M | R0 | HOS | 1118/2715 | 437/927 | 681/1788 | Biopsy | At LM resection | 34 | 16% > 75 | 15% > 75 | |
| Ramos 2015 [24] | CRC | Steat | Cohort | S/M | R0 | HOS | S:511/943 | S:194/421 | S:317/513 | Biopsy | At LM resection | 47.05 | 62.6 | 62.9 | |
| M:253/528 | M:127/264 | M:126/264 | |||||||||||||
| Hayashi 1997 [25] | CRC | Steat | Cohort | S | PR | HOS | 117/839 | 2/121 | 115/718 | Ultrasound | At PR diagnosis | - | - | 58.5 | 61.4 |
| Murono 2013 [26] | CRC | Steat | Cohort | S/M | PR | HOS | S:54/604 | S:2/63 | S:52/541 | CT (HU liver/spleen < 1.1) | Before PR surgery | - | - | 65 | 67.2 |
| M:59/529 | M:1/59 | M:58/470 | |||||||||||||
| Kin Pan Au 2018 [27] | CRC | Vir | Cohort | S | R0 | HOS | 185/304 | 13/21 | 172/283 | Blood Test | Before LM resection | - | - | 61 | 60 |
| Li Destri 2013 [30] | CRC | Vir | Cohort | M | PR | HOS | 44/488 | 1/31 | 43/457 | Blood Test | Before PR diagnosis | 108 | 61 | 66 | |
| Qian 2014 [31] | CRC | Vir | Cohort | S/M | PR | HOS | S:133/1413 | S:7/138 | S:126/1275 | Blood Test | Before PR surgery | 72.3 | 58.5 | 59.2 | |
| M:185/1150 | M:6/131 | M:179/1149 | |||||||||||||
| Zeng 2013 [33] | CRC | Vir | Cohort | S/M | PR | HOS | S:211/2868 | S:14/373 | S:197/2495 | Blood Test | - | 65 | 57 | 61 | |
| M:287/2868 | M:19/373 | M:268/2495 | |||||||||||||
| Kondo 2016 [34] | CRC | Fibr | Cohort | M | PR | HOS | 54/953 | 8/77 | 46/876 | NFS > 0.676 | - | 51.2 | 75.3 | 64.9 | |
| Wu 2017 [35] | Breast | Steat | Cohort | M | PR | HOS | 123/1230 | 27/372 | 96/858 | Ultrasound | At PR diagnosis | 30.7 | 32.4 | 50% > 50 | 35% > 50 |
| Wu 2019 [36] | Lung | Steat | Cohort | M | PR | HOS | 166/1873 | 58/408 | 108/1465 | Ultrasound | At PR diagnosis | 14.5 | 67% > 60 | 51% > 60 | |
| Wei 2013 [37] | Pancreas | Vir | Cohort | S | PR | HOS | 156/460 | 29/63 | 127/397 | Blood Test | At PR diagnosis | 12 | - | - | |
| Li 2019 [38] | NPC | Vir/Steat | Cohort | M | PR | HOS | 64/1367 | 13/123 | 51/492 | Blood Test | At PR diagnosis | 27.8 | 10%≥ 60 | 11%≥ 60 | |
| TOT CRC | 32661 | 5872 | 26780 | ||||||||||||
| TOT NON-CRC | 4930 | 966 | 3212 | ||||||||||||
| TOT | 37591 | 6868 | 29992 | ||||||||||||
| Author/Year | ROB | Selection | Representativeness of EXPOSED Cohort | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Outcome of Interest Not Present at Study Start | Comparability | Comparability on the Basis of the Design or Analysis | Outcome | Assessment of Outcome | Follow-Up Long Enough for Outcomes | Adequacy of Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chiou 2014 [20] | 5/9 | 3/4 | ☼ | ☼ | ☼ | 1/2 | ☼ | 1/3 | ☼ | |||
| Uetsuji 1992 [21] | 4/6 | 3/3 | ☼ | ☼ | ☼ | 1/2 | ☼ | 0/1 | ||||
| Iascone 2005 [22] | 4/6 | 3/3 | ☼ | ☼ | ☼ | 1/2 | ☼ | 0/1 | ||||
| Hamady 2012 [23] | 8/9 | 4/4 | ☼ | ☼ | ☼ | ☼ | 2/2 | ☼☼ | 2/3 | ☼ | ☼ | |
| Ramos 2015 [24] | 8/9 | 4/4 | ☼ | ☼ | ☼ | ☼ | 2/2 | ☼☼ | 2/3 | ☼ | ☼ | |
