Radiation for Oligometastatic Lung Cancer in the Era of Immunotherapy: What Do We (Need to) Know?
Abstract
Simple Summary
Abstract
1. Introduction
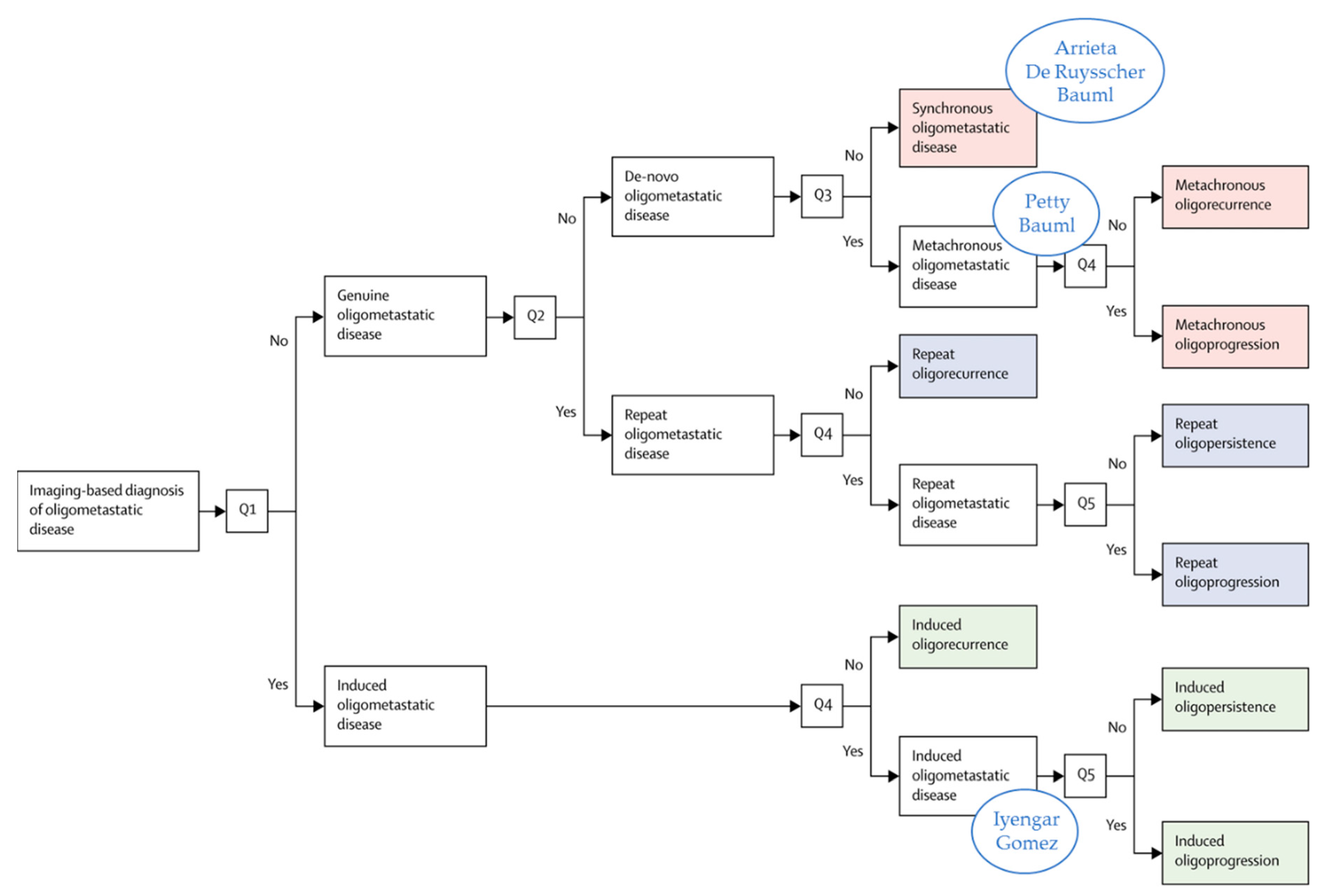
2. Prospective Evidence on the Treatment of Oligometastatic NSCLC before the Era of ICI
3. Prospective Evidence in the Era of ICI
| Author | Iyengar 2017 [12] | Gomez 2016 & 2019 [10,11] | De Ruysscher 2012 & 2018 [13,14] | Arrieta 2019 [15] | Petty 2018 [16] | Collen 2014 [17] | Bauml 2019 [27] |
|---|---|---|---|---|---|---|---|
| Trial type | Single center phase II RCT | Multicenter phase II RCT | Single arm phase II | Single armphase II | Multicenter Single arm phase II | Single arm phase II | Single arm phase II |
| Patients n | 29 | 49 | 39 | 37 | 29 | 26 | 45 |
| Period of inclusion | 2014–2016 | 2012–2016 | 2006–2010 | 2015–2017 | 2010–2015 | NR | 2015–2017 |
