IFN-γ Critically Enables the Intratumoural Infiltration of CXCR3+ CD8+ T Cells to Drive Squamous Cell Carcinoma Regression
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Diet-Based Drug Delivery
2.3. Establishment of the SCC Mouse Model
2.4. In Vitro IFN-γ Treatment
2.5. In Vivo Treatments
2.6. Schedule of In Vivo Depletion Experiments
2.7. Schedule of Anti-IFN-γ and Anti-CXCR3 Treatments
2.8. Cell Isolation from Blood, Lymph Node, and Tumour
2.9. Phenotypic Analysis of Cell Populations by Flow Cytometry
2.10. Chemokine Analysis
2.11. Chimeric Mouse Model
2.12. Statistical Analysis
3. Results
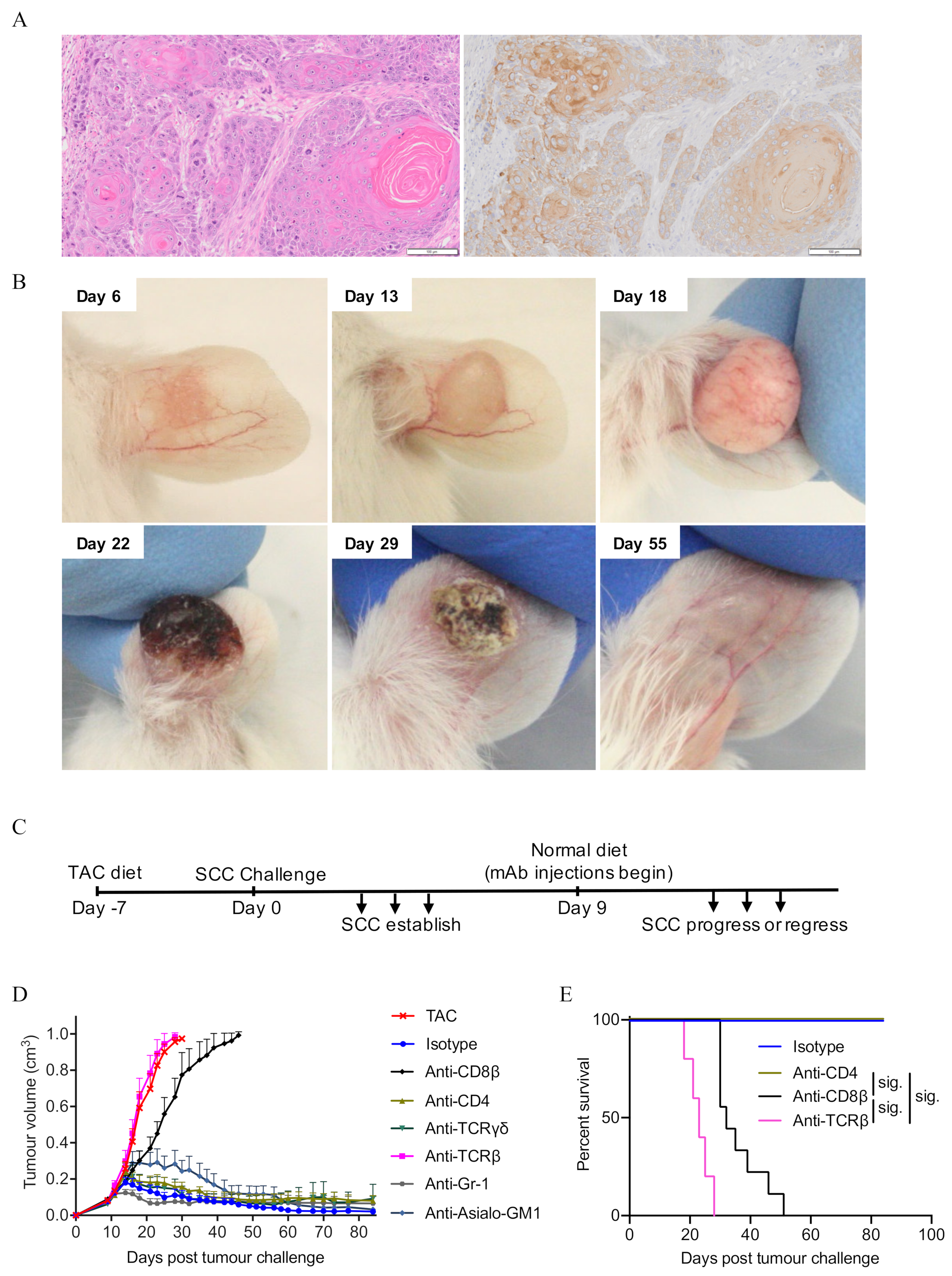
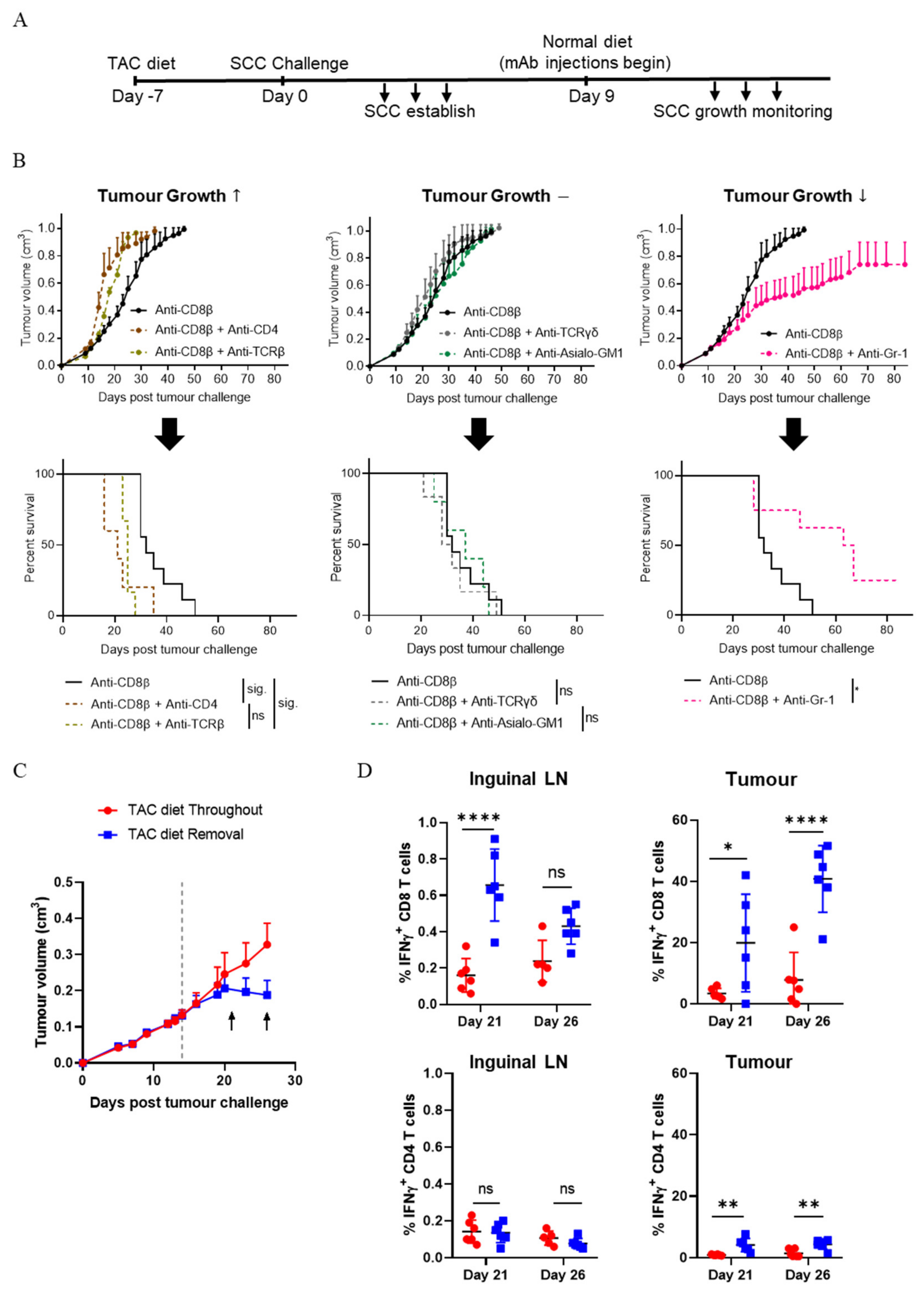
3.1. CD8+ T Cells Are Critical for the Control of SCC
3.2. CD4+ T Cells Contribute to the Control of SCC Growth
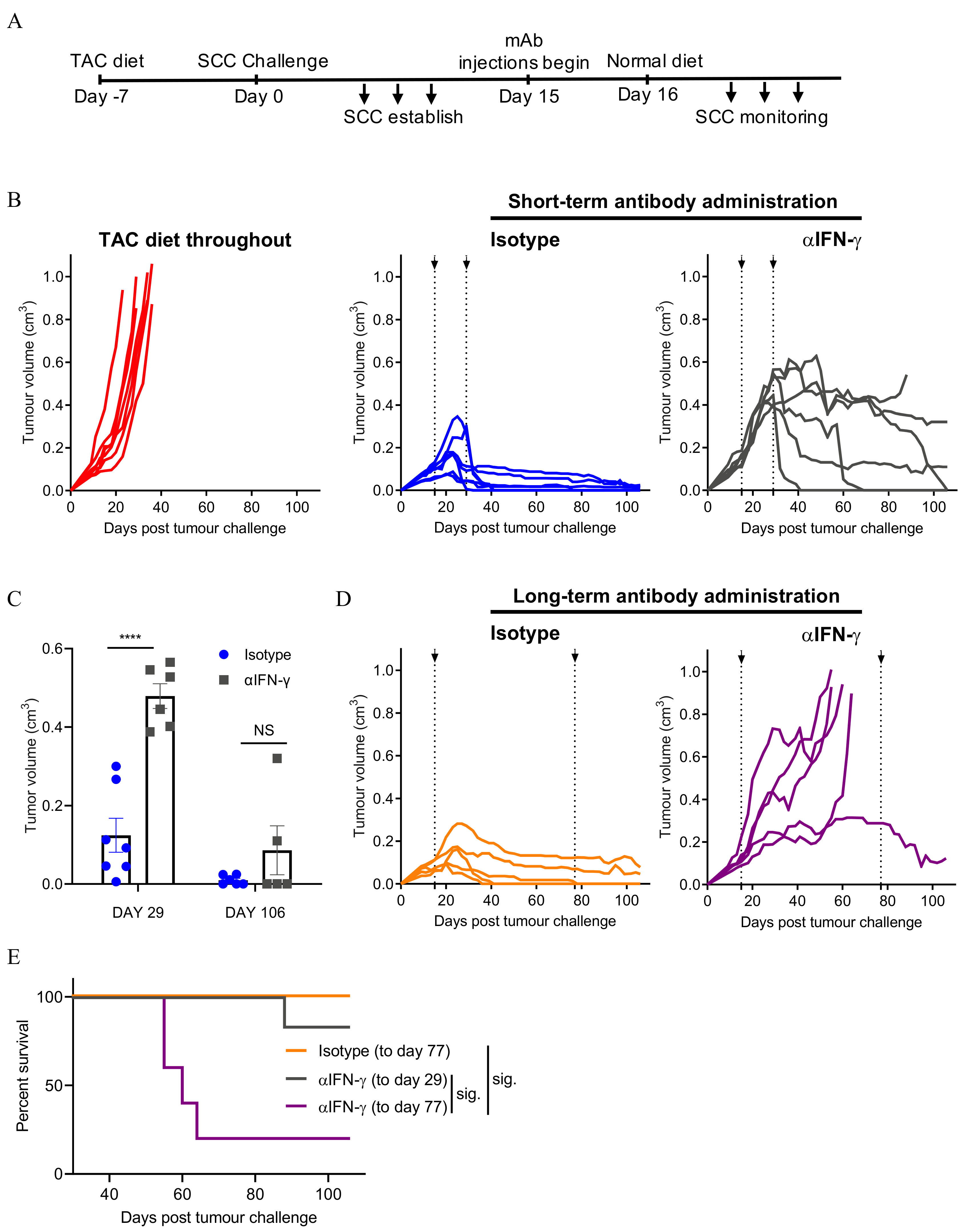
3.3. Neutralising IFN-γ Prevents SCC Regression
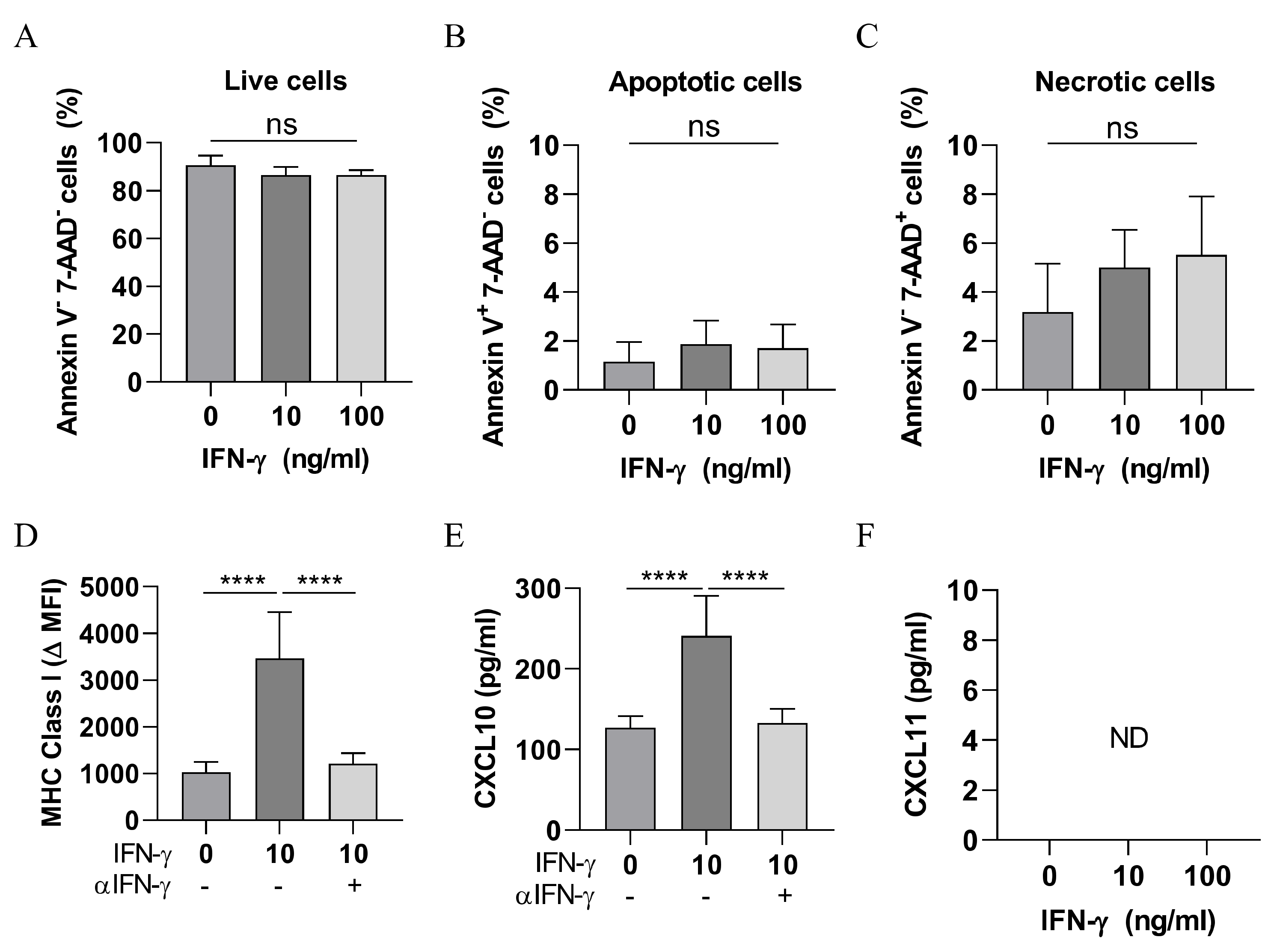
3.4. IFN-γ Is Not Directly Cytotoxic to SCC Cells
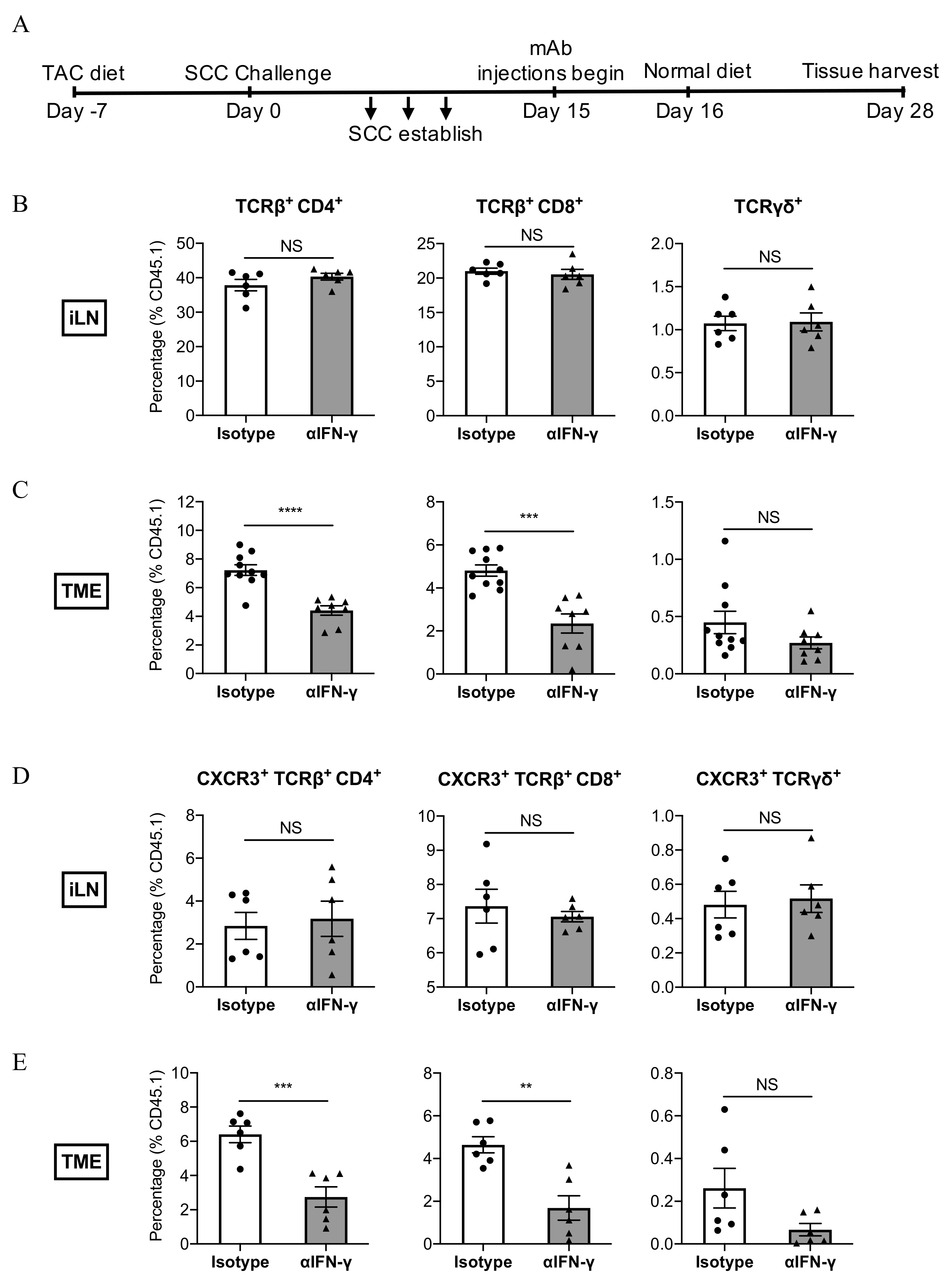
3.5. IFN-γ Neutralisation Impairs the Infiltration of CXCR3+ CD8+ T Cells Into SCC
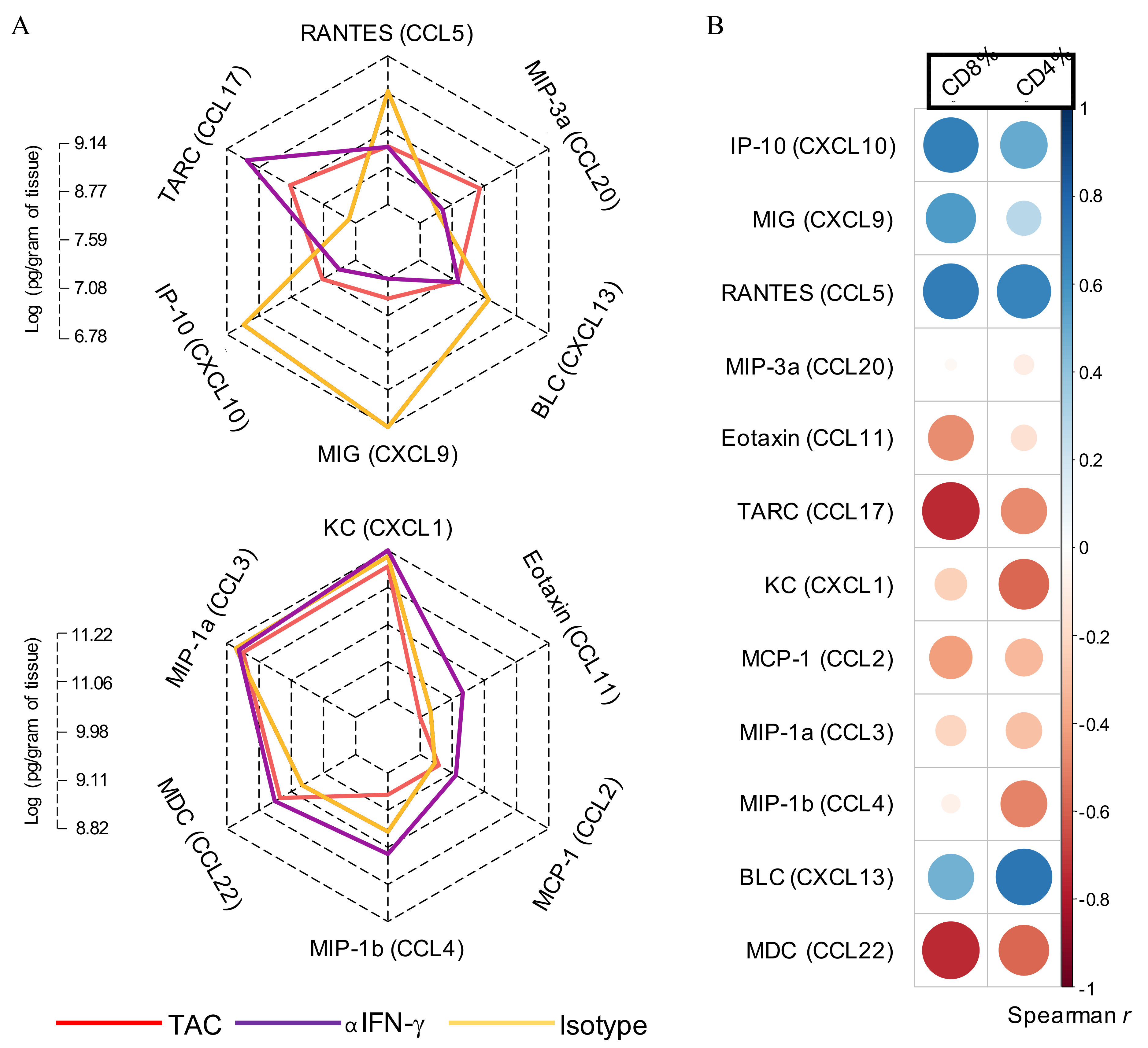
3.6. IFN-γ Neutralisation Diminishes CXCL10 and CCL5 Production Within SCC
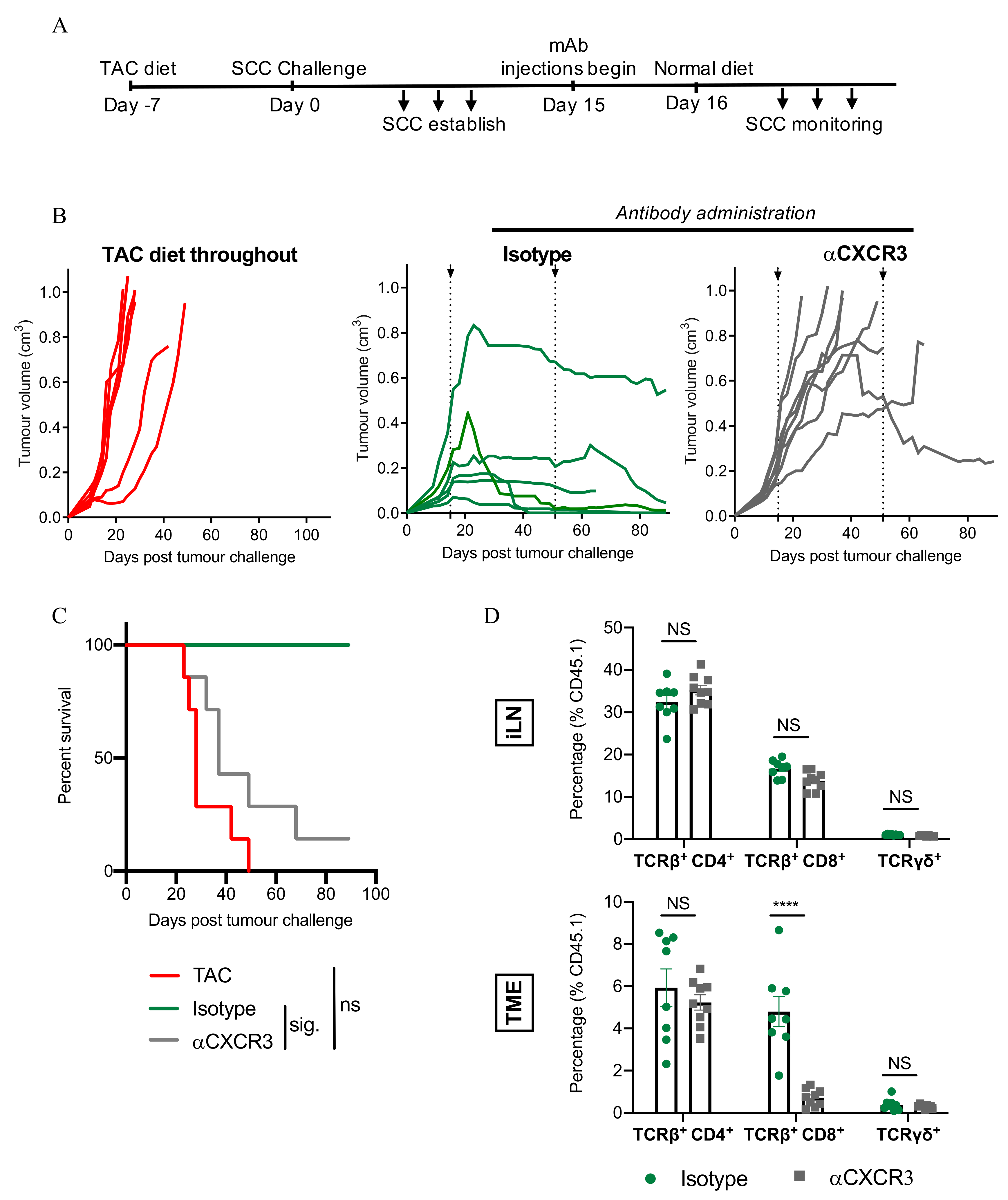
3.7. CXCR3-Blockade Prevents CD8+ T Cell Recruitment and SCC Regression
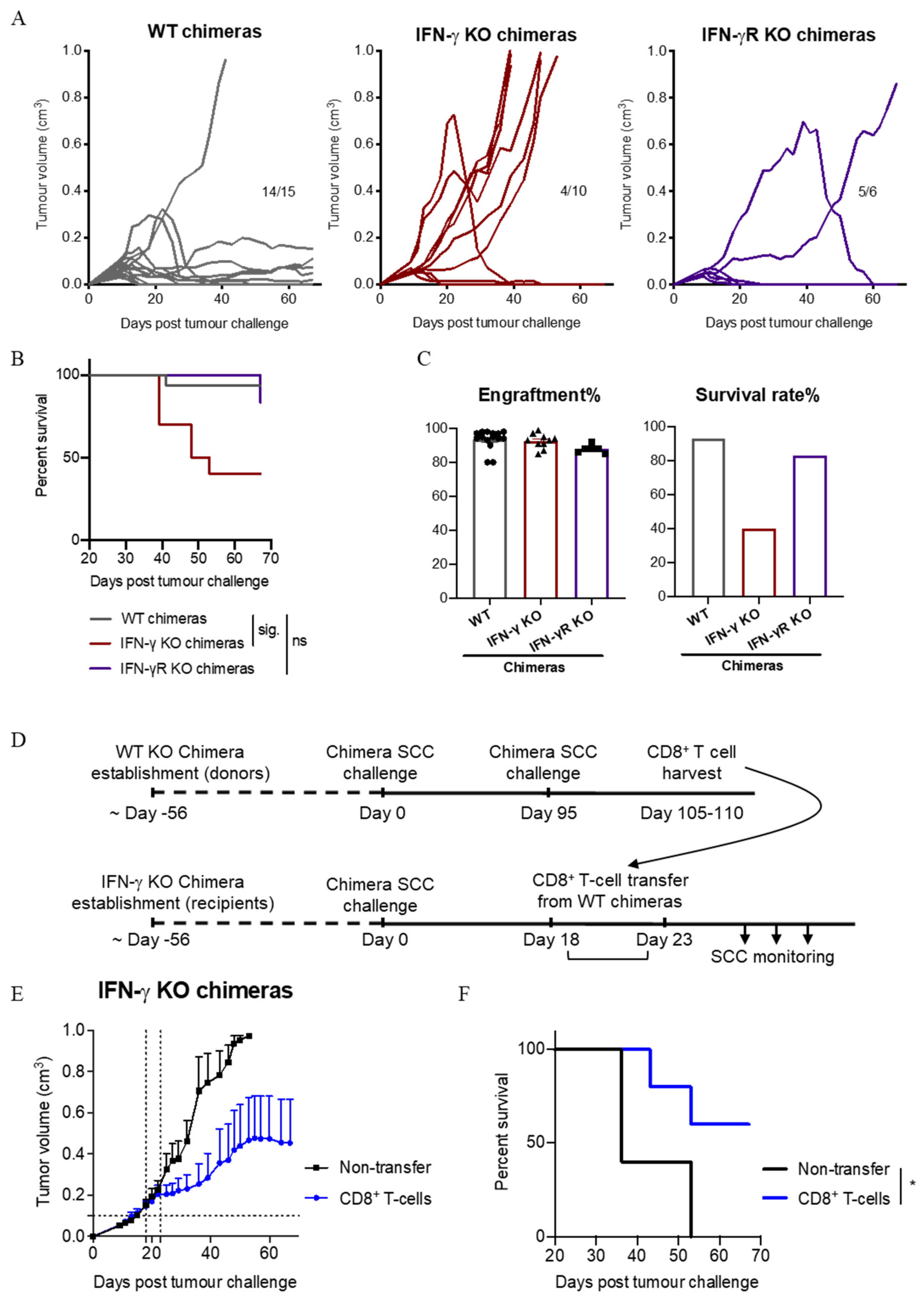
3.8. IFN-γ Secretion by Immune Cells Is Important for SCC Control
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef]
- Jung, J.-W.; Overgaard, N.H.; Burke, M.T.; Isbel, N.; Frazer, I.H.; Simpson, F.; Wells, J.W. Does the nature of residual immune function explain the differential risk of non-melanoma skin cancer development in immunosuppressed organ transplant recipients? Int. J. Cancer 2015, 138, 281–292. [Google Scholar] [CrossRef]