| Hayashi 1997 [25] | 7/9 | 3/4 | ☼ | ☼ | ☼ | 1/2 | ☼ | 3/3 | ☼ | ☼ | ☼ | |
| Murono 2013 [26] | 6/9 | 4/4 | ☼ | ☼ | ☼ | ☼ | 2/2 | ☼☼ | 0/3 | |||
| Kin Pan Au 2018 [27] | 9/9 | 4/4 | ☼ | ☼ | ☼ | ☼ | 2/2 | ☼☼ | 3/3 | ☼ | ☼ | ☼ |
| Huo 2018 [28] | 6/6 | 3/3 | ☼ | ☼ | ☼ | 2/2 | ☼☼ | 1/1 | ☼ | |||
| Iascone 2005 [29] | 4/6 | 3/3 | ☼ | ☼ | ☼ | 1/2 | ☼ | 1/1 | ☼ | |||
| Li Destri 2013 [30] | 7/9 | 3/4 | ☼ | ☼ | ☼ | 1/2 | ☼ | 3/3 | ☼ | ☼ | ☼ | |
| Qian 2014 [31] | 6/9 | 3/4 | ☼ | ☼ | ☼ | 0/2 | 3/3 | ☼ | ☼ | ☼ | ||
| Wang 2012 [32] | 4/6 | 2/3 | ☼ | ☼ | 1/2 | ☼ | 1/1 | ☼ | ||||
| Zeng 2013 [33] | 6/9 | 3/4 | ☼ | ☼ | ☼ | 2/2 | ☼☼ | 1/3 | ☼ | |||
| Kondo 2016 [34] | 3/9 | 1/4 | ☼ | 0/2 | 2/3 | ☼ | ☼ | |||||
| Wu 2017 [35] | 8/9 | 4/4 | ☼ | ☼ | ☼ | ☼ | 1/2 | ☼ | 3/3 | ☼ | ☼ | ☼ |
| Wu 2019 [36] | 7/9 | 4/4 | ☼ | ☼ | ☼ | ☼ | 1/2 | ☼ | 2/3 | ☼ | ☼ | |
| Wei 2013 [37] | 6/9 | 4/4 | ☼ | ☼ | ☼ | ☼ | 0/2 | 2/3 | ☼ | ☼ | ||
| Li 2019 [38] | 9/9 | 4/4 | ☼ | ☼ | ☼ | ☼ | 2/2 | ☼☼ | 3/3 | ☼ | ☼ | ☼ |
| Study | 5-Year Survival Exposed | Median Survival Exposed | 5-Year Survival Non-Exposed | Median Survival Non-Exposed |
|---|---|---|---|---|
| Hamady 2012 [23] | 39.3% | 22 months | 42.8% | 24 months |
| Ramos 2015 [24] | 55.1% | 45.2% | ||
| Qian 2014 [31] | 40.6% | 39.3% | ||
| Zeng 2013 [33] | 56 months | 49 months | ||
| Kondo 2016 [34] | 79.8% | 92.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monelli, F.; Besutti, G.; Djuric, O.; Bonvicini, L.; Farì, R.; Bonfatti, S.; Ligabue, G.; Bassi, M.C.; Damato, A.; Bonelli, C.; et al. The Effect of Diffuse Liver Diseases on the Occurrence of Liver Metastases in Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 2246. https://doi.org/10.3390/cancers13092246
Monelli F, Besutti G, Djuric O, Bonvicini L, Farì R, Bonfatti S, Ligabue G, Bassi MC, Damato A, Bonelli C, et al. The Effect of Diffuse Liver Diseases on the Occurrence of Liver Metastases in Cancer Patients: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(9):2246. https://doi.org/10.3390/cancers13092246
Chicago/Turabian StyleMonelli, Filippo, Giulia Besutti, Olivera Djuric, Laura Bonvicini, Roberto Farì, Stefano Bonfatti, Guido Ligabue, Maria Chiara Bassi, Angela Damato, Candida Bonelli, and et al. 2021. "The Effect of Diffuse Liver Diseases on the Occurrence of Liver Metastases in Cancer Patients: A Systematic Review and Meta-Analysis" Cancers 13, no. 9: 2246. https://doi.org/10.3390/cancers13092246
APA StyleMonelli, F., Besutti, G., Djuric, O., Bonvicini, L., Farì, R., Bonfatti, S., Ligabue, G., Bassi, M. C., Damato, A., Bonelli, C., Pinto, C., Pattacini, P., & Giorgi Rossi, P. (2021). The Effect of Diffuse Liver Diseases on the Occurrence of Liver Metastases in Cancer Patients: A Systematic Review and Meta-Analysis. Cancers, 13(9), 2246. https://doi.org/10.3390/cancers13092246