| Eligibility assessment | After CT | After CT/TKI | Before any treatment | Before any treatment | Before or after CT | Before LAT | After LAT |
| Synchronous | 100% | 94% | 100% | 100% | 0% | 73% | 31% |
| Metachronous | 0% | 6% | 0% | 0% | 100% | 27% | 69% |
| Max n of M+ | 5 | 3 | 5 | 5 | 5 | 5 | 4 |
| Histology nonsquamous squamous | 93% 7% | 90% 10% | 79% 21% | 95% 5% | 78% 22% | 92% 8% | 82% 18% |
| Mean/median age (years) | NR/64 | 63/61 | 62/NR | 56/NR | NR/65 | 62/NR | NR/64 |
| Single metastasis | NR | 65% | 87% | 38% | 11% | 54% | 62% |
| cN2/N3 | NR | 53% | 74% | NR | 40% | 52% | 36% |
| Driver mutations | 0% | 16% | NR | 43% | NR | NR | NR |
| Systemic R/ | CT +/− maint | CT/TKI +/− maint | CT/TKI/no | CT/TKI +/− maint (70%) | CT | CT/TKI/no | ICI |
| Response needed for LAT | At least SD | At least SD | No requirement | At least SD | At least SD | No requirement | - |
| LAT | SABR | RT/S | RT/S | RT/S/RFA | RT | SABR | RT/S/RFA |
| RT dose | 21–27 Gy/1 # 27–33 Gy/3 # 30–38 Gy/5 # (45 Gy/15 #) | NR | EQD2 ≥ 60 Gy | NR | 24–27 Gy/1 # 54 Gy/3 # 50 Gy/5 # 60 Gy/30 # | 50 Gy/10 # | NR |
| FDG-PET-CT | Not mandatory | Not mandatory | At diagnosis | At diagnosis, before inclusion & Follow-up | Not mandatory | Diagnosis & Follow-up | NR |
| Median FUP (m) (range) | 10 (2–30) | 39 (28–61) | Minimum of 84 | 33 | 24 | 16 (33–40) | 25 |
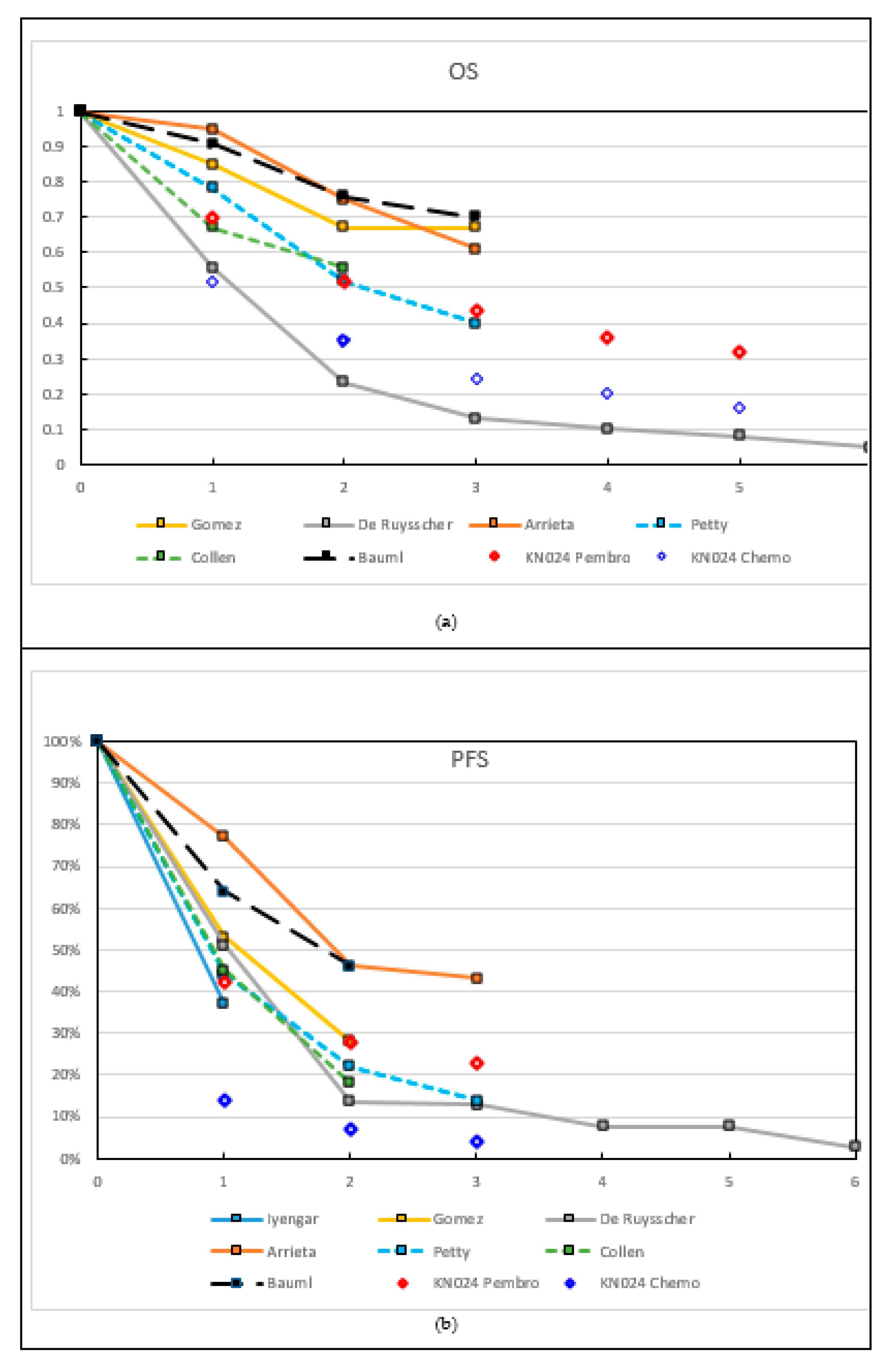
| Median PFS w LAT (m) (95% CI) | 10 | 14 (7–23) | 12 (10–14) | 24 (14–33) | 11 (8–16) | 11.2 | 19 (9–29) |
| Median PFS w/o LAT (m) (95% CI) | 4 | 4 (2–8) | - | - | - | - | - |
| Median OS (m) (95% CI) | Not reached | 41 (19 to not reached) | 14 (8–19) | Not reached | 28 (15–46) | 23 | 41 (27–56) |