- Chockalingam, R.; Downing, C.; Tyring, S.K. Cutaneous Squamous Cell Carcinomas in Organ Transplant Recipients. J. Clin. Med. 2015, 4, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Geissler, E.K. Skin cancer in solid organ transplant recipients: Are mTOR inhibitors a game changer? Transplant. Res. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Lindelof, B. Incidence of skin cancer in 5356 patients following organ transplantation. Br. J. Dermatol. 2000, 143, 513. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.; Bridge, J.A.; Maruthayanar, P.; Overgaard, N.H.; Jung, J.-W.; Simpson, F.; Prow, T.W.; Soyer, H.P.; Frazer, I.H.; Freeman, M.; et al. Comparative Immune Phenotypic Analysis of Cutaneous Squamous Cell Carcinoma and Intraepidermal Carcinoma in Immune-Competent Individuals: Proportional Representation of CD8+ T-Cells but Not FoxP3+ Regulatory T-Cells Is Associated with Disease Stage. PLoS ONE 2014, 9, e110928. [Google Scholar] [CrossRef]
- Tuong, Z.K.; Lewandowski, A.; Bridge, J.A.; Cruz, J.L.G.; Yamada, M.; Lambie, D.; Lewandowski, R.; Steptoe, R.J.; Leggatt, G.R.; Simpson, F.; et al. Cytokine/chemokine profiles in squamous cell carcinoma correlate with precancerous and cancerous disease stage. Sci. Rep. 2019, 9, 17754. [Google Scholar] [CrossRef]
