Efficacy and Safety of Bevacizumab Plus Erlotinib in Patients with Renal Medullary Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Demographic, Clinical, Molecular, and Treatment Characteristics
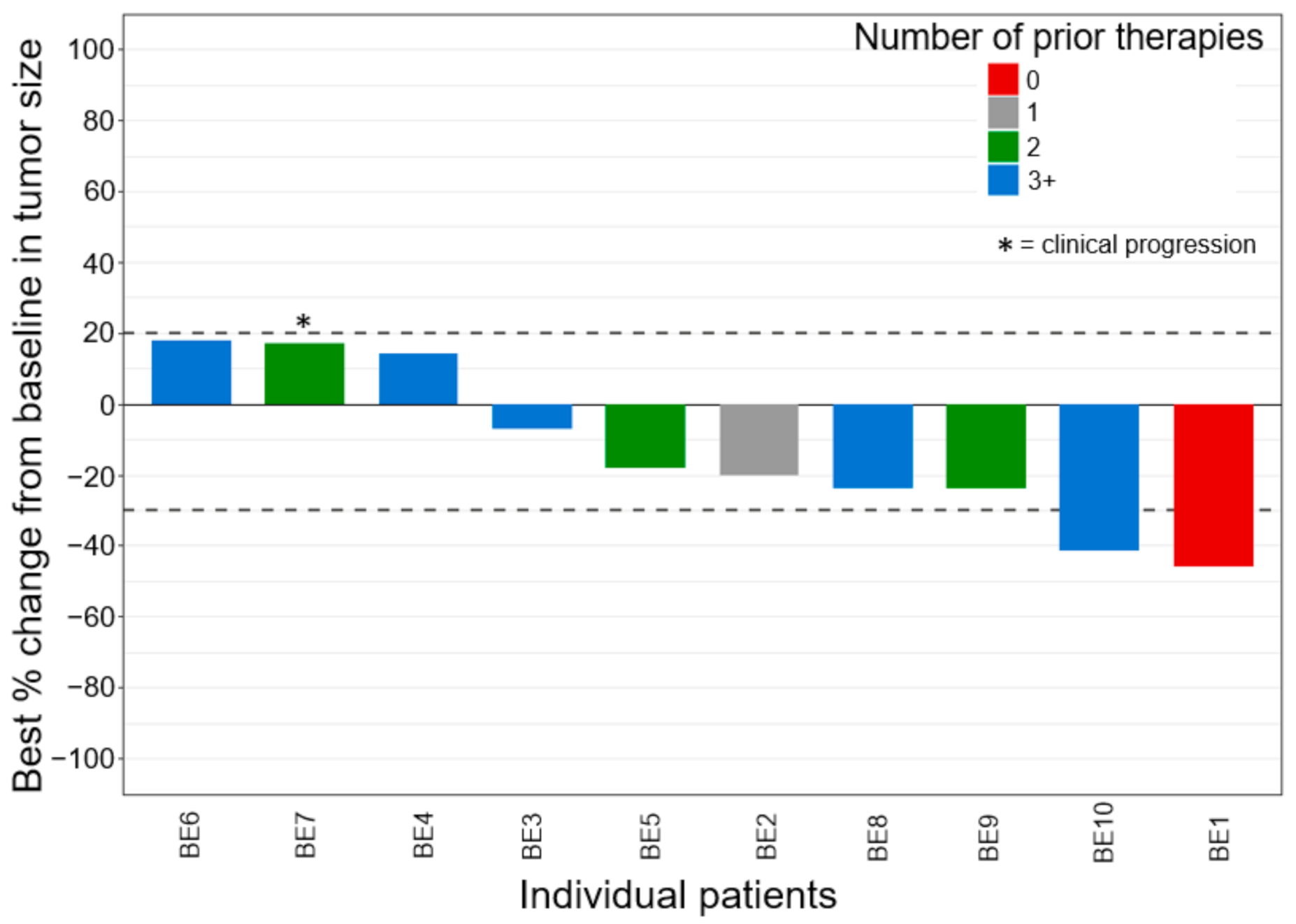
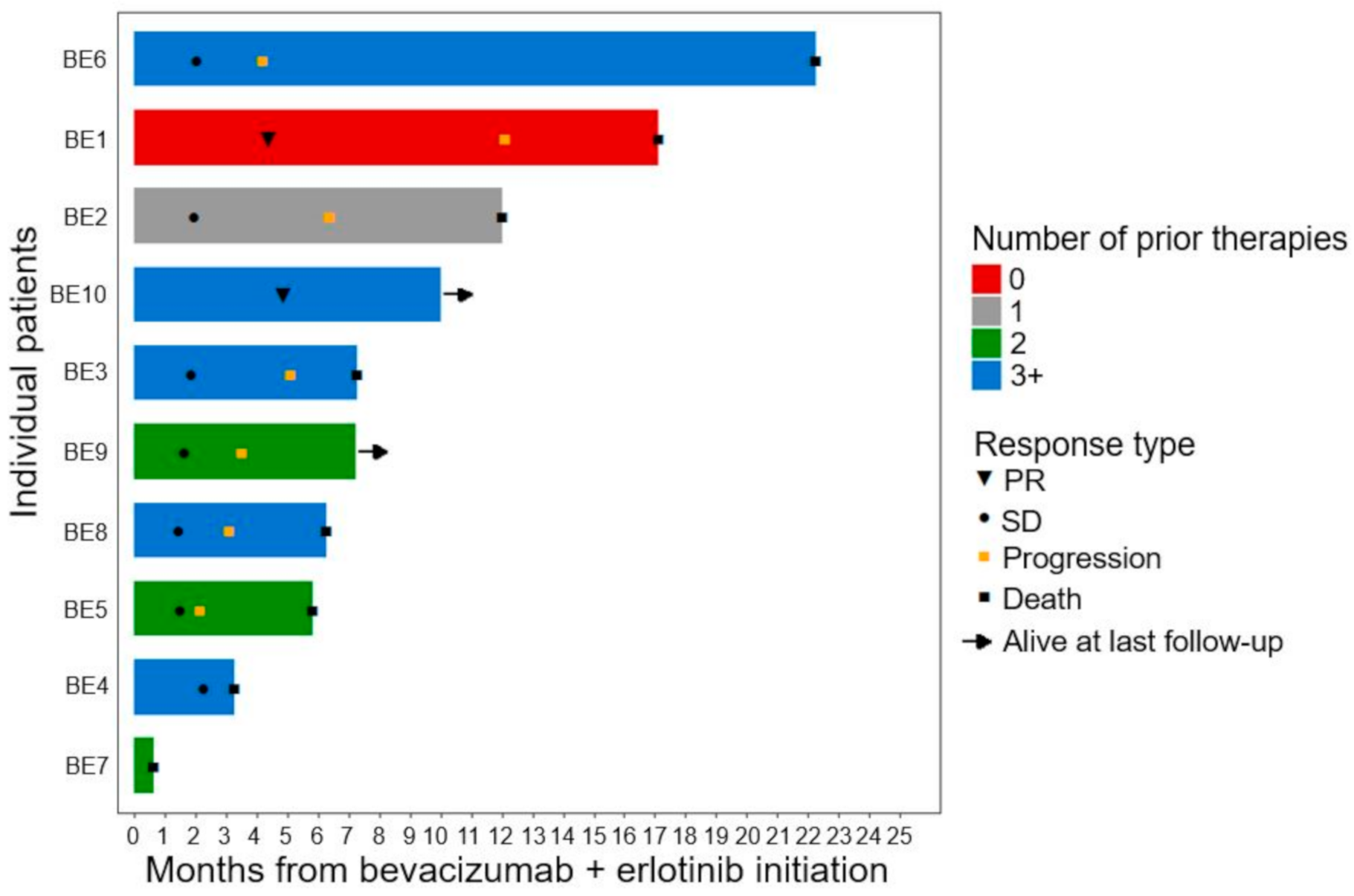
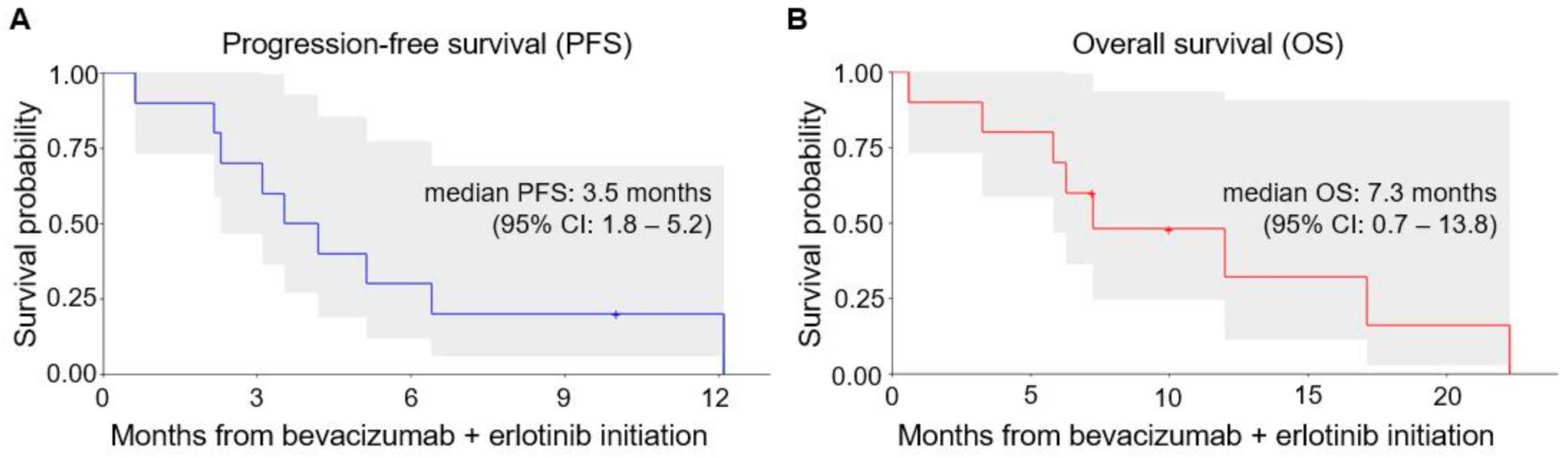
3.2. Efficacy Outcomes
3.3. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Msaouel, P.; Tannir, N.M.; Walker, C.L. A Model Linking Sickle Cell Hemoglobinopathies and SMARCB1 Loss in Renal Medullary Carcinoma. Clin. Cancer Res. 2018, 24, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.Y.; Karam, J.A.; Malouf, G.G.; Rao, P.; Lim, Z.D.; Jonasch, E.; Tannir, N.M.; Gao, J.; Heng, D.Y.; Tamboli, P.; et al. Management and outcomes of patients with renal medullary carcinoma: A multicentre collaborative study. BJU Int. 2017, 120, 782. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Hong, A.L.; Mullen, E.A.; Atkins, M.B.; Walker, C.L.; Lee, C.H.; Tannir, N.M.; Linehan, W.M.; Geller, J.I.; Apolo, A.B.; et al. Updated Recommendations on the Diagnosis, Management, and Clinical Trial Eligibility Criteria for Patients with Renal Medullary Carcinoma. Clin. Genitourin. Cancer 2019, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tannir, N.M.; Plimack, E.; Ng, C.; Tamboli, P.; Bekele, N.B.; Xiao, L.; Jonasch, E.; Pagliaro, L.; Matin, S.; Wood, C.G.; et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur. Urol. 2012, 62, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Zoumpourlis, P.; Genovese, G.; Tannir, N.M.; Msaouel, P. Systemic Therapies for the Management of Non-Clear Cell Renal Cell Carcinoma: What Works, What Doesn’t, and What the Future Holds. Clin. Genitourin. Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Kadoch, C.; Crabtree, G.R. Mammalian SWI/SNF chromatin remodeling complexes and cancer: Mechanistic insights gained from human genomics. Sci. Adv. 2015, 1, e1500447. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Malouf, G.G.; Su, X.; Yao, H.; Tripathi, D.N.; Soeung, M.; Tannir, N.M.; Blando, J.; Srinivasan, S.; Multani, A.S.; et al. Comprehensive Molecular Characterization Identifies Distinct Genomic and Immune Hallmarks of Renal Medullary Carcinoma. Cancer Cell 2020, 37, 720–734.e13. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Rouault, T.A. Molecular pathways: Fumarate hydratase-deficient kidney cancer--targeting the Warburg effect in cancer. Clin. Cancer Res. 2013, 19, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Makinoshima, H.; Takita, M.; Matsumoto, S.; Yagishita, A.; Owada, S.; Esumi, H.; Tsuchihara, K. Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR-mutated lung adenocarcinoma. J. Biol. Chem. 2014, 289, 20813–20823. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Gurram, S.; Harthy, M.A.; Singer, E.A.; Sidana, A.; Shuch, B.M.; Linehan, W.M.; Cowen, E.W.; Choyke, P.L.; Long, L.; et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. J. Clin. Oncol. 2020, 38, 5004. [Google Scholar] [CrossRef]
- Xiao, Z.; Dong, A.; Wang, Y. Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome-Associated Renal Cell Carcinoma Showing High FDG Uptake. Clin. Nucl. Med. 2019, 44, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Karivedu, V.; Jain, A.L.; Eluvathingal, T.J.; Sidana, A. Role of Positron Emission Tomography Imaging in Metabolically Active Renal Cell Carcinoma. Curr. Urol. Rep. 2019, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.S.; Jung, Y.J.; Mole, D.R.; Lee, S.; Torres-Cabala, C.; Chung, Y.L.; Neckers, L.; Trepel, J.; Toro, J.; Linehan, W.M.; et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell 2005, 8, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Verweij, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Luthra, R.; Patel, K.P.; Routbort, M.J.; Broaddus, R.R.; Yau, J.; Simien, C.; Singh, R.R.; Chen, W.; Hatfield, D.Z.; Medeiros, L.J. A Targeted High-Throughput Next-Generation Sequencing Panel for Clinical Screening of Mutations, Gene Amplifications, and Fusions in Solid Tumors. J. Mol. Diagn. 2017, 19, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Keam, B.; Kim, M.; Yoon, S.; Kim, D.; Choi, J.G.; Lee, J.L.; Choi, J.G.; Seo, J.Y.; Park, I. Bevacizumab Plus Erlotinib Combination Therapy for Advanced Hereditary Leiomyomatosis and Renal Cell Carcinoma-Associated Renal Cell Carcinoma: A Multicenter Retrospective Analysis in Korean Patients. Cancer Res. Treat 2019, 51, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Grubb, R.L., 3rd; Franks, M.E.; Toro, J.; Middelton, L.; Choyke, L.; Fowler, S.; Linehan, W.M.; Merino, M.J.; Zbar, B.; Pinto, P.A.; et al. Hereditary leiomyomatosis and renal cell cancer: A syndrome associated with an aggressive form of inherited renal cancer. J. Urol. 2007, 177, 2074–2079; discussion 9–80. [Google Scholar] [CrossRef] [PubMed]
- Menko, F.H.; Maher, E.R.; Schmidt, L.S.; Middelton, L.A.; Aittomäki, K.; Tomlinson, I.; Linehan, W.M.; Richard, S. Hereditary leiomyomatosis and renal cell cancer (HLRCC): Renal cancer risk, surveillance and treatment. Fam. Cancer 2014, 13, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Carlo, M.I.; Khan, H.; Nanjangud, G.J.; Rana, S.; Cimera, R.; Chen, Y.B.; Al-Ahmadie, H.A.; Fine, S.W.; Reuter, V.E.; et al. Distinctive mechanisms underlie the loss of SMARCB1 protein expression in renal medullary carcinoma: Morphologic and molecular analysis of 20 cases. Mod. Pathol. 2019, 32, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total (n = 10) |