| Median OS w/o LAT (m) (95% CI) | 17 NR | 17 (10–40) | - | - | - | - | - |
| 2y PFS | NR | 14% | 46% * | 22% * | NR | NR | |
| 2y OS | NR | 67% * | 23% | 75% * | 52% * | NR | 78% |
| Toxicity w LAT | 4 G3 | 5 G3 | 3 G3 | 8 G3 1 G4 | 0 G3 | 2 G3 | 5 G3 1 G4 |
| Toxicity w/o LAT | 2 G3 1 G4 | 2 G3 | - | - | - | - | - |
4. Challenges and Future Prospects
4.1. Which Oligometastatic Patients Have a Better Prognosis and Will Respond to Therapy?
4.2. How Long Should We Treat Oligometastatic Patients with ICI?
4.3. What Is the Optimal Fractionation and Dose When Combining Radiotherapy with ICI in Oligometastatic Disease?
4.4. What Is the Optimal Target Volume to Irradiate?
4.5. What Is the Best Sequence for Combining ICI and Radiotherapy?
4.6. What Is the Best Local Ablative Treatment–Radiotherapy or Surgery?
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2010, 12, CD007309. [Google Scholar] [CrossRef]
- Stevens, R.; Macbeth, F.; Toy, E.; Coles, B.; Lester, J.F. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst. Rev. 2015, 1, CD002143. [Google Scholar] [CrossRef]
- Mok, T.; Camidge, D.R.; Gadgeel, S.M.; Rosell, R.; Dziadziuszko, R.; Kim, D.W.; Pérol, M.; Ou, S.I.; Ahn, J.S.; Shaw, A.T.; et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. LBA51—KEYNOTE-024 5-year OS update: First-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50%. Ann. Oncol. 2020, 31, S1142–S1215. [Google Scholar] [CrossRef]
- Van Limbergen, E.J.; De Ruysscher, D.K.; Olivo Pimentel, V.; Marcus, D.; Berbee, M.; Hoeben, A.; Rekers, N.; Theys, J.; Yaromina, A.; Dubois, L.J.; et al. Combining radiotherapy with immunotherapy: The past, the present and the future. Br. J. Radiol. 2017, 90, 20170157. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.C.; Hendriks, L.E.L.; Berghmans, T.; Levy, A.; Hasan, B.; Faivre-Finn, C.; Giaj-Levra, M.; Giaj-Levra, N.; Girard, N.; Greillier, L.; et al. Definition of synchronous oligometastatic non-small cell lung cancer-a consensus report. J. Thorac. Oncol. 2019, 14, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-Term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Wanders, R.; van Baardwijk, A.; Dingemans, A.M.; Reymen, B.; Houben, R.; Bootsma, G.; Pitz, C.; van Eijsden, L.; Geraedts, W.; et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: Long-term results of a prospective phase II trial (Nct01282450). J. Thorac. Oncol. 2012, 7, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Wanders, R.; Hendriks, L.E.L.; van Baardwijk, A.; Reymen, B.; Houben, R.; Bootsma, G.; Pitz, C.; van Eijsden, L.; Dingemans, A.C. Progression-Free survival and overall survival beyond 5 years of NSCLC patients with synchronous oligometastases treated in a prospective phase II trial (NCT 01282450). J. Thorac. Oncol. 2018, 13, 1958–1961. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Barrón, F.; Maldonado, F.; Cabrera, L.; Corona-Cruz, J.F.; Blake, M.; Ramírez-Tirado, L.A.; Zatarain-Barrón, Z.L.; Cardona, A.F.; García, O.; et al. Radical consolidative treatment provides a clinical benefit and long-term survival in patients with synchronous oligometastatic non-small cell lung cancer: A phase II study. Lung Cancer 2019, 130, 67–75. [Google Scholar] [CrossRef]
- Petty, W.J.; Urbanic, J.J.; Ahmed, T.; Hughes, R.; Levine, B.; Rusthoven, K.; Papagikos, M.; Ruiz, J.R.; Lally, B.E.; Chan, M.; et al. Long-Term outcomes of a phase 2 trial of chemotherapy with consolidative radiation therapy for oligometastatic non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 527–535. [Google Scholar] [CrossRef]
- Collen, C.; Christian, N.; Schallier, D.; Meysman, M.; Duchateau, M.; Storme, G.; De Ridder, M. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann. Oncol. 2014, 25, 1954–1959. [Google Scholar] [CrossRef]