- Chew, H.Y.; De Lima, P.O.; Cruz, J.L.G.; Banushi, B.; Echejoh, G.; Hu, L.; Joseph, S.R.; Lum, B.; Rae, J.; O’Donnell, J.S.; et al. Endocytosis Inhibition in Humans to Improve Responses to ADCC-Mediating Antibodies. Cell 2020, 180, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Berhane, T.; Halliday, G.; Cooke, B.; Barnetson, R. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br. J. Dermatol. 2002, 146, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R.; Ahmed, R.A. Analysis of the mononuclear inflammatory cell infiltrate in the non-tumorigenic, pre-tumorigenic and tumorigenic keratinocytic hyperproliferative lesions of the skin. Cancer Biol. Ther. 2005, 4, 819–821. [Google Scholar] [CrossRef]
- Bridge, J.A.; Lee, J.C.; Daud, A.; Wells, J.W.; Bluestone, J.A. Cytokines, Chemokines, and Other Biomarkers of Response for Checkpoint Inhibitor Therapy in Skin Cancer. Front. Med. 2018, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and Costs of Skin Cancer Treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kripke, M.L. Antigenicity of Murine Skin Tumors Induced by Ultraviolet Light2. J. Natl. Cancer Inst. 1974, 53, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.; Arina, A.; Engels, B.; Spiotto, M.T.; Sidney, J.; Sette, A.; Karrison, T.G.; Weichselbaum, R.R.; Rowley, D.A.; Schreiber, H. Spleen Cells from Young But Not Old Immunized Mice Eradicate Large Established Cancers. Clin. Cancer Res. 2012, 18, 2526–2533. [Google Scholar] [CrossRef]
- Mullen, C.A.; Urban, J.L.; Van Waes, C.; A Rowley, D.; Schreiber, H. Multiple cancers. Tumor burden permits the outgrowth of other cancers. J. Exp. Med. 1985, 162, 1665–1682. [Google Scholar] [CrossRef]