|---|---|
| Age (Years) at B + E Initiation | |
| Median | 31.5 |
| IQR | 23–36 |
| Range (minimum–maximum) | 20–37 |
| Gender—no. (%) | |
| Male | 9 (90%) |
| Female | 1 (10%) |
| Race | |
| Black | 9 (90%) |
| White, non-Hispanic | 1 (10%) |
| Sickle hemoglobinopathy | |
| Sickle cell trait | 10 (100%) |
| ECOG performance status at B + E initiation—no. (%) | |
| 0 | 1 (10%) |
| 1 | 8 (80%) |
| 2 | 1 (10%) |
| RMC laterality—no. (%) | |
| Left kidney | 3 (30%) |
| Right kidney | 7 (70%) |
| Stage at initial diagnosis of RMC—no. (%) | |
| I–III | 0 (0%) |
| IV | 10 (100%) |
| Prior cytoreductive nephrectomy—no. (%) | |
| Yes | 8 (80%) |
| No | 2 (20%) |
| Sites of metastatic disease at B + E initiation—no (%) | |
| Lung | 10 (100%) |
| Lymph node(s) | 10 (100%) |
| Bone | 4 (40%) |
| Liver | 3 (30%) |
| Other | 8 (80%) |
| Three or more sites of metastatic disease—no. (%) | 9 (90%) |
| Local therapy for metastases—no. (%) | |
| Metastasectomy | 0 (0%) |
| Radiation | 2 (20%) |
| Prior platinum-based chemotherapy—no. (%) | |
| Yes | 9 (90%) |
| No | 1 (10%) |
| Number of prior systemic treatments | |
| Median | 2.5 |
| IQR | 2–4 |
| Range (minimum–maximum) | 0–5 |
| Next generation sequencing of RMC tissues—no. (%) | |
| Yes | 6 (60%) |
| No | 4 (40%) |
| Patient ID | Treatment #1 | Treatment #2 | Treatment #3 | Treatment #4 | Treatment #5 | Treatment #6 | Treatment #7 | NGS |
|---|---|---|---|---|---|---|---|---|
| BE1 | B + E | Sunitinib | CGB | - | - | - | - | N/A |
| BE2 | TC | B + E | CGB | - | - | - | - | N/A |
| BE3 | GD | GC + Imatinib | ddMVAC | GD + Ifos | B + E | - | - | N/A |
| BE4 | GDI | Ipi/Nivo | TC | CGI | B + E | - | - | None |
| BE5 | TC | GDI | B + E | - | - | - | - | None |