- Ashworth, A.B.; Senan, S.; Palma, D.A.; Riquet, M.; Ahn, Y.C.; Ricardi, U.; Congedo, M.T.; Gomez, D.R.; Wright, G.M.; Melloni, G.; et al. An individual patient data meta-analysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin. Lung Cancer 2014, 15, 346–355. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Derks, J.L.; Postmus, P.E.; Damhuis, R.A.; Houben, R.M.; Troost, E.G.; Hochstenbag, M.M.; Smit, E.F.; Dingemans, A.M. Single organ metastatic disease and local disease status, prognostic factors for overall survival in stage IV non-small cell lung cancer: Results from a population-based study. Eur. J. Cancer 2015, 51, 2534–2544. [Google Scholar] [CrossRef]
- Tang, C.; Lee, W.C.; Reuben, A.; Chang, L.; Tran, H.; Little, L.; Gumbs, C.; Wargo, J.; Futreal, A.; Liao, Z.; et al. Immune and circulating tumor DNA profiling after radiation treatment for oligometastatic non-small cell lung cancer: Translational correlatives from a mature randomized phase II trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radio-therapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Blake-Cerda, M.; Lozano-Ruíz, F.; Maldonado-Magos, F.; de la Mata-Moya, D.; Díaz-García, D.; Lara-Mejía, L.; Zatarain-Barrón, Z.L.; Cuevas-Góngora, M.F.; Barron-Barron, F.; Corona-Cruz, J.F.; et al. Consolidative stereotactic ablative radiotherapy (SABR) to intrapulmonary lesions is associated with prolonged progression-free survival and overall survival in oligometastatic NSCLC patients: A prospective phase 2 study. Lung Cancer 2021, 152, 119–126. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year overall survival for patients with advanced non small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Gettinger, S.; Horn, L.; Jackman, D.; Spigel, D.; Antonia, S.; Hellmann, M.; Powderly, J.; Heist, R.; Sequist, L.V.; Smith, D.C.; et al. Five-Year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: Results from the CA209-003 study. J. Clin. Oncol. 2018, 36, 1675–1684. [Google Scholar] [CrossRef]
- Bauml, J.M.; Mick, R.; Ciunci, C.; Aggarwal, C.; Davis, C.; Evans, T.; Deshpande, C.; Miller, L.; Patel, P.; Alley, E.; et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic non–small cell lung cancer a phase 2 trial. JAMA Oncol. 2019, 5, 1283–1290. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.S.M.E.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.U.; Aerts, J.G.J.V.; Bahce, I.; Niemeijer, A.L.N.; Chang, J.Y.; de Groot, P.M.; et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Lang, P.; Gomez, D.R.; Palma, D.A. Local ablative therapies in oligometastatic NSCLC: New data and new directions. Semin Respir. Crit. Care Med. 2020, 41, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Rheinheimer, S.; Heussel, C.P.; Mayer, P.; Gaissmaier, L.; Bozorgmehr, F.; Winter, H.; Herth, F.J.; Muley, T.; Liersch, S.; Bischoff, H.; et al. Oligoprogressive non-small-cell lung cancer under treatment with PD-(L)1 inhibitors. Cancers 2020, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, Y.; Furuta, H.; Uemura, T.; Watanabe, N.; Shimizu, J.; Horio, Y.; Kuroda, H.; Inaba, Y.; Kodaira, T.; Masago, K.; et al. Efficacy of local therapy for oligoprogressive disease after programmed cell death 1 blockade in advanced non-small cell lung cancer. Cancer Sci. 2020, 111, 4442–4452. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Cabiddu, M.; Tomasello, G.; De Stefani, A.; Bruschieri, L.; Vitali, E.; Ghilardi, M.; Borgonovo, K.; Barni, S.; et al. Addition of radiotherapy to the primary tumour in oligometastatic NSCLC: A systematic review and meta-analysis. Lung Cancer 2018, 126, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; Jimenez, E.D.L.M.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.M.; Lukas, R.V.; Goldschmidt, J.; Conkling, P.; Park, K.; Cortinovis, D.; de Marinis, F.; Rittmeyer, A.; Patel, J.D.; von Pawel, J.; et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase III OAK study. Lung Cancer 2019, 128, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. MYSTIC investigators. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Update on the Phase III NEPTUNE Trial of Imfinzi Plus Tremelimumab in Stage IV Non-Small Cell Lung Cancer. Available online: https://www.astrazeneca.com/media-centre/press-releases/2019/update-on-the-phase-iii-neptune-trial-of-imfinzi-plus-tremelimumab-in-stage-iv-non-small-cell-lung-cancer-21082019.html (accessed on 23 February 2021).