- Ward, P.L.; Koeppen, H.; Hurteau, T.; Schreiber, H. Tumor antigens defined by cloned immunological probes are highly polymorphic and are not detected on autologous normal cells. J. Exp. Med. 1989, 170, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Schreiber, K.; Arina, A.; Khodarev, N.N.; Efimova, E.V.; Rowley, N.A.; Weichselbaum, R.R.; Schreiber, H. Progression of cancer from indolent to aggressive despite antigen retention and increased expression of interferon-gamma inducible genes. Cancer Immun. 2011, 11, 2. [Google Scholar] [PubMed]
- Ward, P.L.; Schreiber, H. MHC class I restricted T cells and immune surveillance against transplanted ultraviolet light-induced tumors. Semin. Cancer Biol. 1991, 2, 321–328. [Google Scholar]
- Wenzel, J.; Tomiuk, S.; Zahn, S.; Küsters, D.; Vahsen, A.; Wiechert, A.; Mikus, S.; Birth, M.; Scheler, M.; Von Bubnoff, D.; et al. Transcriptional profiling identifies an interferon-associated host immune response in invasive squamous cell carcinoma of the skin. Int. J. Cancer 2008, 123, 2605–2615. [Google Scholar] [CrossRef]
- Khou, S.; Popa, A.; Luci, C.; Bihl, F.; Meghraoui-Kheddar, A.; Bourdely, P.; Salavagione, E.; Cosson, E.; Rubod, A.; Cazareth, J.; et al. Tumor-Associated Neutrophils Dampen Adaptive Immunity and Promote Cutaneous Squamous Cell Carcinoma Development. Cancers 2020, 12, 1860. [Google Scholar] [CrossRef]
- Fujita, H.; Suárez-Fariñas, M.; Mitsui, H.; Gonzalez, J.; Bluth, M.J.; Zhang, S.; Felsen, D.; Krueger, J.G.; Carucci, J.A. Langerhans Cells from Human Cutaneous Squamous Cell Carcinoma Induce Strong Type 1 Immunity. J. Investig. Dermatol. 2012, 132, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Jinnin, M.; Kajihara, I.; Nakashima, T.; Aoi, J.; Harada, M.; Igata, T.; Masuguchi, S.; Fukushima, S.; Ihn, H. Cytokine expression profiles in the sera of cutaneous squamous cell carcinoma patients. Drug Discov. Ther. 