| BE6 | T | Pembro | TC | B + E | VVC | GDI | CGI | NF2 |
| BE7 | TC | GDI | B + E | - | - | - | - | None |
| BE8 | TC | T | GDI | Ipi/Nivo | CGI | B+E | - | None |
| BE9 | TC | GDI | B + E | --- | - | - | - | None |
| BE10 | Pembro | TC | GDI | B + E | - | - | - | N/A |
| CTCAE Term | All Grades | Grade 3 |
|---|---|---|
| All events | 14 | 1 (7.1%) |
| Acneiform rash | 8 | 1 (12.5%) |
| Anorexia | 1 | 0 |
| Fatigue | 3 | 0 |
| Hypertension | 1 | 0 |
| Proteinuria | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiele, A.J.; Surasi, D.S.; Rao, P.; Sircar, K.; Su, X.; Bathala, T.K.; Shah, A.Y.; Jonasch, E.; Cataldo, V.D.; Genovese, G.; et al. Efficacy and Safety of Bevacizumab Plus Erlotinib in Patients with Renal Medullary Carcinoma. Cancers 2021, 13, 2170. https://doi.org/10.3390/cancers13092170
Wiele AJ, Surasi DS, Rao P, Sircar K, Su X, Bathala TK, Shah AY, Jonasch E, Cataldo VD, Genovese G, et al. Efficacy and Safety of Bevacizumab Plus Erlotinib in Patients with Renal Medullary Carcinoma. Cancers. 2021; 13(9):2170. https://doi.org/10.3390/cancers13092170
Chicago/Turabian StyleWiele, Andrew J., Devaki Shilpa Surasi, Priya Rao, Kanishka Sircar, Xiaoping Su, Tharakeswara K. Bathala, Amishi Y. Shah, Eric Jonasch, Vince D. Cataldo, Giannicola Genovese, and et al. 2021. "Efficacy and Safety of Bevacizumab Plus Erlotinib in Patients with Renal Medullary Carcinoma" Cancers 13, no. 9: 2170. https://doi.org/10.3390/cancers13092170
APA StyleWiele, A. J., Surasi, D. S., Rao, P., Sircar, K., Su, X., Bathala, T. K., Shah, A. Y., Jonasch, E., Cataldo, V. D., Genovese, G., Karam, J. A., Wood, C. G., Tannir, N. M., & Msaouel, P. (2021). Efficacy and Safety of Bevacizumab Plus Erlotinib in Patients with Renal Medullary Carcinoma. Cancers, 13(9), 2170. https://doi.org/10.3390/cancers13092170