- Nabet, B.Y.; Esfahani, M.S.; Moding, E.J.; Hamilton, E.G.; Chabon, J.J.; Rizvi, H.; Steen, C.B.; Chaudhuri, A.A.; Liu, C.L.; Hui, A.B.; et al. Noninvasive early identification of therapeutic benefit from immune checkpoint inhibition. Cell 2020, 183, 363–376.e13. [Google Scholar] [CrossRef] [PubMed]
- Lussier, Y.A.; Khodarev, N.N.; Regan, K.; Corbin, K.; Li, H.; Ganai, S.; Khan, S.A.; Gnerlich, J.L.; Darga, T.E.; Fan, H.; et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS ONE 2012, 7, e50141. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year survival with durvalumab after chemoradiotherapy in stage III NSCLC-an update from the PACIFIC trial. J. Thorac. Oncol. 2021. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Vanneste, B.G.L.; Van Limbergen, E.J.; Dubois, L.; Samarska, I.V.; Wieten, L.; Aarts, M.J.B.; Marcelissen, T.; De Ruysscher, D. Immunotherapy as sensitizer for local radiotherapy. Oncoimmunology 2020, 9, 1832760. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Botticelli, A.; Cirillo, A.; Scagnoli, S.; Cerbelli, B.; Strigari, L.; Cortellini, A.; Pizzuti, L.; Vici, P.; De Galitiis, F.; Di Pietro, F.R.; et al. The agnostic role of site of metastasis in predicting outcomes in cancer patients treated with immunotherapy. Vaccines 2020, 8, 203. [Google Scholar] [CrossRef]
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef]
- Reynders, K.; Illidge, T.; Siva, S.; Chang, J.Y.; De Ruysscher, D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 2015, 41, 503–510. [Google Scholar] [CrossRef]
- Brooks, E.D.; Chang, J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019, 16, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Breen, W.G.; Leventakos, K.; Dong, H.; Merrell, K.W. Radiation and immunotherapy: Emerging mechanisms of synergy. J. Thorac. Dis. 2020, 12, 7011–7023. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv192–iv237. [Google Scholar] [CrossRef]
- Kordbacheh, T.; Honeychurch, J.; Blackhall, F.; Faivre-Finn, C.; Illidge, T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: Building better translational research platforms. Ann. Oncol. 2018, 29, 301–310. [Google Scholar] [CrossRef]
- Patel, S.A.; Minn, A.J. Combination cancer therapy with immune checkpoint blockade: Mechanisms and strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef]
- Pitroda, S.P.; Chmura, S.J.; Weichselbaum, R.R. Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncol. 2019, 20, e434–e442. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.; Wan, I.Y.; Yim, A.P. Impact of video-assisted thoracoscopic major lung resection on immune function. Asian Cardiovasc. Thorac. Ann. 2009, 17, 426–432. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peeters, S.T.H.; Van Limbergen, E.J.; Hendriks, L.E.L.; De Ruysscher, D. Radiation for Oligometastatic Lung Cancer in the Era of Immunotherapy: What Do We (Need to) Know? Cancers 2021, 13, 2132. https://doi.org/10.3390/cancers13092132
Peeters STH, Van Limbergen EJ, Hendriks LEL, De Ruysscher D. Radiation for Oligometastatic Lung Cancer in the Era of Immunotherapy: What Do We (Need to) Know? Cancers. 2021; 13(9):2132. https://doi.org/10.3390/cancers13092132
Chicago/Turabian StylePeeters, Stephanie T. H., Evert J. Van Limbergen, Lizza E. L. Hendriks, and Dirk De Ruysscher. 2021. "Radiation for Oligometastatic Lung Cancer in the Era of Immunotherapy: What Do We (Need to) Know?" Cancers 13, no. 9: 2132. https://doi.org/10.3390/cancers13092132
APA StylePeeters, S. T. H., Van Limbergen, E. J., Hendriks, L. E. L., & De Ruysscher, D. (2021). Radiation for Oligometastatic Lung Cancer in the Era of Immunotherapy: What Do We (Need to) Know? Cancers, 13(9), 2132. https://doi.org/10.3390/cancers13092132