2016, 10, 172–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wakita, D.; Chamoto, K.; Ohkuri, T.; Narita, Y.; Ashino, S.; Sumida, K.; Nishikawa, H.; Shiku, H.; Togashi, Y.; Kitamura, H.; et al. IFN-gamma-dependent type 1 immunity is crucial for immunosurveillance against squamous cell carcinoma in a novel mouse carcinogenesis model. Carcinogenesis 2009, 30, 1408–1415. [Google Scholar] [CrossRef]
- Kaplan, D.H.; Shankaran, V.; Dighe, A.S.; Stockert, E.; Aguet, M.; Old, L.J.; Schreiber, R.D. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7556–7561. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Viarisio, D.; Mueller-Decker, K.; Kloz, U.; Aengeneyndt, B.; Kopp-Schneider, A.; Gröne, H.-J.; Gheit, T.; Flechtenmacher, C.; Gissmann, L.; Tommasino, M. E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice. PLoS Pathog. 2011, 7, e1002125. [Google Scholar] [CrossRef]
- Jung, J.-W.; Veitch, M.; Bridge, J.A.; Overgaard, N.H.; Cruz, J.L.; Linedale, R.; Franklin, M.E.; Saunders, N.A.; Simpson, F.; Frazer, I.H.; et al. Clinically-Relevant Rapamycin Treatment Regimens Enhance CD8+Effector Memory T Cell Function In The Skin and Allow their Infiltration into Cutaneous Squamous Cell Carcinoma. OncoImmunology 2018, 7, e1479627. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cruz, J.L.; Joseph, S.; Pett, N.; Chew, H.Y.; Tuong, Z.K.; Okano, S.; Kelly, G.; Veitch, M.; Simpson, F.; et al. Characterization of 7A7, an anti-mouse EGFR monoclonal antibody proposed to be the mouse equivalent of cetuximab. Oncotarget 2018, 9, 12250–12260. [Google Scholar] [CrossRef]
- Uppaluri, R.; Sheehan, K.C.F.; Wang, L.; Bui, J.D.; Brotman, J.J.; Lu, B.; Gerard, C.; Hancock, W.W.; Schreiber, R.D. Prolongation of Cardiac and Islet Allograft Survival by a Blocking Hamster Anti-Mouse CXCR3 Monoclonal Antibody. Transplantation 2008, 86, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, G.P.; Hufbauer, M.; Kasper, H.U.; Weißenborn, S.J.; Smola, S.; Pfister, H. Spontaneous tumour development in human papillomavirus type 8 E6 transgenic mice and rapid induction by UV-light exposure and wounding. J. Gen. Virol. 2009, 90, 2855–2864. [Google Scholar] [CrossRef]
- Greenhalgh, D.A.; Wang, X.J.; Rothnagel, J.A.; Eckhardt, J.N.; Quintanilla, M.I.; Barber, J.L.; Bundman, D.S.; Longley, M.A.; Schlegel, R.; Roop, D.R. Transgenic mice expressing targeted HPV-18 E6 and E7 oncogenes in the epidermis develop verrucous lesions and spontaneous, rasHa-activated papillomas. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1994, 5, 667–675. [Google Scholar]
- Kang, J.-K.; Kim, J.-H.; Lee, S.-H.; Kim, D.-H.; Kim, H.-S.; Lee, J.-E.; Seo, J.-S. Development of spontaneous hyperplastic skin lesions and chemically induced skin papillomas in transgenic mice expressing human papillomavirus type 16 E6/E7 genes. Cancer Lett. 2000, 160, 177–183. [Google Scholar] [CrossRef]
- Kripke, M.L. Latency, histology, and antigenicity of tumors induced by ultraviolet light in three inbred mouse strains. Cancer Res. 1977, 37, 1395–1400. [Google Scholar] [PubMed]
- Urban, J.L.; Burton, R.C.; Holland, J.M.; Kripke, M.L.; Schreiber, H. Mechanisms of syngeneic tumor rejection. Susceptibility of host-selected progressor variants to various immunological effector cells. J. Exp. Med. 1982, 155, 557–573. [Google Scholar] [CrossRef]
- Fung, J.J. Tacrolimus and transplantation: A decade in review. Transplantation 2004, 77 (Suppl. S9), S41–S43. [Google Scholar] [CrossRef]
- Lai, Y.-P.; Lin, C.-C.; Liao, W.-J.; Tang, C.-Y.; Chen, S.-C. CD4+ T Cell-Derived IL-2 Signals during Early Priming Advances Primary CD8+ T Cell Responses. PLoS ONE 2009, 4, e7766. [Google Scholar] [CrossRef]
- Schietinger, A.; Philip, M.; Liu, R.B.; Schreiber, K.; Schreiber, H. Bystander killing of cancer requires the cooperation of CD4+ and CD8+ T cells during the effector phase. J. Exp. Med. 2010, 207, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Shklovskaya, E.; Terry, A.M.; Guy, T.V.; Buckley, A.; A Bolton, H.; Zhu, E.; Holst, J.; Groth, B.F.D.S. Tumour-specific CD4 T cells eradicate melanoma via indirect recognition of tumour-derived antigen. Immunol. Cell Biol. 2016, 94, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Shin, E.-C.; Perosa, F.; Vacca, A.; Dammacco, F.; Racanelli, V. MHC Class I Antigen Processing and Presenting Machinery: Organization, Function, and Defects in Tumor Cells. J. Natl. Cancer Inst. 2013, 105, 1172–1187. [Google Scholar] [CrossRef]
- Moore, M.B.; Kurago, Z.B.; Fullenkamp, C.A.; Lutz, C.T. Squamous cell carcinoma cells differentially stimulate NK cell effector functions: The role of IL-18. Cancer Immunol. Immunother. 2003, 52, 107–115. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Vanheule, V.; Janssens, R.; Struyf, S.; Proost, P. Overview of the Mechanisms that May Contribute to the Non-Redundant Activities of Interferon-Inducible CXC Chemokine Receptor 3 Ligands. Front. Immunol. 2018, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guan, X.; Ma, X. Interferon regulatory factor 1 is an essential and direct transcriptional activator for interferon {gamma}-induced RANTES/CCl5 expression in macrophages. J. Biol. Chem. 2005, 280, 24347–24355. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, F.; Ping, Y.; Wang, L.; Chen, X.; Wang, D.; Cao, L.; Zhao, S.; Li, B.; Kalinski, P.; et al. Local production of the chemokines CCL5 and CXCL10 attracts CD8+ T lymphocytes into esophageal squamous cell carcinoma. Oncotarget 2015, 6, 24978–24989. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Veitch, M.; Kelly, G.A.; Tuong, Z.K.; Cruz, J.G.; Frazer, I.H.; Wells, J.W. IFN-γ Critically Enables the Intratumoural Infiltration of CXCR3+ CD8+ T Cells to Drive Squamous Cell Carcinoma Regression. Cancers 2021, 13, 2131. https://doi.org/10.3390/cancers13092131
Zeng Z, Veitch M, Kelly GA, Tuong ZK, Cruz JG, Frazer IH, Wells JW. IFN-γ Critically Enables the Intratumoural Infiltration of CXCR3+ CD8+ T Cells to Drive Squamous Cell Carcinoma Regression. Cancers. 2021; 13(9):2131. https://doi.org/10.3390/cancers13092131
Chicago/Turabian StyleZeng, Zhen, Margaret Veitch, Gabrielle A. Kelly, Zewen K. Tuong, Jazmina G. Cruz, Ian H. Frazer, and James W. Wells. 2021. "IFN-γ Critically Enables the Intratumoural Infiltration of CXCR3+ CD8+ T Cells to Drive Squamous Cell Carcinoma Regression" Cancers 13, no. 9: 2131. https://doi.org/10.3390/cancers13092131
APA StyleZeng, Z., Veitch, M., Kelly, G. A., Tuong, Z. K., Cruz, J. G., Frazer, I. H., & Wells, J. W. (2021). IFN-γ Critically Enables the Intratumoural Infiltration of CXCR3+ CD8+ T Cells to Drive Squamous Cell Carcinoma Regression. Cancers, 13(9), 2131. https://doi.org/10.3390/cancers13092131







